Tree-Rings Reveal Accelerated Yellow-Cedar Decline with Changes to Winter Climate after 1980
Abstract
:1. Introduction
2. Materials and Methods
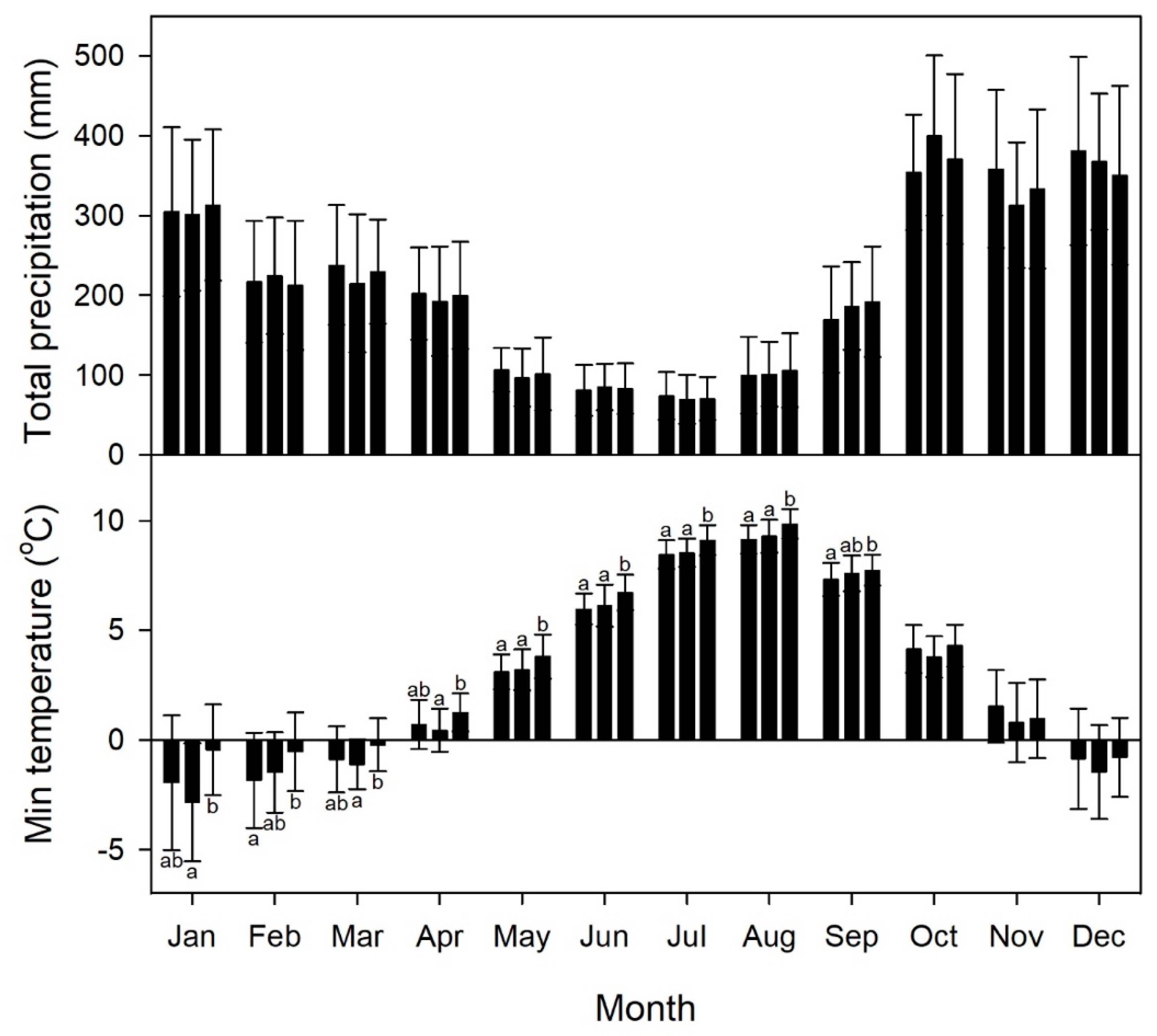
2.1. Study Sites
2.2. Dendrochronological Methods
2.3. Climate-Growth Relations
2.4. Wavelet Transform Analysis
3. Results
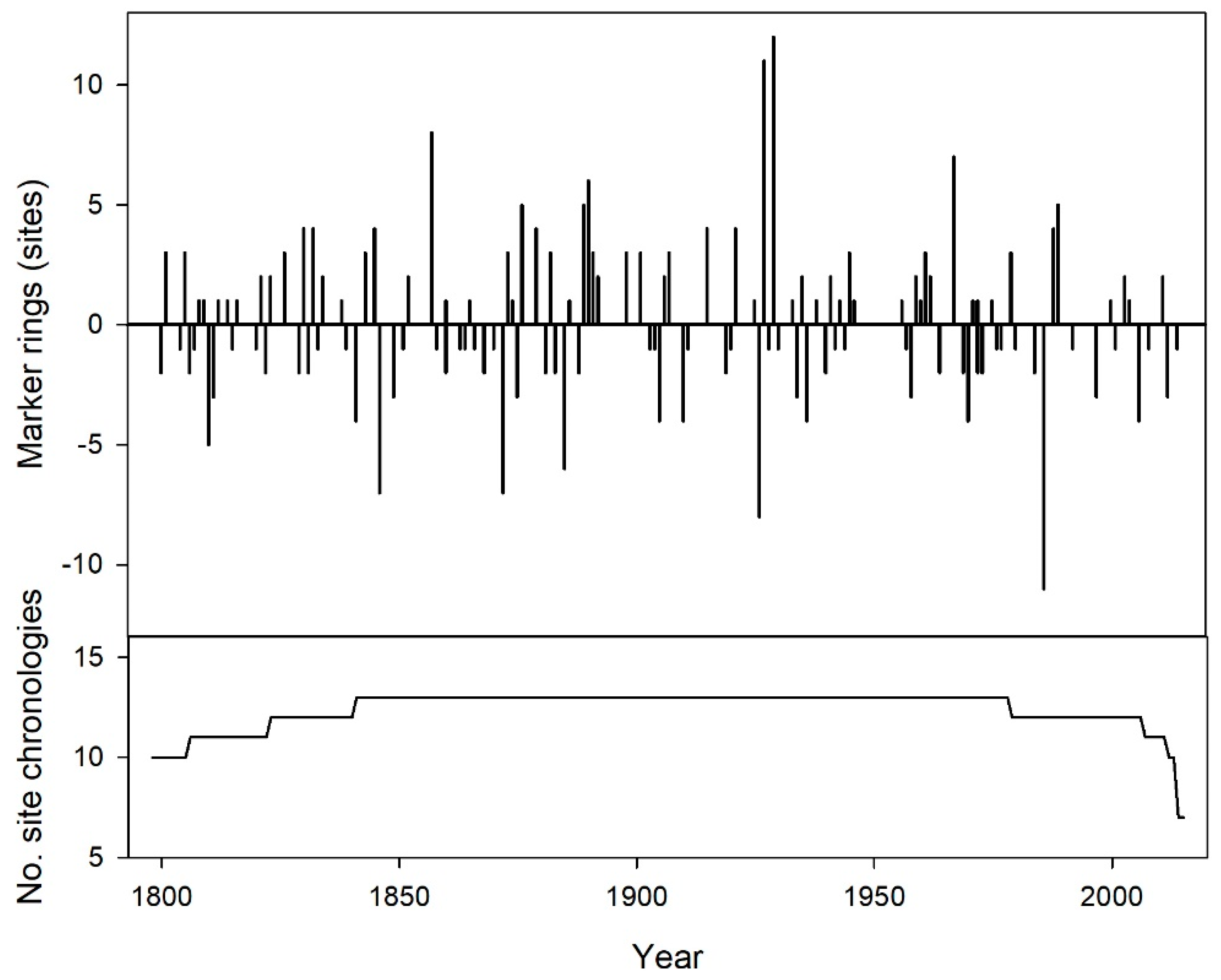
3.1. Site Chronologies
3.2. Regional Chronologies
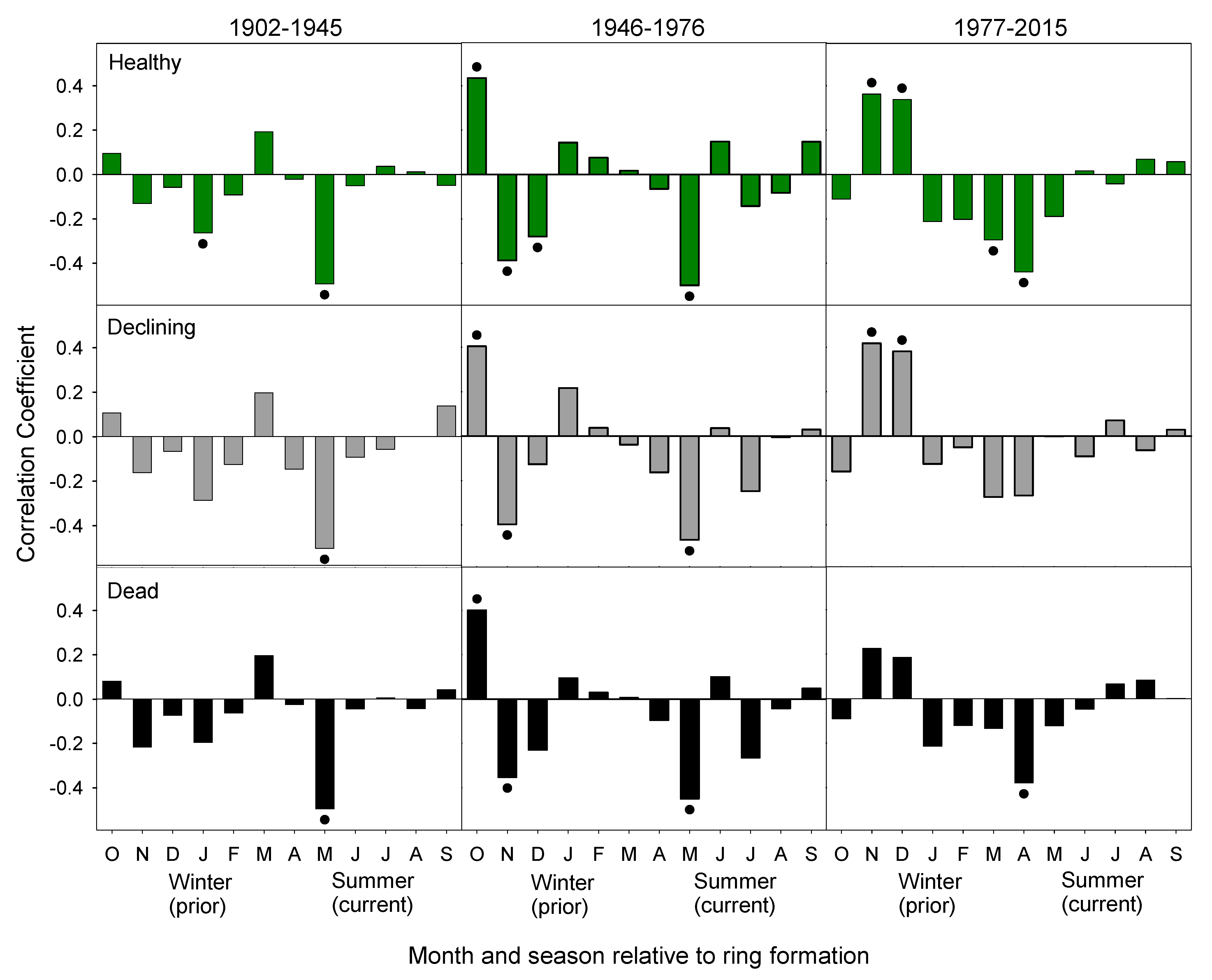
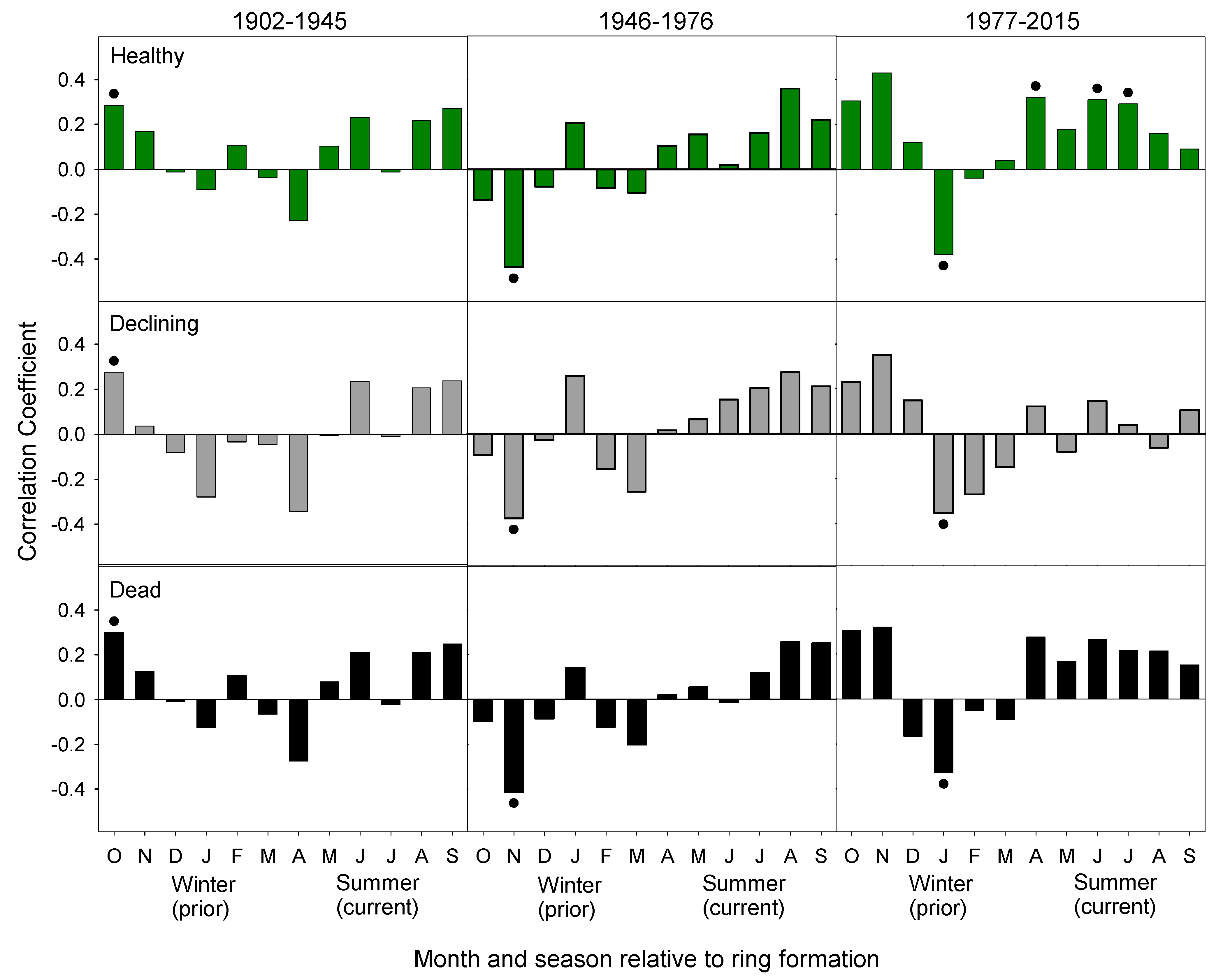
3.3. Climate and Yellow-Cedar Growth
3.4. Temporal Frequency
4. Discussion
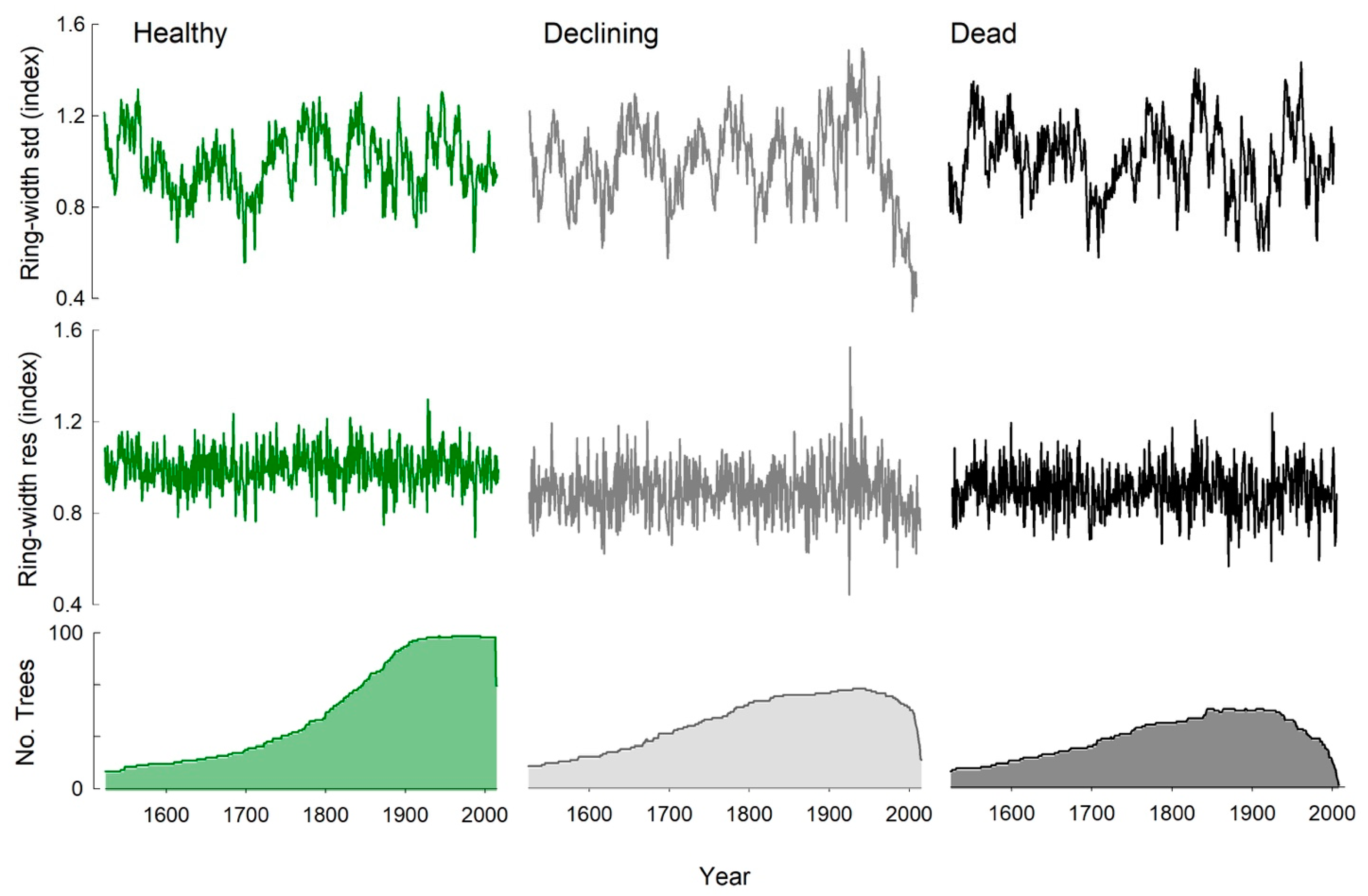
4.1. Common Growth Signal Present across Sites
4.2. Separating Trees by Health Status
4.3. Divergent Growth of Trees of Different Health Classes
4.4. Climatic Drivers of Yellow-Cedar Decline
