Phosphorus Availabilities Differ between Cropland and Forestland in Shelterbelt Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Analysis of Basic Soil Chemical and Physical Properties
2.3. Soil P extraction and Fractionation
2.4. Statistical Analysis
3. Results
3.1. Subsection Soil Properties and P Status in Cropland and Forestland
3.2. Soil P Fractionation in Cropland and Forestland
4. Discussion
4.1. Greater P Build-up in the Surface Soil in the Cropland than in the Forestland
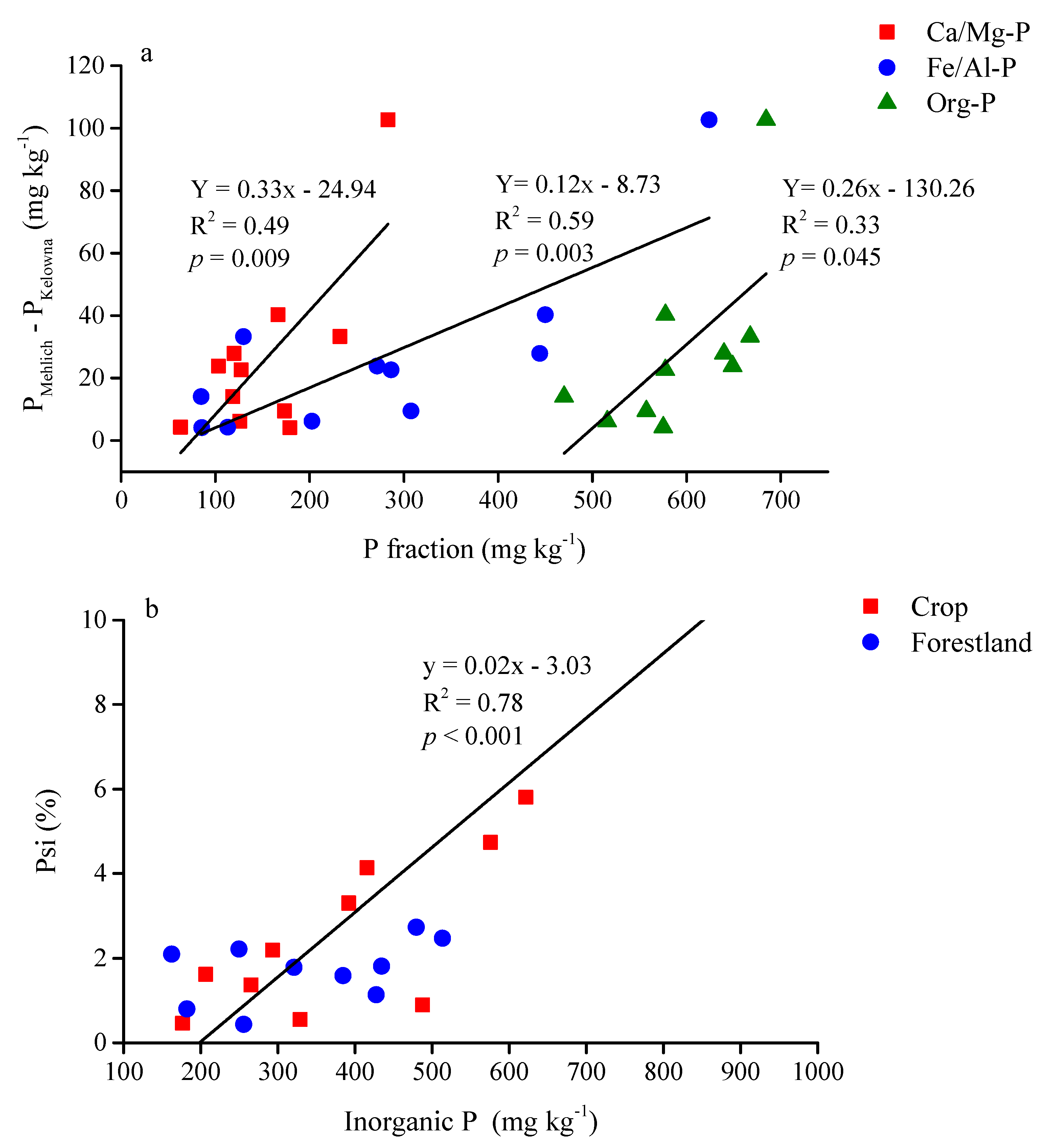
4.2. Soil Test P (PKelowna) is Related to Fe/Al-P
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thevathasan, N.V.; Gordon, A.M.; Bradley, R. Agroforestry research and development in Canada: The way forward. In The Future of Global Land Use; Nair, P.K.R., Garrity, D.P., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 247–283. [Google Scholar]
- McNaughton, K.G. Effects of windbreaks on turbulent transport and microclimate. Agric. Ecosyst. Environ. 1988, 22–23, 17–39. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Soil organic carbon sequestration in agroforestry systems. Rev. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef]
- Kort, J.; Turnock, R. Carbon reservoir and biomass in Canadian prairie shelterbelts. Agrofor. Syst. 1998, 44, 175–186. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Van Rees, K.C.J. Soil organic carbon sequestration by shelterbelt agroforestry systems in Saskatchewan. Can. J. Soil Sci. 2017, 97, 394–409. [Google Scholar] [CrossRef]
- Kowalchuk, T.E.; de Jong, E. Shelterbelts and their effect on crop yield. Can. J. Soil Sci. 1995, 75, 543–550. [Google Scholar] [CrossRef]
- Qiao, Y.; Fan, J.; Wang, Q. Effects of farmland shelterbelts on accumulation of soil nitrate in agro-ecosystems of an oasis in the Heihe River Basin, China. Agric. Ecosyst. Environ. 2016, 235, 182–192. [Google Scholar] [CrossRef]
- Manunta, P.; Kryzanowski, L.; Keyes, D. Preliminary Assessment of Available Soil P in Alberta: Status and Trends; Soil Quality Program, Conservation and Development Branch; Alberta Agriculture, Food and Rural Development: Edmonton, AB, Canada, 2000; p. 64.
- Paterson, B.A.; Olson, B.M.; Bennett, D.R. Alberta Soil Phosphorus Limits Project; Volume 1: Summary and recommendations; Alberta Agriculture, Food and Rural Development: Lethbridge, AB, Canada, 2006; p. 82.
