Global Assessment of Climate-Driven Susceptibility to South American Leaf Blight of Rubber Using Emerging Hot Spot Analysis and Gridded Historical Daily Data
Abstract
:1. Introduction
2. Data and Methods
2.1. Data
2.2. Methods
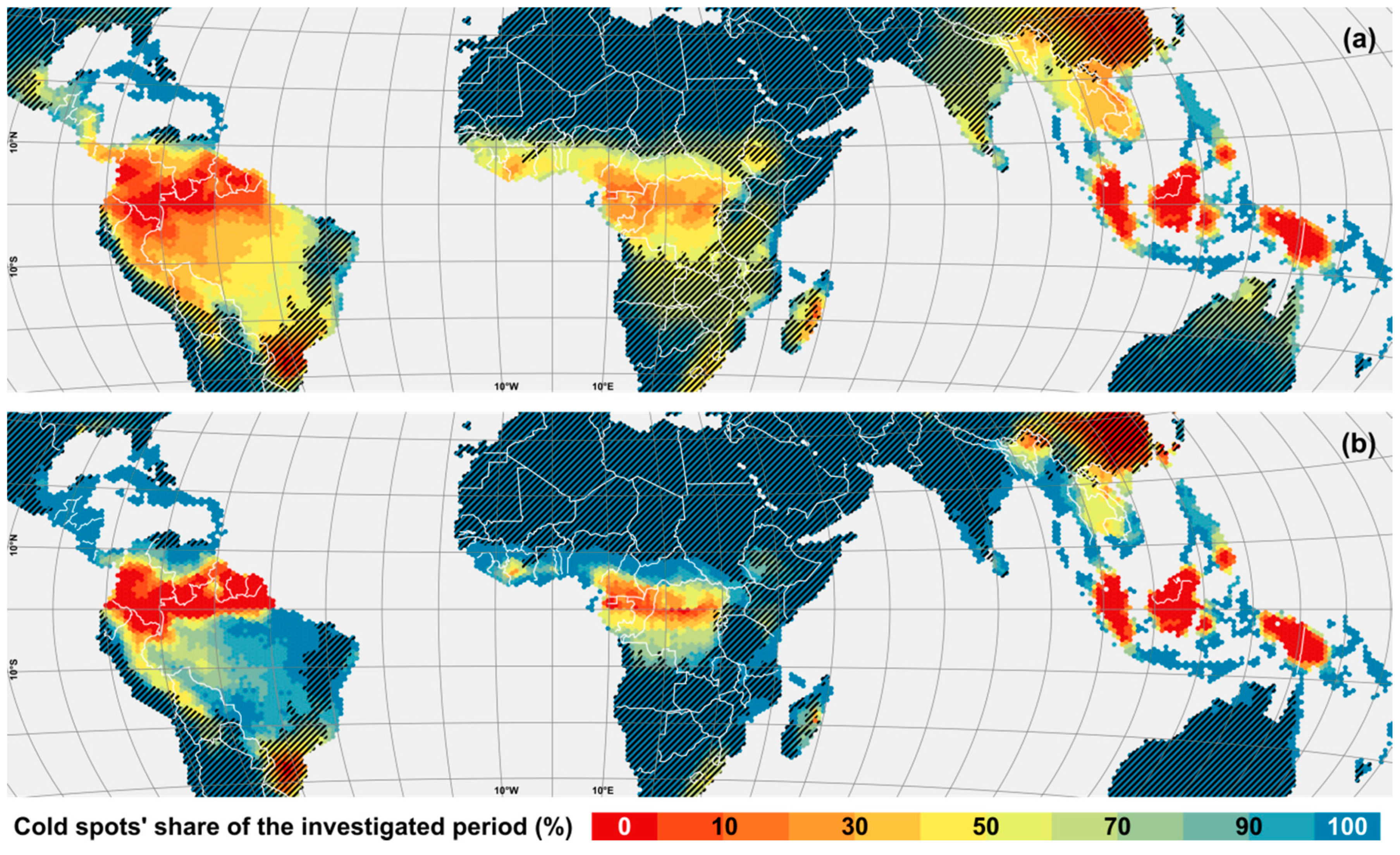
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 9 January 2019).
