Leaf Nitrogen and Phosphorus Stoichiometry of Chinese fir Plantations across China: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Data Analysis
3. Results
3.1. Patterns of the Leaf N and P Concentrations and N:P Ratio of Chinese fir across China
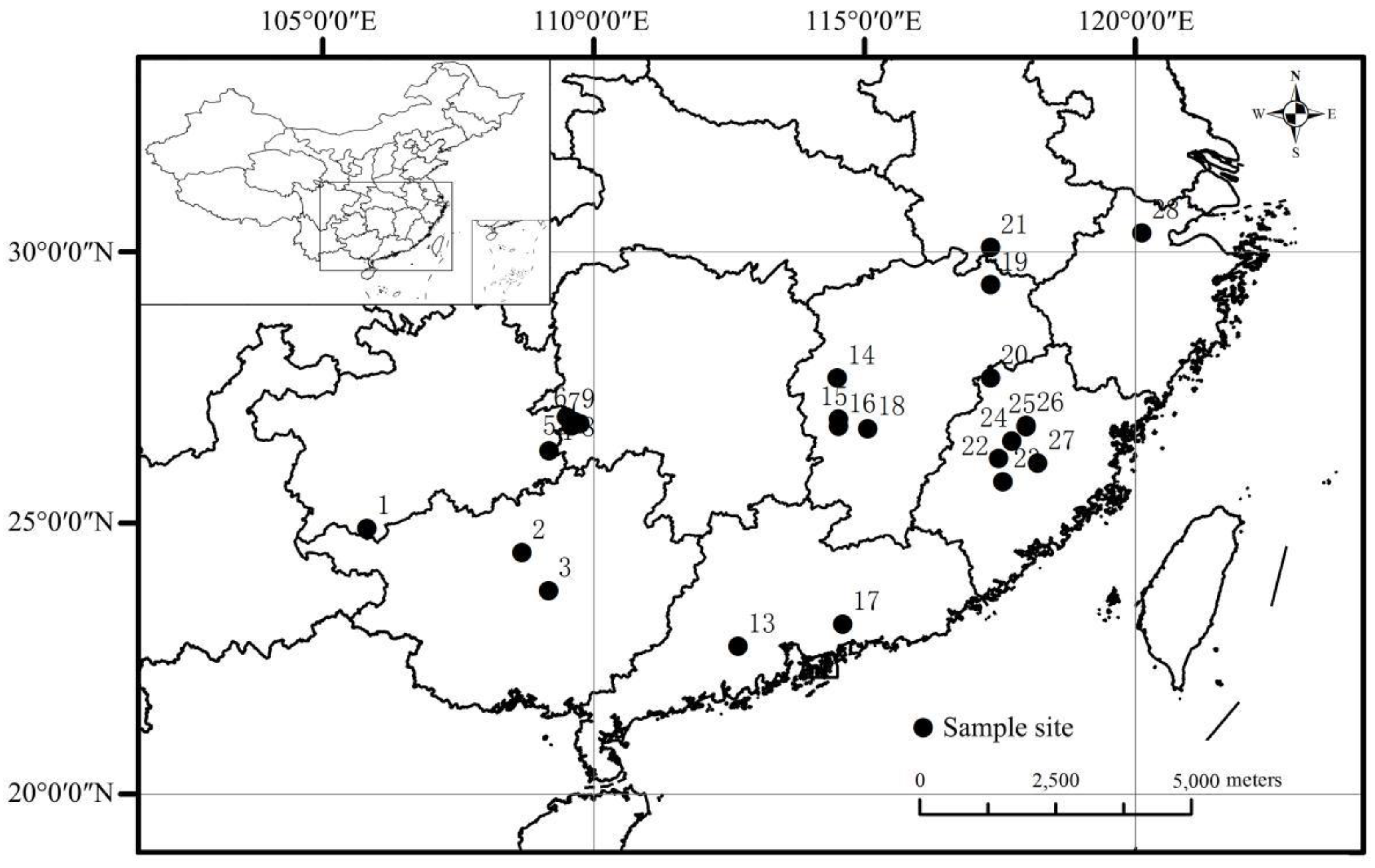
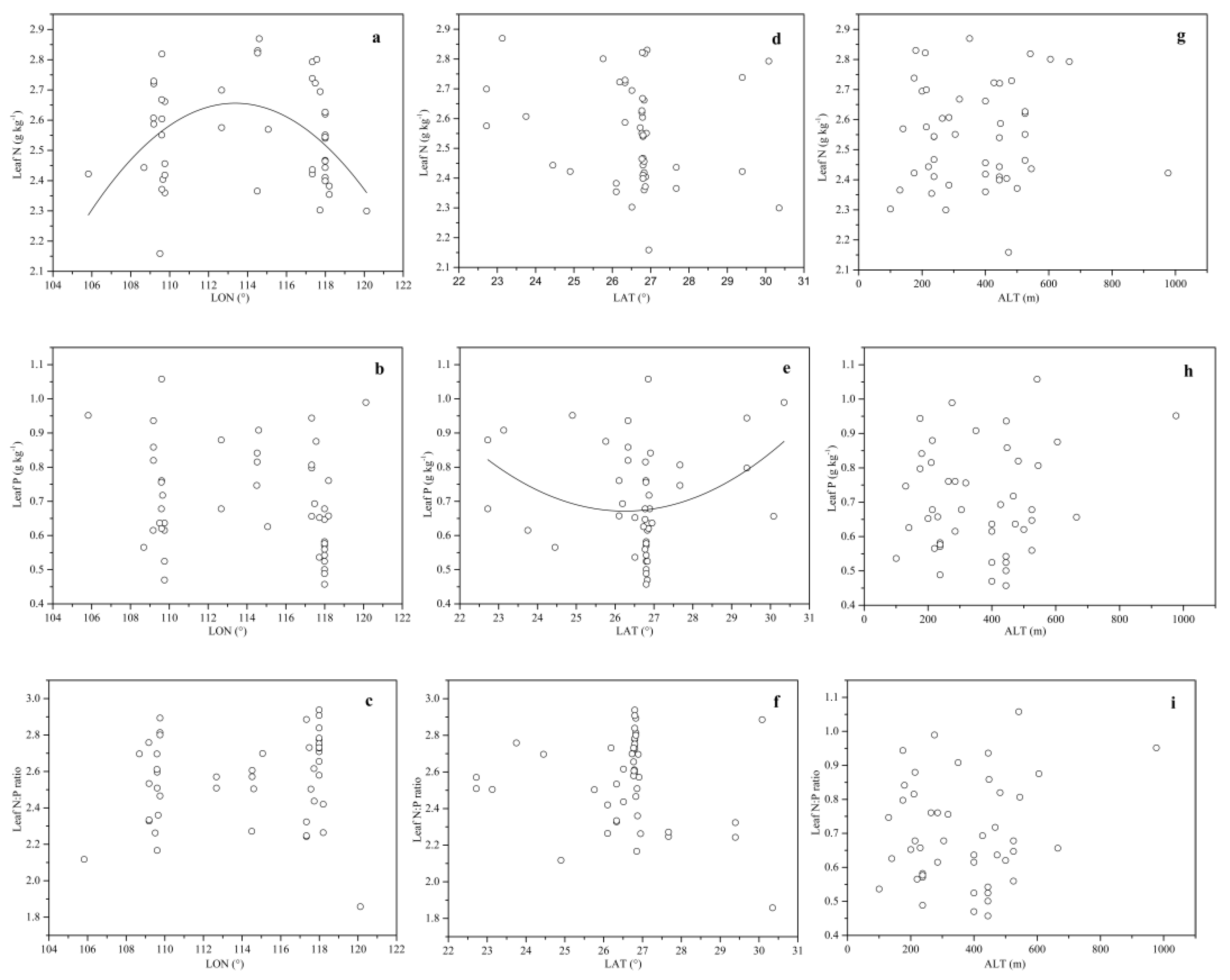
3.2. Variations of the Leaf N and P Concentrations and N:P Ratio with Geographic Factors
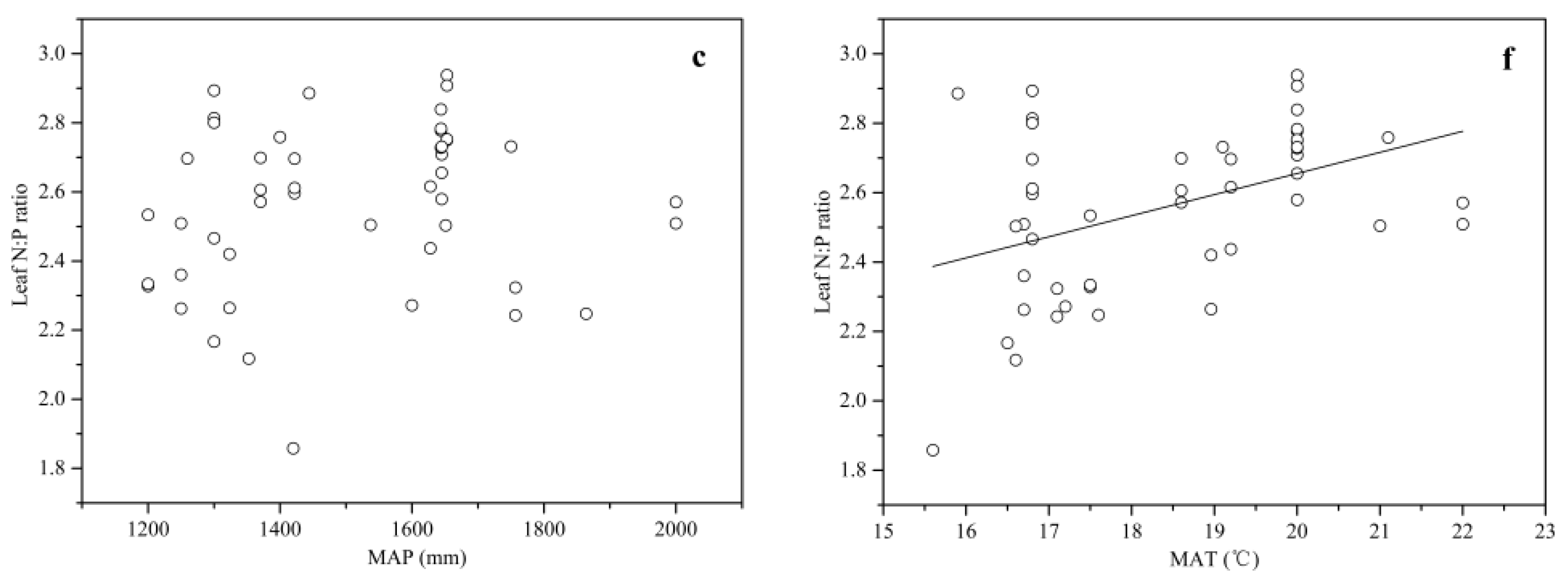
3.3. Variations of the Leaf N and P Concentrations and N:P Ratio with Climatic Factors
3.4. Variations of the Leaf N and P Concentrations and N:P Ratio with Soil Nutrient Concentrations
3.5. Redundancy Analysis (RDA)
4. Discussion
4.1. Patterns of the Leaf N and P Concentrations and N:P Ratio of Chinese Fir across China
4.2. Stand Age Effect on the Leaf N and P Concentrations and N:P Ratio
4.3. Relationships between the Leaf Stoichiometry and Environmental Factors for Chinese Fir
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Sampling Area | Stand Age | Leaf | Soil | Longitude | Latitude | Altitude | MAP | MAT | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | N:P | N | P | ||||||||
| Huitong, Hunan | 8 | 9.59 | 0.89 | 10.7752809 | 1.9 | 0.46 | 109.75 | 26.83 | 400 | 1300 | 16.8 | [47] |
| Huitong, Hunan | 10 | 7.66 | 0.89 | 8.606741573 | 1.4 | 0.29 | 109.5 | 26.95 | 473 | 1250 | 16.7 | [48] |
| Huitong, Hunan | 7.5 | 12.52 | 1.14 | 12.41 | 1.51 | 0.35 | 109.6 | 26.79 | 264 | 1422 | 16.8 | [17] |
| Nanping, Fujian | 5.5 | 12.74 | 0.91 | 14 | 1.47 | 0.3 | 117.99 | 26.77 | 525 | 1645 | 20 | [49] |
| Nanping, Fujian | 5.5 | 11.72 | 0.79 | 15.08 | 1.57 | 0.4189 | 117.99 | 26.8 | 237.5 | 1644 | 20 | [50] |
| Nanping, Fujian | 8 | 10.13 | 0.69 | 14.64 | 1.41 | 0.31 | 117.99 | 26.8 | 444 | 1653 | 20 | [51] |
| Youxi, Fujian | 7.5 | 9.53 | 0.93 | 10.24731183 | 1.5472 | 0.4189 | 118.2 | 26.1 | 230 | 1323.4 | 18.96 | [52] |
| Shanxian, Fujian | 9 | 9 | 0.71 | 12.67605634 | 1.98 | 0.38 | 117.72 | 26.51 | 100 | 1628 | 19.2 | [53] |
| Xuanwu, Guangxi | 8.5 | 12.56 | 0.85 | 14.77647059 | 2.2 | 0.8 | 109.17 | 23.75 | 285 | 1400 | 21.1 | [54] |
| Yunfu, Guangdong | 5 | 16.63 | 1.48 | 11.23648649 | 1.5472 | 0.4189 | 114.6 | 23.13 | 350 | 1537.2 | 21 | [55] |
| Jingdezhen, Jiangxi | 7.5 | 10.27 | 1.22 | 8.418032787 | 0.51 | 0.4189 | 117.33 | 29.39 | 175 | 1756.7 | 17.1 | [56] |
| Liping, Guizhou | 8 | 12.29 | 1.36 | 9.25 | 1.41 | 0.54 | 109.18 | 26.33 | 447.67 | 1200 | 17.5 | [34] |
| Foshan, Guangdong | 5 | 12.14 | 0.97 | 12.07 | 1.5472 | 0.4189 | 112.67 | 22.72 | 213.33 | 2000 | 22 | [57] |
| Taihe, Jiangxi | 10 | 15.95 | 1.32 | 12.08333333 | 1.66 | 0.34 | 114.52 | 26.91 | 180 | 1370.5 | 18.6 | [48] |
| Linan, Zhejiang | 10 | 8.97 | 1.69 | 5.41 | 1.5472 | 0.4189 | 120.12 | 30.35 | 275 | 1420 | 15.6 | [58] |
