Different Responses of Cytoplasmic and Endoplasmic Reticulum Hsp90 Genes from Eogystia hippophaecola (Lepidoptera: Cossidae) to Cold Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Cold Stress Treatments
2.3. Isolation of Total RNA and Synthesis of First-Strand cDNA
2.4. Cloning of EhHsp90 ORFs
2.5. Bioinformatics Analysis
2.6. Expression of EhHsp90 under Different Treatments
3. Results
3.1. Characterization of EhHsp90
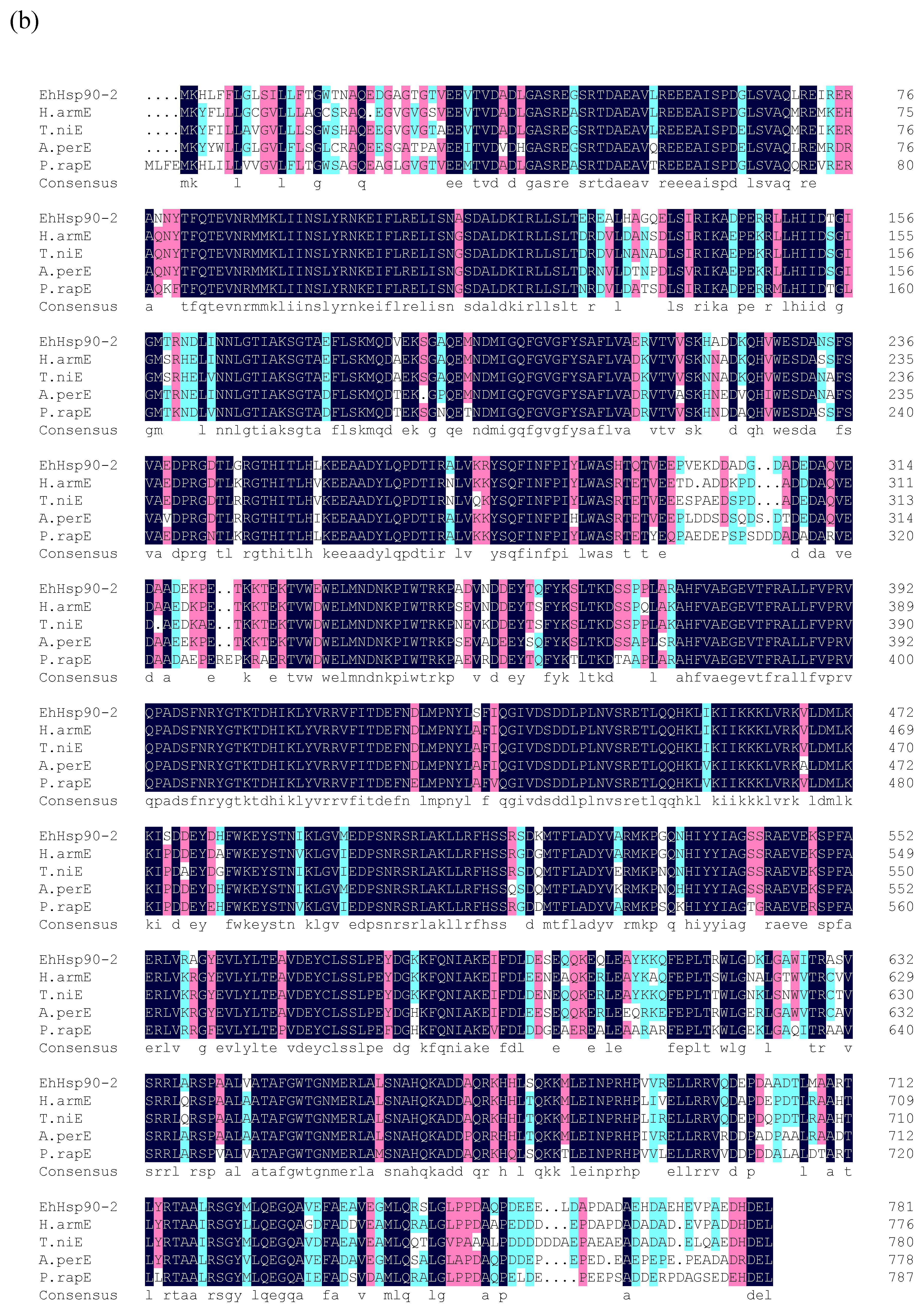
3.2. Phylogenetic Analysis of EhHsp90
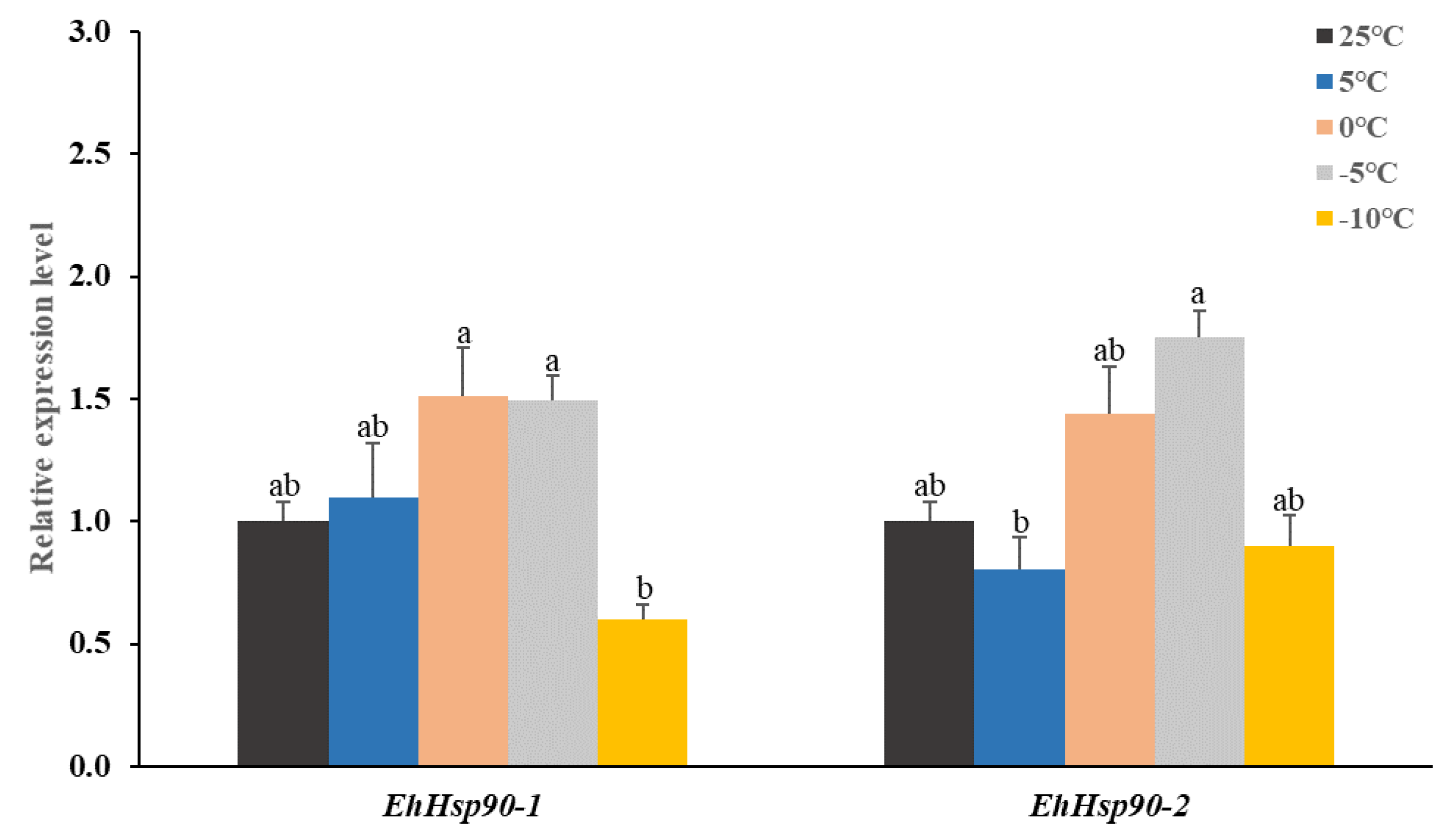
3.3. Expression of EhHsp90 under Different Low-Temperature Treatments
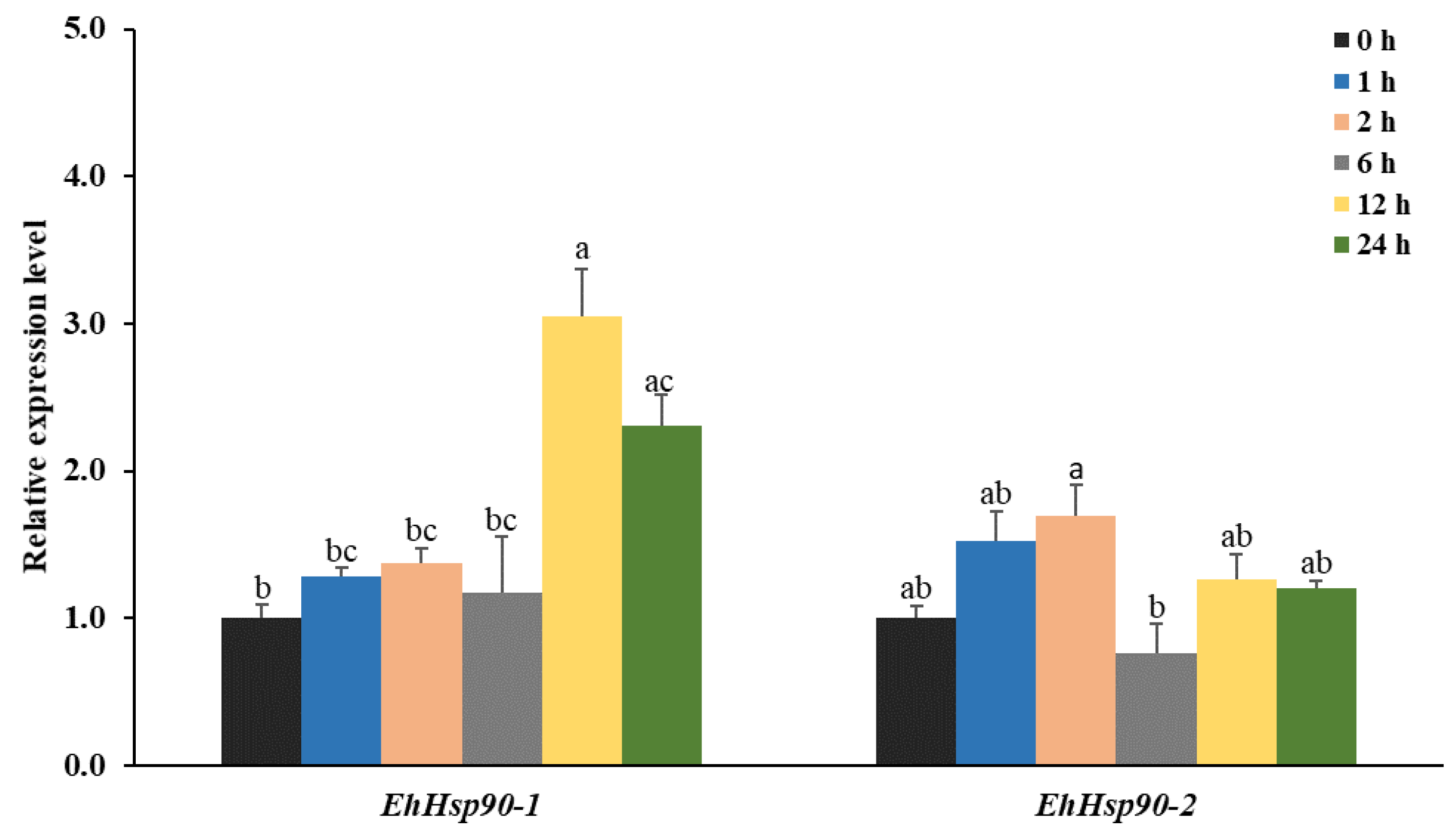
3.4. Expression of EhHsp90 under Different Treatment Times
4. Discussion
4.1. Characterization and Phylogenetic Analysis of EhHsp90
4.2. Expression of EhHsp90 under Different Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations:
| ORF | Open Reading Frame |
| PCR | Polymerase Chain Reaction |
| qRT-PCR | Quantitative real-time PCR |
References
- Joanisse, D.R.; Storey, K.B. Oxidative stress and antioxidants in stress and recovery of cold-hardy insects. J. Insect Biochem. Mol. Biol. 1998, 28, 23–30. [Google Scholar] [CrossRef]
- Matz, J.M.; LaVoi, K.P.; Moen, R.J.; Blake, M.J. Cold-induced heat shock protein expression in rat aorta and brown adipose tissue. Physiol. Behav. 1996, 60, 1369–1374. [Google Scholar] [CrossRef]
- Sejerkilde, M.; Sorensen, J.G.; Loeschcke, V. Effects of cold- and heat hardening on thermal resistance in Drosophila melanogaster. J. Insect Physiol. 2003, 49, 719–726. [Google Scholar] [CrossRef]
- DeVries, A.L. Glycoproteins as biological antifreeze agents in antarctic fishes. Science 1971, 172, 1152–1155. [Google Scholar] [CrossRef]
- Neufeld, D.S.; Leader, L.P. Freezing survival by isolated Malpighian tubules of the New Zealand alpine weta Hemideina maori. J. Exp. Biol. 1998, 201, 227–236. [Google Scholar]