- Da Hora Júnior, B.T.; de Macedo, D.M.; Barreto, R.W.; Evans, H.C.; Mattos, C.R.R.; Maffia, L.A.; Mizubuti, E.S.G. Erasing the Past: A New Identity for the Damoclean Pathogen Causing South American Leaf Blight of Rubber. PLoS ONE 2014, 9, e104750. [Google Scholar] [CrossRef] [PubMed]
- Besse, P.; Seguin, M.; Lebrun, P.; Chevallier, M.H.; Nicolas, D.; Lanaud, C. Genetic diversity among wild and cultivated populations of Hevea brasiliensis assessed by nuclear RFLP analysis. Theor. Appl. Genet. 1994, 88, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Le Guen, V.; Seguin, M.; Mattos, C.R.R. Qualitative resistance of Hevea to Phyllachora huberi P. Henn. Euphytica 2000, 112, 211–217. [Google Scholar] [CrossRef]
- Le Guen, V.; Koop, D.M.; Salgado, L.R.; Déon, M.; Doare, F.; Souza, L.M.; Seguin, M.; Berger, A.; Pujade-Renaud, V.; Garcia, D. Genetic and genomic diversity response of rubber tree to a major fungal disease. In Proceedings of the 11th International Congress of Plant Molecular Biology, Iguazú Falls, Brazil, 25–30 October 2015. [Google Scholar]
- Le Guen, V.; Lespinasse, D.; Oliver, G.; Rodier-Goud, M.; Pinard, F.; Seguin, M. Molecular mapping of genes conferring field resistance to South American Leaf Blight (Microcyclus ulei) in rubber tree. Theor. Appl. Genet. 2003, 108, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Le Guen, V.; Garcia, D.; Mattos, C.R.R.; Clément-Demange, A. Evaluation of field resistance to Microcyclus ulei of a collection of Amazonian rubber tree (Hevea brasiliensis) germplasm. Cropp Breed. Appl. Biotechnol. 2002, 2, 141–148. [Google Scholar] [CrossRef]
- Varghese, Y.A. Germplasm resources and genetic improvement. In Developments in Crop Science; Sethuraj, M.R., Mathew, N.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; Volume 23, pp. 88–115. [Google Scholar]
- Onokpise, O.U. Natural Rubber, Hevea Brasiliensis (Willd. Ex A. Juss.) MüLl. Arg., Germplasm Collection in the Amazon Basin, Brazil: A Retrospective. Econ. Bot. 2004, 58, 544–555. [Google Scholar] [CrossRef]
- Priyadarshan, P.M. Genetic Diversity and Erosion in Hevea Rubber. In Genetic Diversity and Erosion in Plants; Ahuja, M.R., Jain, S.M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 8, pp. 233–267. ISBN 978-3-319-25953-6. [Google Scholar]
- Barrès, B.; Carlier, J.; Seguin, M.; Fenouillet, C.; Cilas, C.; Ravigné, V. Understanding the recent colonization history of a plant pathogenic fungus using population genetic tools and Approximate Bayesian Computation. Heredity 2012, 109, 269–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyot, J.; Le Guen, V. A Review of a Century of Studies on South American Leaf Blight of the Rubber Tree. Plant Dis. 2018, 102, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Häuser, I.; Martin, K.; Germer, J.; He, P.; Blagodatskiy, S.; Liu, H.; Krauss, M.; Rajaona, A.; Shi, M.; Langenberger, G.; et al. Environmental and socio-economic impacts of rubber cultivation in the Mekong region: Challenges for sustainable land use. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Le Guen, V.; Guyot, J.; Mattos, C.R.R.; Seguin, M.; Garcia, D. Long lasting rubber tree resistance to Microcyclus ulei characterized by reduced conidial emission and absence of teleomorph. Crop Prot. 2008, 27, 1498–1503. [Google Scholar] [CrossRef]
- Evans, H.C. Invasive neotropical pathogens of tree crops. In Tropical Mycology: Micromycetes; Watling, R., Frankland, J.C., Ainsworth, A.M., Isaac, S., Robinson, C.H., Eds.; CABI Pub: Wallingford, Oxon, UK; New York, NY, USA, 2002; Volume 2, pp. 83–112. ISBN 0-85199-543-8. [Google Scholar]
- Onokpise, O.; Louime, C. The Potential of the South American Leaf Blight as a Biological Agent. Sustainability 2012, 4, 3151–3157. [Google Scholar] [CrossRef] [Green Version]
- Miedaner, T. Henry Ford musste kapitulieren. In Pflanzenkrankheiten, die die Welt beweg(t)en; Springer: Berlin/Heidelberg, Germany, 2017; pp. 211–234. ISBN 978-3-662-49903-0. [Google Scholar]