- Zhang, M.; Wright, R.; Heaney, D.; Vanderwe, D. Comparison of different phosphorus extraction and determination methods using manured soils. Can. J. Soil Sci. 2004, 84, 469–475. [Google Scholar] [CrossRef]
- McKenzie, R.H.; Kryzanowski, L.; Cannon, K.; Solberg, E.; Penney, D.; Coy, G.; Heaney, D.; Harapiak, J.; Flore, N. Field Evaluation of Laboratory Tests for Soil Phosphorus; Alberta Agricultural Research Institute Report No. 90 M230; Alberta Innovation and Science: Edmonton, AB, Canada, 1995. [Google Scholar]
- Howard, A.E. Agronomic thresholds for soil phosphorus in Alberta: A review. In Alberta Soil Phosphorus Limits Project; Alberta Agriculture, Food and Rural Development: Lethbridge, AB, Canada, 2016; Volume 5, p. 42. [Google Scholar]
- McKenzie, R.H.; Stewart, J.W.B.; Dormaar, J.F.; Schaalje, G.B. Long-term crop rotation and fertilizer effects on phosphorus transformations: I. In a Chernozemic soil. Can. J. Soil Sci. 1992, 72, 569–579. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, R.H.; Bremer, E. Relationship of soil phosphorus fractions to phosphorus soil tests and fertilizer response. Can. J. Soil Sci. 2003, 83, 443–449. [Google Scholar] [CrossRef]
- Chirino-Valle, M.R.D.; Condron, L.M. Impact of different tree species on soil phosphorus immediately following grassland afforestation. J. Soil Sci. Plant Nutr. 2016, 16, 477–489. [Google Scholar] [CrossRef]
- Li, J.; Richter, D.; Mendoza, A.; Heine, P. Four-decade responses of soil trace elements to an aggrading old-field forest: B, Mn, Zn, Cu, and Fe. Ecology 2008, 89, 2911–2923. [Google Scholar] [CrossRef]
- Liversley, S.J.; Gregory, P.J.; Buresh, R.J. Competition in tree row Agroforestry systems. 3. Soil water distribution and dynamics. Plant Soil 2004, 264, 129–139. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Heenan, D.P.; Marschner, P.; McNeill, A.M. Long-term effects of crop rotation, stubble management and tillage on soil phosphorus dynamics. Soil Res. 2006, 44, 611–618. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Kleinman, P.J.A.; McDowell, R.W.; Gitau, M.; Bryant, R.B. Modeling phosphorus transport in agricultural watersheds: Processes and possibilities. J. Soil Water Conserv. 2002, 57, 425–439. [Google Scholar]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Tiessen, H.; Stewart, J.W.B.; Cole, C.V. Pathways of phosphorus transformations in soils of differing pedogenesis. Soil Sci. Soc. Am. J. 1984, 48, 853–858. [Google Scholar] [CrossRef]