- Cai, Q.L.; Lu, X.Y.; Ma, J. Molecular cloning and expression profiling of a cold stress-related chitinase gene Mpcht19 in the desert beetle Microdera punctipennis (Coleoptera: Tenebrionidae). Acta Entomol. Sin. 2017, 60, 286–296. [Google Scholar]
- Maki, L.R.; Galyan, E.L.; Chang-Chien, M.M.; Caldwell, D.R. Ice nucleation induced by pseudomonas syringae. Appl. Microbiol. 1974, 28, 456–459. [Google Scholar]
- Goto, S.G.; Kimura, M.T. Heat- and cold-shock responses and temperature adaptations in subtropical and temperate species of Drosophila. J. Insect Physiol. 1998, 44, 1233–1239. [Google Scholar] [CrossRef]
- Goto, S.G. A novel gene that is up-regulated during recovery from cold shock in Drosophila melanogaster. Gene 2001, 270, 259–264. [Google Scholar] [CrossRef]
- Parsell, D.A.; Lindquist, S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993, 27, 437–496. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2003, 6, 1025–1037. [Google Scholar] [CrossRef]
- Arya, R.; Mallik, M.; Lakhotia, S.C. Heat shock genes—Integrating cell survival and death. J. Biosci. 2007, 32, 595–610. [Google Scholar] [CrossRef]
- Beissinger, M.; Buchner, J. How chaperones fold proteins. Biol. Chem. 1998, 379, 245–259. [Google Scholar]
- Young, J.C.; Agashe, V.R.; Siegers, K.; Hartl, F.U. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 2004, 5, 781–791. [Google Scholar] [CrossRef]
- Qiao, L.; Wu, J.X.; Qin, D.Z.; Liu, X.C.; Lu, Z.C.; Lv, L.Z.; Pan, Z.L.; Chen, H.; Li, G.W. Gene expression profiles of heat shock proteins 70 and 90 from Empoasca onukii (Hemiptera: Cicadellidae) in response to temperature stress. J. Insect Sci. 2015, 15, 49. [Google Scholar] [CrossRef]
- Sonoda, S.; Ashfaq, M.; Tsumuki, H. Cloning and nucleotide sequencing of three heat shock protein genes (hsp90, hsc70, and hsp19.5) from the diamondback moth, Plutella xylostella (L.) and their expression in relation to developmental stage and temperature. Arch. Insect Biochem. Physiol. 2006, 62, 80–90. [Google Scholar] [CrossRef]