| Huitong, Hunan | 14 | 13.32 | 0.85 | 15.67058824 | 1.9 | 0.46 | 109.75 | 26.83 | 400 | 1300 | 16.8 | [47] |
| Huitong, Hunan | 20 | 9.71 | 0.86 | 11.29069767 | 1.21 | 0.21 | 109.6 | 26.86 | 500 | 1250 | 16.7 | [48] |
| Huitong, Hunan | 17.5 | 11.82 | 0.97 | 13.82 | 1.59 | 0.35 | 109.59 | 26.89 | 304.5 | 1422 | 16.8 | [17] |
| Nanping, Fujian | 14 | 12.83 | 0.97 | 13.22680412 | 1.27 | 0.33 | 117.99 | 26.77 | 525 | 1645 | 20 | [49] |
| Nanping, Fujian | 14 | 10.79 | 0.77 | 14.3 | 2.08 | 0.3467 | 117.99 | 26.8 | 237.5 | 1644 | 20 | [50] |
| Nanping, Fujian | 14 | 11.68 | 0.65 | 17.88 | 1.9 | 0.37 | 117.99 | 26.8 | 444 | 1653 | 20 | [51] |
| Nanping, Fujian | 20 | 10.43 | 1.24 | 8.46 | 1.26 | 0.36 | 117.33 | 27.67 | 545 | 1864 | 17.6 | [59] |
| Youxi Fujian | 17.5 | 9.83 | 1.14 | 8.622807018 | 1.406 | 0.3467 | 118.2 | 26.1 | 285 | 1323.4 | 18.96 | [52] |
| Sanming, Fujian | 12 | 13.8 | 0.92 | 10.44 | 1.406 | 0.3467 | 117.73 | 26.51 | 200 | 1628 | 19.2 | [60] |
| Fenyi, Jiangxi | 19 | 9.65 | 1.11 | 8.693693694 | 1.406 | 0.3467 | 114.5 | 27.67 | 130 | 1600 | 17.2 | [61] |
| Jingdezhen, Jiangxi | 20 | 14.46 | 1.57 | 9.210191083 | 1.13 | 0.3467 | 117.33 | 29.39 | 175 | 1756.7 | 17.1 | [56] |
| Yishan, Guangxi | 11 | 10.51 | 0.76 | 13.82894737 | 0.96 | 0.2 | 108.68 | 24.45 | 220 | 1259.6 | 19.2 | [62] |
| Taihe, Jiangxi | 20 | 12.06 | 0.87 | 13.86206897 | 0.99 | 0.12 | 115.06 | 26.73 | 140 | 1370.5 | 18.6 | [48] |
| Liping, Guizhou | 16 | 14.19 | 1.55 | 9.32 | 1.18 | 0.38 | 109.18 | 26.33 | 445 | 1200 | 17.5 | [34] |
| Foshan, Guangdong | 11 | 13.87 | 1.41 | 11.29 | 1.406 | 0.3467 | 112.67 | 22.72 | 213.33 | 2000 | 22 | [57] |
| Anlong, Guizhou | 11 | 10.27 | 1.59 | 7.31 | 1.406 | 0.3467 | 105.82 | 24.9 | 976.8 | 1352.8 | 16.6 | [63] |
| Nanping, Fujian | 21 | 11.82 | 0.97 | 12.18556701 | 1.21 | 0.26 | 117.99 | 26.77 | 525 | 1645 | 20 | [49] |
| Nanping, Fujian | 21 | 11.73 | 0.78 | 15.17 | 2.45 | 0.446 | 117.99 | 26.8 | 237.5 | 1644 | 20 | [50] |
| Nanping, Fujian | 21 | 10.51 | 0.72 | 14.68 | 1.95 | 0.32 | 117.99 | 26.8 | 444 | 1653 | 20 | [51] |
| Huitong, Hunan | 25 | 13.41 | 1.13 | 12.62 | 1.77 | 0.36 | 109.59 | 26.79 | 318 | 1422 | 16.8 | [17] |
| Huitong, Hunan | 25 | 10.66 | 0.69 | 15.44927536 | 1.9 | 0.46 | 109.75 | 26.83 | 400 | 1300 | 16.8 | [47] |
| Shitai, Anhui | 20 | 15.33 | 0.928 | 16.91 | 3.57 | 0.83 | 117.33 | 30.08 | 665 | 1444 | 15.9 | [64] |
| Huitong, Hunan | 30 | 10.07 | 1.05 | 9.59047619 | 1.9 | 0.28 | 109.65 | 26.87 | 467 | 1250 | 16.7 | [48] |
| Huitong, Hunan | 29 | 15.76 | 1.88 | 7.73 | 0.6 | 0.23 | 109.6 | 26.85 | 541.6 | 1300 | 16.5 | [65] |
| Datian, Fujian | 26 | 15.46 | 1.4 | 11.22 | 0.71 | 0.4 | 117.56 | 25.76 | 605.3 | 1651 | 16.6 | [65] |
| Taihe, Jiangxi | 30 | 15.81 | 1.26 | 12.54761905 | 1.99 | 0.37 | 114.52 | 26.78 | 210 | 1370.5 | 18.6 | [48] |
| Liping, Guizhou | 28 | 14.32 | 1.27 | 11.6 | 1.38 | 0.47 | 109.18 | 26.33 | 482.67 | 1200 | 17.5 | [34] |