4.5. Consistent with Alaska Hypothesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H. (Ted); et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Peng, C.; Ma, Z.; Lei, X.; Zhu, Q.; Chen, H.; Wang, W.; Liu, S.; Li, W.; Fang, X.; Zhou, X. A drought-induced pervasive increase in tree mortality across Canada’s boreal forests. Nat. Clim. Chang. 2011, 1, 467–471. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Navarro-Cerrillo, R.M.; Camarero, J.J.; Fernández-Cancio, Á. Selective drought-induced decline of pine species in southeastern Spain. Clim. Chang. 2012, 113, 767–785. [Google Scholar] [CrossRef]
- Meddens, A.J.H.; Hicke, J.A.; Macalady, A.K.; Buotte, P.C.; Cowles, T.R.; Allen, C.D. Patterns and causes of observed piñon pine mortality in the southwestern United States. New Phytol. 2015, 206, 91–97. [Google Scholar] [CrossRef]
- Michaelian, M.; Hogg, E.H.; Hall, R.J.; Arsenault, E. Massive mortality of aspen following severe drought along the southern edge of the Canadian boreal forest. Glob. Chang. Biol. 2011, 17, 2084–2094. [Google Scholar] [CrossRef]
- Lloyd, A.H.; Bunn, A.G. Responses of the circumpolar boreal forest to 20th century climate variability. Environ. Res. Lett. 2007, 2, 045013. [Google Scholar] [CrossRef]
- Buma, B.; Hennon, P.E.; Harrington, C.A.; Popkin, J.R.; Krapek, J.; Lamb, M.S.; Oakes, L.E.; Saunders, S.; Zeglen, S. Emerging climate-driven disturbance processes: Widespread mortality associated with snow-to-rain transitions across 10° of latitude and half the range of a climate-threatened conifer. Glob. Chang. Biol. 2016, 23, 2903–2914. [Google Scholar] [CrossRef]
- Susiluoto, S.; Berninger, F. Interactions between morphological and physiological drought responses in Eucalyptus microtheca. Silva Fenn. 2007, 41, 221. [Google Scholar] [CrossRef]