- Feng, H.; Wang, L.; Liu, Y.; He, L.; Li, M.; Lu, W.; Xue, C. Molecular characterization and expression of a heat shock protein gene (HSP90) from the carmine spider mite, Tetranychus cinnabarinus (Boisduval). J. Insect Sci. 2010, 10, 112. [Google Scholar] [CrossRef]
- Jiang, X.; Zhai, H.; Wang, L.; Luo, L.; Sappington, T.W.; Zhang, L. Cloning of the heat shock protein 90 and 70 genes from the beet armyworm, Spodoptera exigua, and expression characteristics in relation to thermal stress and development. Cell Stress Chaperones 2012, 17, 67–80. [Google Scholar] [CrossRef]
- Li, H.B.; Du, Y.Z. Molecular cloning and characterization of an Hsp90/70 organizing protein gene from Frankliniella occidentalis (Insecta: Thysanoptera, Thripidae). Gene 2013, 520, 148–155. [Google Scholar] [CrossRef]
- Wang, H.; Li, K.; Zhu, J.Y.; Fang, Q.; Ye, G.Y.; Wang, H.; Li, K.; Zhu, J.Y. Cloning and expression pattern of heat shock protein genes from the endoparasitoid wasp, Pteromalus puparum in response to environmental stresses. Arch. Insect Biochem. Physiol. 2012, 79, 247–263. [Google Scholar] [CrossRef]
- Sonoda, S.; Tsumuki, H. Induction of heat shock protein genes by chlorfenapyr in cultured cells of the cabbage armyworm, Mamestra brassicae. Pestic. Biochem. Physiol. 2007, 89, 185–189. [Google Scholar] [CrossRef]
- Shu, Y.; Du, Y.; Wang, J. Molecular characterization and expression patterns of Spodoptera litura heat shock protein 70/90, and their response to zinc stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 158, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Kayukawa, T.; Monteiro, A.; Ishikawa, Y. The expression of the HSP90 gene in response to winter and summer diapauses and thermal-stress in the onion maggot, Delia antiqua. Insect Mol. Biol. 2010, 14, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.P.; Li, A.; Yocum, G.D.; Robich, R.M.; Hayward, S.A.; Denlinger, D.L. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc. Natl. Acad. Sci. USA 2007, 104, 11130–11137. [Google Scholar] [CrossRef]