| Huitong, Hunan | 45.5 | 10.23 | 0.6 | 17.05 | 1.98 | 0.28 | 109.75 | 26.83 | 400 | 1300 | 16.8 | [47] |
| Nanping, Fujian | 46 | 10.76 | 0.75 | 14.34666667 | 1.71 | 0.27 | 117.99 | 26.77 | 525 | 1645 | 20 | [49] |
| Nanping, Fujian | 46 | 10.14 | 0.63 | 16.0952381 | 2.24 | 0.28 | 117.99 | 26.8 | 237.5 | 1644 | 20 | [50] |
| Nanping, Fujian | 46 | 10.01 | 0.58 | 17.32 | 1.99 | 0.29 | 117.99 | 26.8 | 444 | 1653 | 20 | [51] |
| Sanming, Fujian | 50 | 14.22 | 1 | 14.35 | 1.98 | 0.28 | 117.48 | 26.19 | 427.1 | 1750 | 19.1 | [18] |
References
- Niklas, K.J.; Owens, T.; Reich, P.B.; Edward, D.C. NitrogeN:Phosphorus leaf stoichiometry and the scaling of plant growth. Ecol. Lett. 2005, 8, 636–642. [Google Scholar] [CrossRef]
- Branco, P.; Stomp, M.; Egas, M.; Huisman, J. Evolution of Nutrient Uptake Reveals a Trade-Off in the Ecological Stoichiometry of Plant-Herbivore Interactions. Am. Nat. 2010, 176, E162–E176. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wanek, W.; Zhou, C.P.; Richter, A. Nutrient limitation of alpine plants: Implications from leaf N:P stoichiometry and leaf δ15N. J. Soil Sci. Plant Nutr. 2014, 177, 378–387. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Q.S.; Elser, J.J.; He, N.P.; Wu, H.H.; Zhang, G.M.; Wu, J.G.; Bai, Y.F.; Han, X.G. Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecol. Lett. 2010, 13, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, Q.C.; An, S.S.; Dong, Y.Y.; Li, Y.Y. Ecological stoichiometry in leaf and litter under different vegetation types of Zhifanggou watershed on the Loess Plateau, China. Environ. Sci. 2015, 36, 1084–1091. [Google Scholar]
- Esmeijer-liu, A.J.; Aerts, R.; Kürschner, W.M.; Bobbink, R.; Lotter, A.F.; Verhoeven, J.T.A. Nitrogen enrichment lowers Betula pendula green and yellow leaf stoichiometry irrespective of effects of elevated carbon dioxide. Plant Soil 2009, 316, 311–322. [Google Scholar] [CrossRef]
- Kang, H.Z.; Zhuang, H.L.; Wu, L.L.; Liu, Q.L.; Shen, G.R.; Berg, B.; Man, R.Z.; Liu, C.J. Variation in leaf nitrogen and phosphorus stoichiometry in Picea abies across Europe: An analysis based on local observations. For. Ecol. Manag. 2011, 261, 195–202. [Google Scholar] [CrossRef]
- Wu, T.G.; Dong, Y.; Yu, M.K.; Wang, G.G.; Zeng, D.H. Leaf nitrogen and phosphorus stoichiometry of Quercus species across China. For. Ecol. Manag. 2012, 284, 116–123. [Google Scholar] [CrossRef]
- Lin, Y.M.; Chen, A.M.; Yan, S.W.; Rafay, L.; Du, K.; Wang, D.J.; Ge, Y.G.; Li, J. Available soil nutrients and water content affect leaf nutrient concentrations and stoichiometry at different ages of Leucaena leucocephala forests in dry-hot valley. J. Soil. Sediment 2019, 19, 511–521. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Xia, C.X.; Yu, D.; Wu, Z.G. Low-temperature induced leaf elements accumulation in aquatic macrophytes across Tibetan Plateau. Ecol. Eng. 2015, 75, 1–8. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Reich, P.B.; Woodward, F.I.; Wang, Z.H. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 2011, 14, 788–796. [Google Scholar] [CrossRef] [PubMed]