- Motavalli, P.P.; Miles, R.J. Soil phosphorus fractions after 111 years of animal manure and fertilizers applications. Biol. Fertil. Soils 2002, 36, 35–42. [Google Scholar] [CrossRef]
- Castillo, M.S.; Wright, A.L. Microbial activity and phosphorus availability in a subtropical soil under different land uses. World J. Agric. Sci. 2008, 4, 314–320. [Google Scholar]
- Garcia-Montiel, D.C.; Nelly, C.H.; Melillo, J.; Thomas, S.; Steudler, P.A.; Cerri, C.C. Soil phosphorus transformation following forest clearing for pasture in the Brazilian Amazon. Soil Sci. Soc. Am. J. 2000, 64, 1792–1804. [Google Scholar] [CrossRef]
- Paul, K.I.; Polglase, P.J.; Nyakuengama, J.G.; Khanna, P.K. Change in soil carbon following afforestation. For. Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Frossard, E.; Stewart, J.W.B.; St. Arnaud, R.J. Distribution and mobility of phosphorus in grassland and forest soils of Saskatchewan. Can. J. Soil Sci. 1989, 69, 401–416. [Google Scholar] [CrossRef]
- Vaithiyanathan, P.; Correll, D.L. The Rhode River Watershed: Phosphorus Distribution and Export in Forest and Agricultural Soils. J. Environ. Qual. 1992, 21, 280–288. [Google Scholar] [CrossRef]
- Frossard, E.; Condron, L.M.; Oberson, A.; Sinaj, S.; Fardeau, J.C. Processes governing phosphorus availability in temperate soils. J. Environ. Qual. 2000, 29, 15–23. [Google Scholar] [CrossRef]
- Environment Canada. Alberta Weather Condition. 2012. Available online: http://weather.gc.ca/forecast/canada/index e.html?id=AB (accessed on 2 May 2017).
- Soil Classification Working Group. The Canadian System of Soil Classification; NRC Research Press: Ottawa, ON, Canada, 1998; p. 187. [Google Scholar]
- Baah-Acheamfour, M.; Chang, S.X.; Cameron, N.C.; Bork, E.W. Carbon pool size and stability are affected by trees and grassland cover types within agroforestry systems of western Canada. Agric. Ecosyst. Environ. 2015, 213, 105–113. [Google Scholar] [CrossRef]
- Lim, S.S.; Baah-Acheamfour, M.; Choi, W.J.; Arshad, M.A.; Fatemi, F.; Banerjee, S.; Carlyle, C.N.; Bork, E.W.; Park, H.J.; Chang, S.X. Soil organic carbon stocks in three Canadian agroforestry systems from surface organic to deeper mineral soils. For. Ecol. Manag. 2018, 417, 103–109. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; Francis and Taylor Group LLC/CRC Press: Boca Raton, FL, USA, 2006; pp. 334887–342742. [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Qian, P.; Schoenau, J.J.; Karamanos, R.E. Simultaneous extraction of available phosphorus and potassium with a new soil test: A modification of the Kelowna extraction. Commun. Soil Sci. Plant Anal. 1994, 25, 627–635. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution methods for the determination of phosphate in natural water. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Ige, D.V.; Akinremi, O.O.; Flaten, D.N.; Ajiboye, B.; Kashem, M.A. Phosphorus sorption capacity of alkaline Manitoba soils and its relationship to soil properties. Can. J. Soil Sci. 2005, 85, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, P.J.A.; Sharpley, A.N. Estimation soil phosphorus sorption saturation from Mehlich-3 data. Commun. Soil Sci. Plant Anal. 2002, 33, 1825–1839. [Google Scholar] [CrossRef]
- Nair, V.D.; Graetz, D.A.; Portier, K.M. Forms of phosphorus in soil profiles from dairies of south Florida. Soil Sci. Soc. Am. J. 1995, 59, 1244–1249. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis Part 3—Chemical Methods; SSSA Book Ser. 5.3; SSSA, ASA: Madison, WI, USA, 1996. [Google Scholar]
- Baah-Acheamfour, M.; Cameron, N.C.; Bork, E.W.; Chang, S.X. Forest and perennial herbland cover reduce microbial respiration but increase root respiration in agroforestry systems. Agric. For. Meteorol. 2020, 280, 107790. [Google Scholar] [CrossRef]
- Sharpley, A.N. Depth of surface soil-runoff interaction as affected by rainfall, soil slope and management. Soil Sci. Soc. Am. J. 1985, 49, 1010–1015. [Google Scholar] [CrossRef]
- McCollum, R.E. Buildup and decline in soil-phosphorus—30-year trends on a Typic Umprabuult. Agron. J. 1991, 83, 77–85. [Google Scholar] [CrossRef]
- Spohn, M.; Kuzyakov, Y. Phosphorus mineralization can be driven by microbial need for carbon. Soil Biol. Biochem. 2013, 61, 69–75. [Google Scholar] [CrossRef]
- Safford, L.O.; Bell, S. Biomass of fine roots in a white spruce plantation. Can. J. For. Res. 1972, 2, 169–172. [Google Scholar] [CrossRef]
- Soon, Y.K. Solubility and retention of phosphate in soils of the northwestern Canadian prairie. Can. J. Soil Sci. 1991, 71, 453–463. [Google Scholar] [CrossRef]
- Sims, J.T.; Maguire, R.O.; Leyten, A.B.; Gartley, K.L.; Pautler, M.C. Evaluation of Mehlich 3 as an agri-environmental soil phosphorus test for the mid-Atlantic United States of America. Soil Sci. Soc. Am. J. 2002, 66, 2016–2032. [Google Scholar] [CrossRef]