- Gkouvitsas, T.; Kontogiannatos, D.; Kourti, A. Cognate Hsp70 gene is induced during deep larval diapause in the moth Sesamia nonagrioides. Insect Mol. Biol. 2009, 18, 253–264. [Google Scholar] [CrossRef]
- Zhang, Q.; Denlinger, D.L. Molecular characterization of heat shock protein 90, 70 and 70 cognate cDNAs and their expression patterns during thermal stress and pupal diapause in the corn earworm. J. Insect Physiol. 2010, 56, 138–150. [Google Scholar] [CrossRef]
- Jones, C.; Anderson, S.; Singha, U.K.; Chaudhuri, M. Protein phosphatase 5 is required for Hsp90 function during proteotoxic stresses in Trypanos. Brucei. Parasitol. Res. 2008, 102, 835–844. [Google Scholar] [CrossRef]
- Csermely, P.; Schnaider, T.; Soti, C.; Prohaszka, Z.; Nardai, G. The 90-kDa molecular chaperone family: Structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998, 79, 129–168. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, D.; Monteiro, A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genom. 2006, 7, 156. [Google Scholar]
- Boher, F.; Trefault, N.; Piulachs, M.D. Biogeographic origin and thermal acclimation interact to determine survival and hsp90 expression in Drosophila species submitted to thermal stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 162, 391–396. [Google Scholar] [CrossRef]
- Colinet, H.; Lee, S.F.; Hoffmann, A. Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult Drosophila melanogaster. FEBS J. 2010, 277, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Fukumoto, K.; Izumi, Y.; Yoshida, H.; Tsumuki, H. Cloning of heat shock protein genes (hsp90 and hsc70) and their expression during larval diapause and cold tolerance acquisition in the rice stem borer, Chilo suppressalis Walker. Arch. Insect Biochem. Physiol. 