- He, J.S.; Wang, L.; Flynn, D.F.B.; Wang, X.P.; Ma, W.H.; Fang, J.Y. Leaf nitrogen:phosphorus stoichiometry across Chinese grassland biomes. Oecologia 2008, 155, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.J.; Yu, G.R.; Tao, B.; Wang, S.Q. Leaf nitrogen and phosphorus stoichiometry across 654 terrestrial plant species in NSTEC. Environ. Sci. 2007, 28, 2665–2673. [Google Scholar]
- Li, M.H.; Ritchie, G.A. Eight hundred years of clonal forestry in China: I. traditional afforestation with Chinese fir (Cunninghamia lanceolata (Lamb.) Hook.). New For. 1999, 18, 131–142. [Google Scholar]
- Chen, A.N.; Wang, G.J.; Chen, C.; Li, S.Y.; Li, W.J. Variation in the N and P stoichiometry of leaf-root-soil during stand development in a Cunninghamia lanceolata plantation in subtropical China. Acta Ecol. Sin. 2018, 38, 4027–4036. [Google Scholar]
- Meng, Q.Q.; Ge, L.L.; Yang, X.M.; Wang, J.; Lin, Y.; He, Z.M.; Qiu, L.J.; Hu, H.T. Seasonal variation of C, N, and P stoichiometric characteristics in leaves of two plantations in Sanming, Fujian. Chin. J. Appl. Environ. Biol. 2019, 25, 1–10. [Google Scholar]
- Cao, J.; Yan, W.D.; Xiang, W.D.; Chen, X.Y.; Lei, P.F. Stoichiometry characterization of soil C, N, and P of Chinese fir plantations at three different ages in Huitong, Hunan province, China. Sci. Silv. Sin. 2015, 51, 1–8. [Google Scholar]
- Majarenkov, V.; Legendre, P. Nonlinear redundancy analysis and canonical correspondence analysis based on polynomial regression. Ecology 2002, 83, 1146–1161. [Google Scholar] [CrossRef]
- Hedin, L.O. Global organization of terrestrial plant-nutrient interactions. Proc. Natl. Acad. Sci. USA 2004, 101, 10849–10850. [Google Scholar] [CrossRef] [PubMed]
- Güsewell, S.; Koerselman, W. Variation in nitrogen and phosphorus concentrations of wetland plant. Perspect. Plant Ecol. 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Zhang, L.X.; Bai, Y.F.; Han, X.G. Application of N: P Stoichiometry to Ecology Studies. Acta Bot. Sin. 2003, 45, 1009–1018. [Google Scholar]
- Niklas, K.J. Plant Allometry, Leaf Nitrogen and Phosphorus Stoichiometry, and Interspecific Trends in Annual Growth Rates. Ann. Bot. 2005, 97, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Koreselman, W.; Meuleman, A.M. The vegetation N:P ratio: A new tool to detect the Nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Zheng, L.J.; Huang, Z.Q.; He, Z.M.; Liu, R.Q.; Xiao, H.Y.; Du, T. δ15N in fine roots of Cunninghamia lanceolata plantations of different ages and implications for soil nitrogen cycling rates. Acta Ecol. Sin. 2016, 36, 2185–2191. [Google Scholar]