- LebaiJuri, M.; Othman, S.; Wan Mashol, W.Z.; Ismail, R. Comparative feasibility of gamma, electron beam and x-rays facilities at the Kuala Lumpur International airport (KLIA), Sepang, Malaysia. In Proceedings of the INC ’97—International Nuclear Conference: A New Era in Nuclear Science and Technology—The Challenge of the 21st Century, Kuala Lumpur, Malaysia, 27–28 October 1997; pp. 173–185. [Google Scholar]
- Asna, B.O.; Ho, H.L. Managing Invasive Species: The Threat to Oil-palm and Rubber—The Malaysian Plant Quarantine Regulatory Perspective. In Proceedings of the Unwelcome Guests—Proceedings of the Asia-Pacific Forest Invasive Species Conference, Kunming, China, 17–23 August 2003; pp. 32–38. [Google Scholar]
- Hashim, I. South American Leaf Blight (Microcyclus ulei) of Hevea rubber. In Protection against South American Leaf Blight of Rubber in Asia and the Pacific Region; FAO: Bangkok, Thailand, 2012; Volume II, pp. 21–133. ISBN 978-92-5-107228-8. [Google Scholar]
- Furtado, E.L.; da Cunha, A.R.; Alvares, C.A.; Bevenuto, J.A.Z.; Passos, J.R. Ocorrência de epidemia do mal das folhas em regiões de “escape” do Brasil. Arq. Inst. Biol. 2015, 82, 1–6. [Google Scholar] [CrossRef]
- Rivano, F.; Maldonado, L.; Simbaña, B.; Lucero, R.; Gohet, E.; Cevallos, V.; Yugcha, T. Suitable rubber growing in Ecuador: An approach to South American leaf blight. Ind. Crops Prod. 2015, 66, 262–270. [Google Scholar] [CrossRef]
- Francl, L.J. The Disease Triangle: A Plant Pathological Paradigm Revisited. Plant Health Instr. 2001. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; ISBN 978-0-12-044565-3. [Google Scholar]
- Chee, K.H. Factors affecting discharge, germination and viability of spores of Microcyclus ulei. Trans. Br. Mycol. Soc. 1976, 66, 499–504. [Google Scholar] [CrossRef]
- Langford, M.H. South American Leaf Blight of Hevea Rubber Trees; US Dept. of Agriculture: Washington, DC, USA, 1945; Volume 882.
- Guyot, J.; Condina, V.; Doaré, F.; Cilas, C.; Sache, I. Role of ascospores and conidia in the initiation and spread of South American leaf blight in a rubber tree plantation. Plant Pathol. 2014, 63, 510–518. [Google Scholar] [CrossRef]
- Gasparotto, L.; Junqueira, N.T.V. Ecophysiological variability of Microcyclus ulei, causal agent of rubber tree leaf blight. Fitopatol. Bras. 1994, 19, 22–28. [Google Scholar]
- Holliday, P. Dispersal of conidia of Dothidella ulei from Hevea brasiliensis. Ann. Appl. Biol. 1969, 63, 435–447. [Google Scholar] [CrossRef]
- Guyot, J.; Condina, V.; Doaré, F.; Cilas, C.; Sache, I. Segmentation applied to weather-disease relationships in South American leaf blight of the rubber tree. Eur. J. Plant Pathol. 2010, 126, 349–362. [Google Scholar] [CrossRef]
- Lieberei, R. South American Leaf Blight of the Rubber Tree (Hevea spp.): New Steps in Plant Domestication using Physiological Features and Molecular Markers. Ann. Bot. 2007, 100, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Marattukalam, J.G.; Saraswathyamma, C.K. Propagation and planting. In Developments in Crop Science; Sethuraj, M.R., Mathew, N.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; Volume 23, pp. 164–199. [Google Scholar]
- Priyadarshan, P.M. Biology of Hevea Rubber; CABI: Wallingford, Oxfordshire, UK; Cambridge, MA, USA, 2011; ISBN 978-1-84593-666-2. [Google Scholar]
- Fang, Y.; Mei, H.; Zhou, B.; Xiao, X.; Yang, M.; Huang, Y.; Long, X.; Hu, S.; Tang, C. De novo Transcriptome Analysis Reveals Distinct Defense Mechanisms by Young and Mature Leaves of Hevea brasiliensis (Para Rubber Tree). Sci. Rep. 2016, 6, 33151. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Cazaux, E.; Rivano, F.; D’Auzac, J. Chemical and structural barriers to Microcyclus ulei, the agent of South American leaf blight, in Hevea spp. Eur. J. For. Pathol. 1995, 25, 282–292. [Google Scholar] [CrossRef]