2006, 63, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Eletto, D.; Dersh, D.; Argon, Y. GRP94 in ER quality control and stress responses. Semin. Cell Dev. Biol. 2010, 21, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Eletto, D.; Argon, Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta. 2012, 1823, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, B.; Dai, J.; Srivastava, P.K.; Zammit, D.J.; Lefrancois, L.; Li, Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity 2007, 26, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.Z.; Zhou, Y.; Fang, D.Q.; Chen, S.L. Chinese Cossidae; Beijing Tianze Press: Beijing, China, 1990. [Google Scholar]
- Yakovlev, R.V. A revision of carpenter moths of the genus Holcocerus Staudinger, 1884(s. l.). Eversmannia 2006, 1, 1–104. [Google Scholar]
- Zong, S.X.; Jia, F.Y.; Luo, Y.Q.; Zhi-Chun, X.U.; Zhang, L.S. Harm characteristics and population dynamics of Holcocerus hippophaecolus. J. Beijing For. Univ. 2005, 27, 70–74. [Google Scholar]
- Zong, S.X. Studies on the Bio-ecological Characteristics of Seabuckthorn Carpenter Moth: Holcocerus hippophaecolus (Lepidoptera: Cossidae). Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2006. [Google Scholar]
- Li, W.B.; Cui, M.M.; Hu, P.; Tao, J.; Zong, S.X. Cloning and Expression Analysis of the Heat-Shock Protein 70 Gene in Eogystia hippophaecolus (Lepidoptera: Cossidae). Ann. Entomol. Soc. Am. 2017, 110, 528–535. [Google Scholar]
- Cui, M.; Hu, P.; Wang, T.; Tao, J.; Zong, S. Differential transcriptome analysis reveals genes related to cold tolerance in seabuckthorn carpenter moth, Eogystia hippophaecolus. PLoS ONE 2017, 12, e0187105. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.A.; Strimmer, K.; Vingron, M.; Haeseler, A.V. TREE-PUZZLE: Maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 2002, 18, 502–504. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, S. Primer Premier 5. Biotech. Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Hu, P.; Tao, J.; Cui, M.; Gao, C.; Lu, P.; Luo, Y. Antennal transcriptome analysis and expression profiles of odorant binding proteins in Eogystia hippophaecolus (Lepidoptera: Cossidae). BMC Genom. 2016, 17, 651. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.S. Phylogenetic analysis of the 90 kDa heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol. Biol. Evol. 1995, 12, 1063–1073. [Google Scholar]