- Zheng, L.J.; Huang, Z.Q.; He, Z.M.; Wang, X.Y.; Liu, Z.M. Influence of forest and foliar ages on the composition of stable carbon and nitrogen isotope of Cunninghamia lanceolata in subtropical China. Sci. Silv. Sin. 2015, 51, 22–28. [Google Scholar]
- Chen, F.; Niklas, K.J.; Liu, Y.; Fang, X.M.; Wan, S.Z.; Wang, H.M. Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol. 2015, 35, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.L.; Addo-Danso, S.D.; Wu, P.F.; Li, S.B.; Zou, X.H.; Ma, X.Q. Leaf resorption efficiency in relation to foliar and soil nutrient concentrations and stoichiometry of Cunninghamia lanceolata with stand development in southern China. J. Soil. Sediment 2016, 16, 1448–1459. [Google Scholar] [CrossRef]
- Liang, X.Y.; Liu, S.R.; Wang, H.; Wang, J.X. Variation of carbon and nitrogen stoichiometry along a chronosequence of natural temperature forest in northeastern China. J. Plant Ecol. 2018, 11, 339–350. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.N.; Wang, J.Y.; Guo, Z.W.; Wang, G.; Zeng, D.H.; Wu, T.G. Tree stoichiometry and nutrient resorption along a chronosequence of Metasequoia glyptostroboides forests in coastal China. For. Ecol. Manag. 2018, 430, 445–450. [Google Scholar] [CrossRef]
- Matzek, V.; Vitousek, P.M. N:P stoichiometry and protein: RNA ratios in vascular plants: An evaluation of the growth-rate hypothesis. Ecol. Lett. 2010, 12, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Ubach, A.; Sardans, J.; Pérez-Trujillo, M.; Estiarte, M.; Peñuelas, J. Strong relationship between elemental stoichiometry and metabolome on plants. Proc. Natl. Acad. Sci. USA 2012, 109, 4181–4186. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Yu, M.F.; Huang, Z.S.; Shi, J.H.C. C, N, and P stoichiometry and their interaction with plants, litter, and soil in a Cunninghamia lanceolata plantations with different ages. Acta Ecol. Sin. 2018, 38, 7772–7781. [Google Scholar]
- Waal, D.V.; Verschoor, A.M.; Verspagen, J.M.H.; Donk, E.V.; Huisman, J. Climate-driven changes in the ecological stoichiometry of aquatic ecosystems. Front. Ecol. Environ. 2010, 8, 145–152. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Dijkstra, F.A.; Zhang, K.; Li, X.; Tan, H.; Li, G. Leaf nitrogen and phosphorus of temperate desert plants in response to climate and soil nutrient availability. Sci. Rep. 2014, 4, 6932. [Google Scholar] [CrossRef]
- Chen, Y.H.; Han, W.X.; Tang, L.Y.; Tang, Z.Y.; Fang, J.Y. Leaf nitrogen and phosphorus concentrations of woody plants differ in response to climate, soil and plant growth form. Ecography 2013, 36, 178–184. [Google Scholar] [CrossRef]
- Zhang, S.B.; Zhang, J.L.; Slik-Ferry, J.W.; Cao, K.F. Leaf element concentrations of terrestrial plants across China are influenced by taxonomy and the environment. Glob. Ecol. Biogeogr. 2012, 21, 809–818. [Google Scholar] [CrossRef]