- Hennon, P.E.; D’Amore, D.V.; Schaberg, P.G.; Wittwer, D.T.; Shanley, C.S. Shifting climate, altered niche, and a dynamic conservation strategy for yellow-cedar in the north Pacific coastal rainforest. BioScience 2012, 62, 147–158. [Google Scholar] [CrossRef]
- Hennon, P.; Amore, D.D.; Johnson, A.; Schaberg, P.G.; Hawley, G.; Beier, C.; Sink, S.; Juday, G. Climate warming, reduced snow, and freezing injury could explain the demise of yellow-cedar in southeast Alaska, USA. World Resour. Rev. 2006, 18, 24. [Google Scholar]
- Hennon, P.E.; D’Amore, D.V.; Witter, D.T.; Lamb, M.B. Influence of forest canopy and snow on microclimate in a declining yellow-dedar forest of southeast Alaska. Northwest Sci. 2010, 84, 73–87. [Google Scholar] [CrossRef]
- Schaberg, P.G.; Hennon, P.E.; D’Amore, D.V.; Hawley, G.J. Influence of simulated snow cover on the cold tolerance and freezing injury of yellow-cedar seedlings. Glob. Chang. Biol. 2008, 14, 1282–1293. [Google Scholar] [CrossRef]
- D’Amore, D.V.; Hennon, P.E. Evaluation of soil saturation, soil chemistry, and early spring soil and air temperatures as risk factors in yellow-cedar decline. Glob. Chang. Biol. 2006, 12, 524–545. [Google Scholar] [CrossRef]
- Stan, A.B.; Maertens, T.B.; Daniels, L.D.; Zeglen, S. Reconstructing population dynamics of yellow-cedar in declining stands: Baseline information from tree rings. Tree-Ring Res. 2011, 67, 13–25. [Google Scholar] [CrossRef]
- Banner, A.; MacKenzie, W.H.; Pojar, J.; MacKinnon, A.; Saunders, S.C.; Klassen, H. A Field Guide to Ecosystem Classification and Identification for Haida Gwaii; Land management handbook; Province of British Columbia, Ministry of Forests: Victoria, BC, Canada, 2014; ISBN 978-0-7726-6768-7.