- Von Sperber, C.; Stallforth, R.; Du Preez, C.; Amelung, W. Changes in soil phosphorus pools during prolonged arable cropping in semiarid grasslands. Eur. J. Soil Sci. 2017, 68, 462–471. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, H.; Hao, F.; Ouyang, W.; Liu, S.; Lin, C. The influence of land-use change on the forms of phosphorus in soil profiles from the Sanjiang Plain of China. Geoderma 2012, 189–190, 207–214. [Google Scholar] [CrossRef]
- Costa, M.; Gama-Rodrigues, A.; Gonçalves, J.; Gama-Rodrigues, E.; Sales, M.; Aleixo, S. Labile and non-labile fractions of phosphorus and its transformations in soil under eucalyptus plantations, Brazil. Forests 2016, 7, 15. [Google Scholar] [CrossRef]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley and Sons: New York, NY, USA, 1979. [Google Scholar]
- Reddy, D.D.; Rao, A.S.; Takkar, P.N. Effects of repeated manure and fertilizer phosphorus additions on soil phosphorus dynamics under a soybean-wheat rotation. Biol. Fertil. Soils 1999, 28, 150–155. [Google Scholar] [CrossRef]
- Xavier, F.A.D.S.; Almeida, E.F.; Cardoso, I.M. Soil phosphorus distribution in sequentially extracted fractions in tropical coffee-agroecosystems in the Atlantic Forest biome, Southeastern Brazil. Nutr. Cycl. Agroecosyst. 2011, 89, 31–44. [Google Scholar] [CrossRef]
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.S. Land use and soil factors affecting accumulation of phosphorus species in temperate soils. Geoderma 2015, 257, 29–39. [Google Scholar] [CrossRef]

| Land-Use Type | Soil Properties | |||||
|---|---|---|---|---|---|---|
| Sand | Silt | Clay | Total C | pH | Bulk Density (Mg m−3) | |
| (g kg−1) | ||||||
| Cropland | 27.74(14.76) | 46.73(5.88) | 25.51(11.0) | 47.88(9.75) | 6.07(0.85) | 1.47(0.10)a 2 |
| Forestland | 29.50(10.07) | 44.07(12.8) | 26.44(6.96) | 65.75(23.95) | 6.04(0.67) | 1.24(0.20)b |
| Land-use type | Soil Properties | |||||
| Cation exchange capacity (cmolc kg−1) | Ca | Mg | Al | Fe | Mn | |
| (mg kg−1) 1 | ||||||
| Cropland | 41.58(11.93) | 4.83(0.84) | 0.83(0.66) | 0.57(0.18) | 0.30(0.84) | 0.06(0.84) |
| Forestland | 42.91(12.80) | 4.98(0.77) | 0.69(0.18) | 0.52(0.06) | 0.24(0.05) | 0.06(0.03) |
| Land-Use Type | Soil Property | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sand | Clay | Total C | pH | Al 1 | Fe 1 | Mn 1 | Fe/Al-P 1 | Org-P 1 | |
| (g kg−1) | (mg kg−1) | ||||||||
| Cropland | −0.77 | 0.77 | 0.94 | −0.82 | NS | NS | NS | NS | −0.77 |