- Wu, Z.L. (Ed.) Cunninghmia Lancelata; China Forestry Publishing House: Beijing, China, 1984; pp. 309–311. [Google Scholar]
- Reich, P.B.; Oleksyn, J.; Wright, I.J. Leaf phosphorus influences the photosynthesis-nitrogen relation: A cross-biome analysis of 314 species. Oecologia 2009, 160, 207–212. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.L.; Wang, T.L.; Fang, S.Z. Leaf nitrogen and phosphorus stoichiometry of Cyclocarya paliurus across China. Forests 2018, 9, 771. [Google Scholar] [CrossRef]
- Lin, Y.L.; Mao, W.; Zhao, X.Y.; Zhang, T.H. Leaf nitrogen and phosphorus stoichiometry in typical desert and desertified region, north China. Chin. Environ. Sci. 2010, 31, 1716–1725. [Google Scholar]
- Rentería, L.Y.; Jaramillo, V.J. Rainfall drives leaf traits and leaf nutrient resorption in a tropical dry forest in Mexico. Oecologia 2011, 165, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Moles, A.T.; Perkins, S.E.; Laffan, S.W.; Flores-Moreno, H.; Awasthy, M.; Tindall, M.L.; Sack, L.; Pitman, A.; Kattge, J.; Aarssen, L.W.; et al. Which is a better predictor of plant traits: Temperature or precipitation? J. Veg. Sci. 2014, 25, 1167–1180. [Google Scholar] [CrossRef]
- Du, M.Y.; Fan, S.H.; Liu, G.L.; Feng, H.Y.; Guo, B.H.; Tang, X.L. Stoichiometric characteristics of carbon, nitrogen and phosphorus in Phyllostachys edulis forests of China. Chin. J. Plant Ecol. 2016, 40, 760–774. [Google Scholar]
- Reimann, C.; Englmaier, P.; Fabian, K.; Gough, L.; Lamothe, P.; Smith, D. Biogeochemical plant-soil interaction: Variable element composition in leaves of four plant species collected along a south-north transect at the southern tip of Norway. Sci. Total Environ. 2015, 506, 480–495. [Google Scholar] [CrossRef]
- Pan, W.T.; Tian, D.L.; Li, L.C.; Gao, Z.H. Studies on the nutrient cycling in the Chinese fir plantations (I) The yield structure and nutrient dynamics of Chinese fir forests in different growth stages. J. Cent. South For. Coll. 1981, 1, 1–21. [Google Scholar]
- Yang, Z.A. A Study of Root Characteristics and Nutrients of Different-aged Chinese Fir Plantations. Master’s Thesis, Northwest A & F University, Xianyang, China, May 2014. [Google Scholar]
- Liu, Z.M. Research on the Nutrient Dynamics and Internal Absorption of Cunninghamia lanceolota Foliar. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, April 2014. [Google Scholar]
- Kong, L.L.; Huang, Z.Q.; He, Z.M.; Zheng, L.J.; Liu, Z.M.; Wang, M.H. Variations of water use efficiency and foliar nutrient concentrations in Cunninghamia lanceolata plantations at different ages. Chin. J. Appl. Ecol. 2017, 28, 1069–1076. [Google Scholar]
- Qiu, L.J.; Hu, H.T.; Lin, B.P.; Wang, F.L.; Lin, Y.; He, Z.M.; Liu, Z.M. Nutrient resorption efficiency and C:N:P stoichiometry of Cunninghamia lanceolata plantations with different ages. J. Northwest For. Univ. 2017, 32, 22–27. [Google Scholar]