- Comeau, V. Freezing to Death in a Warming Climate: Drivers of Yellow-Cedar Decline on Haida Gwaii; University of British Columbia: Vancouver, BC, Canada, 2019. [Google Scholar]
- Cailleret, M.; Jansen, S.; Robert, E.M.R.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Chang. Biol. 2017, 23, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, M.M.; Daniels, L.D.; Larson, B.C. Temporal patterns of radial growth in declining Austrocedrus chilensis forests in Northern Patagonia: The use of tree-rings as an indicator of forest decline. For. Ecol. Manag. 2012, 265, 62–70. [Google Scholar] [CrossRef]
- Chavardès, R.D.; Daniels, L.D.; Waeber, P.O.; Innes, J.L.; Nitschke, C.R. Unstable climate−growth relations for white spruce in southwest Yukon, Canada. Clim. Chang. 2012, 116, 593–611. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Mantua, N.J.; Hare, S.R. The Pacific Decadal Oscillation. J. Oceanogr. 2002, 58, 35–44. [Google Scholar] [CrossRef]
- Wiles, G.C.; Charlton, J.; Wilson, R.J.S.; D’Arrigo, R.; Buma, B.; Krapek, J.; Gaglioti, B.V.; Wiesenberg, N.; Oelkers, R. Yellow-Cedar Blue Intensity Tree Ring Chronologies as Records of Climate, Juneau, Alaska, USA. Can. J. For. Res. 2019, 49, 1483–1492. [Google Scholar] [CrossRef]
- Larsson, L. CooRecorder; Cybis Elektronik & Data: Saltsjöbaden, Sweden, 2014. [Google Scholar]
- Larsson, L. CDendro; Cybis Elektronik & Data: Saltsjöbaden, Sweden, 2014. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Bunn, A.G.; Biondo, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Zang, C. dplR: Dendrochronology Program Library in R. 2017. Available online: https://rdrr.io/cran/dplR/ (accessed on 1 November 2017).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Melvin, T.M.; Briffa, K.R. CRUST: Software for the implementation of Regional Chronology Standardisation: Part 1. Signal-Free RCS. Dendrochronologia 2014, 32, 7–20. [Google Scholar] [CrossRef]
- Melvin, T.M.; Briffa, K.R. A “signal-free” approach to dendroclimatic standardisation. Dendrochronologia 2008, 26, 71–86. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Wigley, T.M.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Spittlehouse, D.; Carroll, C. Locally downscaled and spatially customizable climate data for historical and future periods for North America. PLoS ONE 2016, 11, e0156720. [Google Scholar] [CrossRef]
- Blasing, T.J.; Solomon, A.M.; Duvick, D.N. Response functions revisited. Tree-Ring Bull. 1984, 1–15. [Google Scholar]
- Zang, C.; Biondi, F. treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G.P. A practical guide to wavelet analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Roesch, A.; Schmidbauer, H. WaveletComp: Computational Wavelet Analysis. 2018. Available online: https://rdrr.io/cran/WaveletComp/man/WaveletComp-package.html (accessed on 1 November 2018).
- National Centers for Environmental Information Pacific Decadal Oscillation (PDO); National Oceanic and Atmospheric Administration: Washington, DC, USA, 2019.