| (0.07) | (0.07) | (<0.01) | (0.04) | (0.07) | |||||
| Forestland | −0.82 | NS 2 | NS | −0.82 | 0.88 | 0.77 | −0.82 | 0.77 | NS |
| (0.04) | (0.04) | (0.01) | (0.07) | (0.04) | (0.07) | ||||
| Land-Use Type | Depth (cm) | Water-P | Ca/Mg-P a | Fe/Al-P 1 | Org-P 1 | Residual P | Total P |
|---|---|---|---|---|---|---|---|
| (mg kg−1) | |||||||
| Cropland | 0–10 | 7.68 a | 170.0 a | 328.0 aA 2 | 626.3 aA | 1339.1 a | 2467.0 a |
| 10–30 | 1.97 b | 130.0 a | 203.8 b | 489.7 b | 1272.2 a | 2097.7 a | |
| Forestland | 0–10 | 6.82 a | 149.6 a | 215.6 aB | 581.5 aB | 1326.3 a | 2279.9 a |
| 10–30 | 1.74 b | 100.6 b | 225.6 a | 501.9 a | 1049.9 a | 1879.8 a | |
| Land-Use Type | Soil Test P Method | P Fraction 1 | Linear Regression Model | R2 | p-Value |
|---|---|---|---|---|---|
| Cropland | Kelowna | Fe/Al-P | Y = 0.22x − 9.77 | 0.50 | 0.005 |
| Ca/Mg-P | Y = 0.41x − 20.14 | 0.24 | 0.051 | ||
| Inorganic P | Y = 0.19x − 30.68 | 0.55 | 0.003 | ||
| Mehlich-3 | Fe/Al-P | Y = 0.37x − 31.27 | 0.67 | <0.001 | |
| Ca/Mg-P | Y = 0.88x − 65.52 | 0.48 | 0.007 | ||
| Inorganic P | Y = 0.32x − 70.98 | 0.80 | <0.001 | ||
| Forestland | Kelowna | Fe/Al-P | Y = 0.09x − 4.96 | 0.45 | 0.009 |
| Inorganic P | Y = 0.08x − 12.75 | 0.47 | <0.001 | ||
| Org-P | Y = 0.09x − 32.52 | 0.24 | 0.051 | ||
| Mehlich-3 | Ca/Mg-P | Y = 0.39x − 12.23 | 0.22 | 0.061 | |
| Inorganic P | Y = 0.15x − 16.61 | 0.29 | 0.031 |
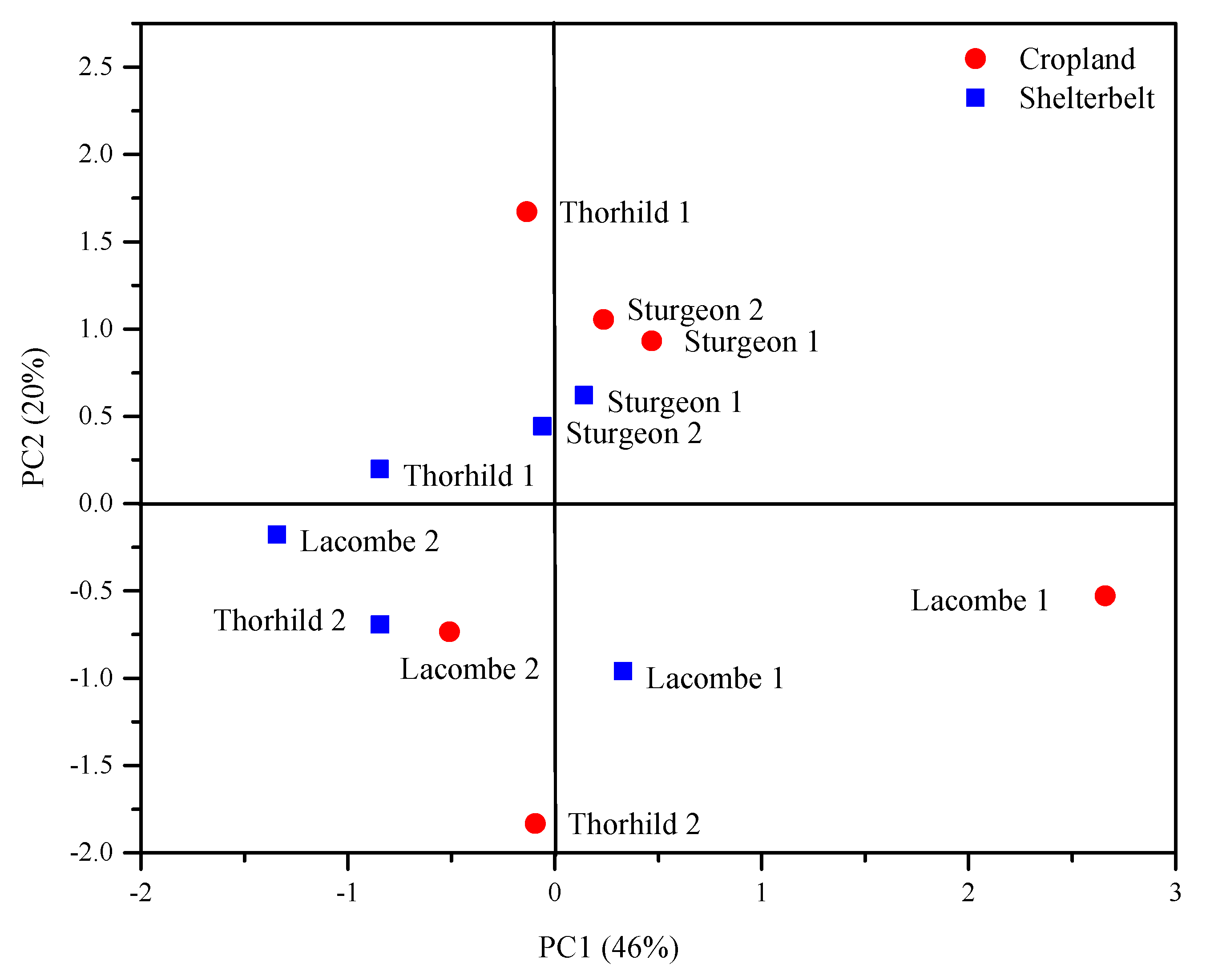
| Soil Property a | PC1 b | PC2 |
|---|---|---|
| PKelowna | 0.76 | 0.20 |
| PMehlich | 0.89 | 0.29 |
| Fe/Al-P | 0.92 | −0.24 |
| Ca/Mg-P | 0.72 | 0.35 |
| Org-P | 0.78 | 0.37 |
| Fe | 0.70 | −0.22 |
| Al | 0.42 | −0.84 |
| Ca | −0.30 | 0.78 |
| Eigenvalue | 4.11 | 1.82 |
| % Variance | 46 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manimel Wadu, M.C.W.; Ma, F.; Chang, S.X. Phosphorus Availabilities Differ between Cropland and Forestland in Shelterbelt Systems. Forests 2019, 10, 1001. https://doi.org/10.3390/f10111001
Manimel Wadu MCW, Ma F, Chang SX. Phosphorus Availabilities Differ between Cropland and Forestland in Shelterbelt Systems. Forests. 2019; 10(11):1001. https://doi.org/10.3390/f10111001
Chicago/Turabian StyleManimel Wadu, Mihiri C.W., Fengxiang Ma, and Scott X. Chang. 2019. "Phosphorus Availabilities Differ between Cropland and Forestland in Shelterbelt Systems" Forests 10, no. 11: 1001. https://doi.org/10.3390/f10111001