- Ma, X.Q.; Liu, A.Q.; Ma, Z. A comparative study on nutrient accumulation and distribution of different generations of Chinese fir plantations. Chin. J. Appl. Ecol. 2000, 11, 501–506. [Google Scholar]
- Lei, X.M. The Influence of Simulated Nitrogen Deposition and Understory Removal on Available N and P Dynamics in a Cunninghamia lanceolata Plantation. Master’s Thesis, Nanchang Institute of Technology, Nanchang, China, December 2017. [Google Scholar]
- Pan, W.C.; Tian, D.L.; Lei, Z.X.; Kang, W.X. Studies on the nutrient cycling in the Chinese fir plantations (II) Content, accumulation rate and biological cycling of nutrient elements in the fast-growing Chinese fir forest in the hill regions. J. Cent. South For. Coll. 1983, 3, 1–17. [Google Scholar]
- Liu, K.C.; Zeng, T.X. An investigation on the translocation and cycling of main nutrient elements in young mixed stand of Cunninghamia lanceolata and Michelia macclurei. For. Res. 1990, 3, 618–623. [Google Scholar]
- Zhong, A.L.; Xiong, W.Y. Seasonal changes of nutrient concentrations and nutrient interactions in needles of Chinese fir plantations. J. Nanjing For. Univ. 1993, 17, 1–8. [Google Scholar]
- Xian, G.B.; Huang, Y.H.; Xian, W.G.; Wang, M.; Tang, H.H.; Gan, X.H. Ecological stoichiometry characteristics of leaf nitrogen, phosphorus and sulfur of trees species of plantation on South China. Guangdong For. Sci. Technol. 2015, 31, 28–34. [Google Scholar]
- Wang, Q.; Zhang, J.B.; Lei, Z.F.; Liu, M.; Li, Q.; Huang, H.H.; Song, X.Z. Effects of simulated nitrogen deposition and phosphorus addition on ecological stoichiometry of Chinese fir leaves. Chin. J. Ecol. 2019, 38, 368–375. [Google Scholar]
- Liao, Z.H. Comparative analyses of macro nutrient elements in various organs of twenty-year-old Cunninghamia lanceolata under different site conditions. J. Fujian For. Sci. Tech. 1999, 26, 22–25. [Google Scholar]
- Liu, W.F.; Fan, H.B.; Zhang, Z.W.; Yang, Y.L.; Wang, Q.Q.; Xu, L. Foliar nutrient contents of Chinese fir in response to simulated nitrogen deposition. Chin. J. Appl. Environ. Biol. 2008, 14, 319–323. [Google Scholar]
- Nie, D.P. A comparasion of the productivity and nutrient cycling of Chinese fir plantation in different site conditions. For. Res. 1993, 6, 643–649. [Google Scholar]
- Xue, L.; Luo, S. Concentration and distribution of nutrients in an artificial Cunninghamia lanceolata stand ecosystem at Yishan. J. South China Agric. Univ. 2002, 23, 24–26. [Google Scholar]
- Lv, W.Q.; Zhou, C.Y.; Yan, J.H.; Li, S.J. Leaf C, N, and P stoichiometry for four typical artificial forests in the karst region of Guihou Province. J. Zhejiang A F Univ. 2016, 33, 984–990. [Google Scholar]
- Liu, F.; Luo, R.Y.; Jiang, J.P. Soil nutritive conditions and tree growth of Chinese fir. J. Nanjing For. Univ. 1991, 15, 41–46. [Google Scholar]