- Ramya, T.N.; Surolia, N.; Surolia, A. 15-Deoxyspergualin modulates Plasmodium falciparum heat shock protein function. Biochem. Biophys. Res. Commun. 2006, 348, 585–592. [Google Scholar] [CrossRef]
- Pearl, L.H.; Prodromou, C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 2006, 75, 271–294. [Google Scholar] [CrossRef]
- Daugaard, M.; Rohde, M.; Jaattela, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.Y.; Wu, G.X.; Ye, G.Y.; Hu, C. Heat shock protein genes (hsp20, hsp75 and hsp90) from Pieris rapae: Molecular cloning and transcription in response to parasitization by Pteromalus puparum. Insect Sci. 2013, 20, 183–193. [Google Scholar] [CrossRef]
- Cheng, W.; Li, D.; Wang, Y.; Liu, Y.; Zhu-Salzman, K. Cloning of heat shock protein genes (hsp70, hsc70 and hsp90) and their expression in response to larval diapause and thermal stress in the wheat blossom midge, Sitodiplosis Mosellana. J. Insect Physiol. 2016, 95, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.P.; Denlinger, D.L. Heat-shock protein 90 is down-regulated during pupal diapause in the flesh fly, Sarcophaga crassipalpis, but remains responsive to thermal stress. Insect Mol. Biol. 2000, 9, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sømme, L. Cold Hardiness in Terrestrial Arthropods. Eur. J. Entomol 1999, 96, 1–10. [Google Scholar]
- Tian, B.; Xu, L.; Tao, W.; Zong, S. Comparison of the supercooling capacity in three species of Cossidae overwintering larvae. Plant Prot. 2017, 43, 113–118. [Google Scholar]
- Bashir, S.; Bashir, H.; Shuja Qadri, S. Heat Shock and the Heat Shock Proteins: An Overview. Int. J. Med. Sci. Public Health 2013, 2, 489–494. [Google Scholar]