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Chicago Press: Chicago, IL, USA, 1996. [Google Scholar]
- Wiles, G.C.; Mennett, C.R.; Jarvis, S.K.; D’Arrigo, R.D.; Wiesenberg, N.; Lawson, D.E. Tree-ring investigations into changing climatic responses of yellow-cedar, Glacier Bay, Alaska. Can. J. For. Res. 2012, 42, 814–819. [Google Scholar] [CrossRef]
- Kellner, A.M.E.; Laloque, C.P.; Smith, D.J.; Harestadl, A.S. Chronological dating of high-elevation dead and dying trees on Northern Vancouver Island, British Columbia. Northwest Sci. 2000, 74, 242–247. [Google Scholar]
- Laroque, C.P.; Smith, D.J. Tree-ring analysis of yellow-cedar (Chamaecyparis nootkatensis) on Vancouver Island, British Columbia. Can. J. For. Res. 1999, 29, 115–123. [Google Scholar] [CrossRef]
- Hennon, P.E.; McKenzie, C.M.; D’Amore, D.V.; Wittwer, D.T.; Mulvey, R.L.; Lamb, M.S.; Biles, F.E.; Cronn, R.C. A Climate Adaptation Strategy for Conservation and Management of Yellow-Cedar in Alaska; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2016; p. 382.
- Beier, C.M.; Sink, S.E.; Hennon, P.E.; D’Amore, D.V.; Juday, G.P. Twentieth-century warming and the dendroclimatology of declining yellow-cedar forests in southeastern Alaska. Can. J. For. Res. 2008, 38, 1319–1334. [Google Scholar] [CrossRef]
- Parish, R. Mount Cain, Alaska Yellow-Cedar Tree-Ring Record (CANA175); NOAA/NGDC Paleoclimatology: Boulder, CO, USA, 2005. [Google Scholar]
- Rodríguez-Catón, M.; Villalba, R.; Srur, A.M.; Luckman, B. Long-term trends in radial growth associated with Nothofagus pumilio forest decline in Patagonia: Integrating local- into regional-scale patterns. For. Ecol. Manag. 2015, 339, 44–56. [Google Scholar] [CrossRef]
- Wong, C.M.; Daniels, L.D. Novel forest decline triggered by multiple interactions among climate, an introduced pathogen and bark beetles. Glob. Chang. Biol. 2017, 23, 1926–1941. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.E.; Cook, E.R.; Larson, D.W. Constrained growth, cambial mortality, and dendrochronology of ancient Thuja occidentalis on cliffs of the Niagara Escarpment: An eastern version of bristlecone pine? Int. J. Plant Sci. 1992, 153, 117–127. [Google Scholar] [CrossRef]
- Daniels, L.D.; Dobry, J.; Klinka, K.; Feller, M.C. Determining year of death of logs and snags of Thuja plicata in southwestern coastal British Columbia. Can. J. For. Res. 1997, 27, 1132–1141. [Google Scholar] [CrossRef]
- Amoroso, M.M.; Daniels, L.D. Cambial mortality in declining Austrocedrus chilensis forests: Implications for stand dynamics studies. Can. J. For. Res. 2010, 40, 885–893. [Google Scholar] [CrossRef]
- Hennon, P.E.; Shaw, C.G.; Hansen, E.M. Symptoms and fungal associations of declining Chamaecyparis nootkatensis in southeast Alaska. Plant Dis. 1990, 74, 267–273. [Google Scholar] [CrossRef]
- Nemani, R.R.; Keeling, C.D.; Hashimoto, H.; Jolly, W.M.; Piper, S.C.; Tucker, C.J.; Myneni, R.B.; Running, S.W. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 2003, 300, 1560–1563. [Google Scholar] [CrossRef] [Green Version]
- Wilmking, M.; Juday, G.P. Longitudinal variation of radial growth at Alaska’s northern treeline—recent changes and possible scenarios for the 21st century. Glob. Planet. Chang. 2005, 47, 282–300. [Google Scholar] [CrossRef]
- Driscoll, W.W.; Wiles, G.C.; D’Arrigo, R.D.; Wilmking, M. Divergent tree growth response to recent climatic warming, Lake Clark National Park and Preserve, Alaska. Geophys. Res. Lett. 2005, 32, L20703. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, A.H.; Bunn, A.G.; Berner, L. A latitudinal gradient in tree growth response to climate warming in the Siberian taiga. Glob. Chang. Biol. 2011, 17, 1935–1945. [Google Scholar] [CrossRef]