- Fan, J. Stand Structure Influence on Phosphorus Functional Fractions and Vegetative Organ Nitrogen and Phosphorus Stoichiometric Ratio in Cunninghamia lanceolata Plantations. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, June 2015. [Google Scholar]




| Age Groups | Age Range (a) | N | P | N:P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (mg g−1) | SD (mg g−1) | CV | Mean (mg g−1) | SD (mg g−1) | CV | Mean | SD | CV | ||
| AAG (n = 47) | 11.94 | 2.20 | 0.184 | 1.04 | 0.32 | 0.306 | 12.29 | 2.97 | 0.242 | |
| YAG (n = 15) | ≤10 | 11.45 | 2.52 | 0.221 | 1.06 | 0.30 | 0.282 | 11.46 | 2.73 | 0.239 |
| MAG (n = 16) | 11–20 | 11.83 | 1.72 | 0.145 | 1.08 | 0.31 | 0.288 | 11.70 | 3.04 | 0.260 |
| NMAG (n = 6) | 21–25 | 12.24 | 1.84 | 0.150 | 0.87 | 0.17 | 0.195 | 14.50 | 1.79 | 0.124 |
| MAG (n = 5) | 26–35 | 14.28 | 2.43 | 0.170 | 1.37 | 0.31 | 0.226 | 10.54 | 1.90 | 0.180 |
| OMAG (n = 5) | ≥36 | 11.07 | 1.78 | 0.160 | 0.71 | 0.17 | 0.224 | 15.83 | 1.43 | 0.090 |
| F | 2.01 | 4.06 | 4.604 | |||||||
| P | 0.111 | 0.007 | 0.004 | |||||||
| Axis | Axis I | Axis II | Axis III | Axis IV |
|---|---|---|---|---|
| Eigenvalues Explained variation of the leaf stoichiometry | 0.350 | 0.058 | 0.001 | 0.326 |
| Correlations between the leaf stoichiometry and influencing factors | 0.721 | 0.427 | 0.363 | 0.000 |
| Cumulative explained variation of the leaf stoichiometry | 0.350 | 0.408 | 0.409 | 0.735 |
| Cumulative explained variation of the relations between the leaf stoichiometry and influencing factors | 0.855 | 0.997 | 1.000 | 0.000 |
| Canonical eigenvalues | 0.409 | |||
| Sum of all eigenvalues | 1 | |||
| Data Source | Species Type | Species Number | N mg g−1 | P mg g−1 | N:P | Reference |
|---|---|---|---|---|---|---|
| Present study | Chinese fir | 1 | 11.94 | 1.04 | 12.29 | |
| Europe | Picea abies L. | 1 | 13.37 | 1.41 | 9.76 | [7] |
| China | Quercus L. | 13 | 17.27 | 1.54 | 13.96 | [8] |
| NSTEC | Whole trees | 654 | 19.09 | 1.56 | 15.39 | [15] |
| China | Whole trees | 753 | 20.24 | 1.45 | 16.35 | [10] |
| Global | Whole trees | 1251 | 20.1 | 1.77 | 13.8 | [11] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, R.; Zhou, B.; Jiang, L.; Ge, X.; Cao, Y.; Yang, Z. Leaf Nitrogen and Phosphorus Stoichiometry of Chinese fir Plantations across China: A Meta-Analysis. Forests 2019, 10, 945. https://doi.org/10.3390/f10110945
Tong R, Zhou B, Jiang L, Ge X, Cao Y, Yang Z. Leaf Nitrogen and Phosphorus Stoichiometry of Chinese fir Plantations across China: A Meta-Analysis. Forests. 2019; 10(11):945. https://doi.org/10.3390/f10110945
Chicago/Turabian StyleTong, Ran, Benzhi Zhou, Lina Jiang, Xiaogai Ge, Yonghui Cao, and Zhenya Yang. 2019. "Leaf Nitrogen and Phosphorus Stoichiometry of Chinese fir Plantations across China: A Meta-Analysis" Forests 10, no. 11: 945. https://doi.org/10.3390/f10110945