- Yang, J.; Baloch, N.A.; Dong, F.; University, N.A. cDNA Cloning and Induction of Heat Shock Protein Hsp90 from Myth. Sep. Chin. J. Biol. Control 2017, 33, 623–630. [Google Scholar]
- Zhang, Y.; Gu, S.; Li, C.; Sang, M.; Wu, W.; Yun, X.; Hu, X.; Li, B. Identification and characterization of novel ER-based hsp90 gene in the red flour beetle, Tribolium castaneum. Cell Stress Chaperones 2014, 19, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Silbermann, R.; Tatar, M. Reproductive Costs of Heat Shock Protein in Transgenic Drosophila Melanogaster. Evolution 2000, 54, 2038–2045. [Google Scholar] [CrossRef] [PubMed]




| The Characteristics of Hsp90s | EhHsp90-1 | EhHsp90-2 |
|---|---|---|
| ORF | 2154 bp | 2346 bp |
| Amino acid residues | 717 aa | 781 aa |
| Molecular weight | 82.51 kDa | 88.85 kDa |
| A theoretical isoelectric point | 5.01 | 5.12 |
| Instability index | 40.15 | 39.85 |
| Hydrophilic protein | −0.67 | −0.57 |
| Signal peptide | no | aa 1–20 |
| The heat shock domain | aa 191–713 | aa 262–780 |
| N-terminal ATPase domain | aa 35–189 | aa 100–260 |
| The number of Hsp90 family signature segments | 5 | 4 |
| C-terminal region | EEVD | HDEL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Tao, J.; Li, Y.; Xu, Y.; Liu, X.; Zong, S. Different Responses of Cytoplasmic and Endoplasmic Reticulum Hsp90 Genes from Eogystia hippophaecola (Lepidoptera: Cossidae) to Cold Stress. Forests 2019, 10, 1039. https://doi.org/10.3390/f10111039
Wang Q, Tao J, Li Y, Xu Y, Liu X, Zong S. Different Responses of Cytoplasmic and Endoplasmic Reticulum Hsp90 Genes from Eogystia hippophaecola (Lepidoptera: Cossidae) to Cold Stress. Forests. 2019; 10(11):1039. https://doi.org/10.3390/f10111039
Chicago/Turabian StyleWang, Qianqian, Jing Tao, Yurong Li, Yabei Xu, Xinhai Liu, and Shixiang Zong. 2019. "Different Responses of Cytoplasmic and Endoplasmic Reticulum Hsp90 Genes from Eogystia hippophaecola (Lepidoptera: Cossidae) to Cold Stress" Forests 10, no. 11: 1039. https://doi.org/10.3390/f10111039