- Myneni, R.B.; Keeling, C.D.; Tucker, C.J.; Asrar, G.; Nemani, R.R. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 1997, 386, 698–702. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Ji, Y.; Li, Z.; Li, M.; Zhang, Y. Temperature signals in tree-ring width and divergent growth of Korean pine response to recent climate warming in northeast Asia. Trees 2017, 31, 415–427. [Google Scholar] [CrossRef]
- Salzer, M.W.; Hughes, M.K.; Bunn, A.G.; Kipfmueller, K.F. Recent unprecedented tree-ring growth in bristlecone pine at the highest elevations and possible causes. Proc. Natl. Acad. Sci. USA 2009, 106, 20348–20353. [Google Scholar] [CrossRef] [Green Version]
- D’Arrigo, R.D.; Kaufmann, R.K.; Davi, N.; Jacoby, G.C.; Laskowski, C.; Myneni, R.B.; Cherubini, P. Thresholds for warming-induced growth decline at elevational tree line in the Yukon Territory, Canada. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Wilson, R.; Liepert, B.; Cherubini, P. On the ‘Divergence Problem’ in Northern Forests: A review of the tree-ring evidence and possible causes. Glob. Planet. Chang. 2008, 60, 289–305. [Google Scholar] [CrossRef]
- Schneider, L.; Esper, J.; Timonen, M.; Büntgen, U. Detection and evaluation of an early divergence problem in northern Fennoscandian tree-ring data. Oikos 2014, 123, 559–566. [Google Scholar] [CrossRef]
- Wilmking, M.; Juday, G.P.; Barber, V.A.; Zald, H.S.J. Recent climate warming forces contrasting growth responses of white spruce at treeline in Alaska through temperature thresholds. Glob. Chang. Biol. 2004, 10, 1724–1736. [Google Scholar] [CrossRef]
- Schaberg, P.G.; D’Amore, D.V.; Hennon, P.E.; Halman, J.M.; Hawley, G.J. Do limited cold tolerance and shallow depth of roots contribute to yellow-cedar decline? For. Ecol. Manag. 2011, 262, 2142–2150. [Google Scholar] [CrossRef]
- Buma, B. Transitional climate mortality: Slower warming may result in increased climate-induced mortality in some systems. Ecosphere 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Stewart, I.T.; Cayan, D.R.; Dettinger, M.D. Changes in snowmelt runoff timing in western North America under a ‘business as usual’ climate change scenario. Clim. Chang. 2004, 62, 217–232. [Google Scholar] [CrossRef]


| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| 2 | 0.28 | |||||||||||
| 3 | 0.14 | 0.48 | ||||||||||
| 4 | (0.08) | 0.68 | 0.56 | |||||||||
| 5 | 0.11 | 0.31 | 0.24 | 0.48 | ||||||||
| 6 | 0.24 | 0.43 | 0.51 | 0.43 | 0.42 | |||||||
| 7 | 0.17 | 0.53 | 0.45 | 0.71 | 0.38 | 0.35 | ||||||
| 8 | 0.23 | 0.48 | 0.36 | 0.53 | 0.23 | 0.23 | 0.60 | |||||
| 9 | 0.28 | 0.78 | 0.44 | 0.82 | 0.53 | 0.51 | 0.73 | 0.65 | ||||
| 10 | 0.26 | 0.69 | 0.43 | 0.79 | 0.46 | 0.43 | 0.64 | 0.53 | 0.86 | |||
| 11 | 0.28 | 0.44 | 0.08 | 0.47 | 0.41 | 0.27 | 0.50 | 0.66 | 0.73 | 0.51 | ||
| 12 | 0.33 | 0.63 | 0.38 | 0.58 | 0.36 | 0.47 | 0.62 | 0.56 | 0.76 | 0.69 | 0.56 | |
| 13 | 0.31 | 0.30 | 0.37 | 0.18 | 0.50 | 0.60 | 0.30 | 0.35 | 0.32 | 0.21 | 0.46 | 0.28 |
| Group | n | Full Length of | n ≥ 10 | Mean Series | Mean | EPS ≥ 0.85 |
|---|---|---|---|---|---|---|
| Chronology | (year) | Intercorrelation | Sensitivity | (year) | ||
| Healthy | 99 | 1324−2015 | 1521 | 0.381 | 0.260 | 1759 |
| Declining | 64 | 1282−2015 | 1500 | 0.377 | 0.273 | 1741 |
| Dead | 57 | 1297−2008 | 1524 | 0.377 | 0.260 | 1759 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comeau, V.M.; Daniels, L.D.; Knochenmus, G.; Chavardès, R.D.; Zeglen, S. Tree-Rings Reveal Accelerated Yellow-Cedar Decline with Changes to Winter Climate after 1980. Forests 2019, 10, 1085. https://doi.org/10.3390/f10121085
Comeau VM, Daniels LD, Knochenmus G, Chavardès RD, Zeglen S. Tree-Rings Reveal Accelerated Yellow-Cedar Decline with Changes to Winter Climate after 1980. Forests. 2019; 10(12):1085. https://doi.org/10.3390/f10121085
Chicago/Turabian StyleComeau, Vanessa M., Lori D. Daniels, Garrett Knochenmus, Raphaël D. Chavardès, and Stefan Zeglen. 2019. "Tree-Rings Reveal Accelerated Yellow-Cedar Decline with Changes to Winter Climate after 1980" Forests 10, no. 12: 1085. https://doi.org/10.3390/f10121085