- Da Silva, K.R.; Cecílio, R.A.; Xavier, A.C.; Pezzopane, J.R.M.; Garcia, G.D.O. Zoneamento edafoclimático para a cultura da seringueira no Espírito Santo. Irriga 2013, 18, 1. [Google Scholar] [CrossRef]
- De Camargo, Â.P.; Marin, F.R.; de Camargo, M.B.P. Zoneamento Climático da Heveicultura no Brasil; Embrapa Monitoramento por Satélite; Embrapa (Brazilian Agricultural Research Corporation): Campinas, São Paulo, Brazil, 2003; p. 19. [Google Scholar]
- Jaimes, Y.; Rojas, J.; Cilas, C.; Furtado, E.L. Suitable climate for rubber trees affected by the South American Leaf Blight (SALB): Example for identification of escape zones in the Colombian middle Magdalena. Crop Prot. 2016, 81, 99–114. [Google Scholar] [CrossRef]
- Roy, C.B.; Newby, Z.-J.; Mathew, J.; Guest, D.I. A climatic risk analysis of the threat posed by the South American leaf blight (SALB) pathogen Microcyclus ulei to major rubber producing countries. Eur. J. Plant Pathol. 2017, 148, 129–138. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Ord, J.K.; Getis, A. Local Spatial Autocorrelation Statistics: Distributional Issues and an Application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- CPC Global Unified Gauge-Based Analysis of Daily Precipitation. Available online: https://www.esrl.noaa.gov/psd/data/gridded/data.cpc.globalprecip.html (accessed on 21 August 2018).
- NCEP. NCEP Global Reanalysis Daily Surface Relative Humidity Data. Available online: https://www.esrl.noaa.gov/psd/data/gridded/data.ncep.reanalysis.surface.html (accessed on 21 August 2018).
- Ashouri, H.; Sorooshian, S.; Hsu, K.-L.; Bosilovich, M.G.; Lee, J.; Wehner, M.F.; Collow, A. Evaluation of NASA’s MERRA Precipitation Product in Reproducing the Observed Trend and Distribution of Extreme Precipitation Events in the United States. J. Hydrometeorol. 2015, 17, 693–711. [Google Scholar] [CrossRef]
- Cui, W.; Dong, X.; Xi, B.; Kennedy, A. Evaluation of Reanalyzed Precipitation Variability and Trends Using the Gridded Gauge-Based Analysis over the CONUS. J. Hydrometeorol. 2017, 18, 2227–2248. [Google Scholar] [CrossRef]
- You, Q.; Min, J.; Lin, H.; Pepin, N.; Sillanpää, M.; Kang, S. Observed climatology and trend in relative humidity in the central and eastern Tibetan Plateau: RELATIVE HUMIDITY IN THE TP. J. Geophys. Res. Atmospheres 2015, 120, 3610–3621. [Google Scholar] [CrossRef]
- Villamil-Otero, G.A.; Zhang, J.; He, J.; Zhang, X. Role of extratropical cyclones in the recently observed increase in poleward moisture transport into the Arctic Ocean. Adv. Atmospheric Sci. 2018, 35, 85–94. [Google Scholar] [CrossRef]
- Dessler, A.E.; Davis, S.M. Trends in tropospheric humidity from reanalysis systems. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Golbon, R.; Cotter, M.; Sauerborn, J. Climate change impact assessment on the potential rubber cultivating area in the Greater Mekong Subregion. Environ. Res. Lett. 2018, 13, 084002. [Google Scholar] [CrossRef]
- Nelson, T.A.; Boots, B. Detecting spatial hot spots in landscape ecology. Ecography 2008, 31, 556–566. [Google Scholar] [CrossRef]
- Harris, N.L.; Goldman, E.; Gabris, C.; Nordling, J.; Minnemeyer, S.; Ansari, S.; Lippmann, M.; Bennett, L.; Raad, M.; Hansen, M.; et al. Using spatial statistics to identify emerging hot spots of forest loss. Environ. Res. Lett. 2017, 12, 024012. [Google Scholar] [CrossRef] [Green Version]
- Holliday, P. South American leaf blight (Microcyclus ulei) of Hevea brasiliensis. Phytopathol. Pap. 1970, 12, 31. [Google Scholar]
- Liyanage, A.d.S.; Jacob, C.K. Diseases of economic importance in rubber. In Developments in Crop Science; Sethuraj, M.R., Mathew, N.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; Volume 23, pp. 324–359. [Google Scholar]
- Yeang, H.-Y. Synchronous flowering of the rubber tree (Hevea brasiliensis) induced by high solar radiation intensity. New Phytol. 2007, 175, 283–289. [Google Scholar] [CrossRef] [PubMed]
- De Lemos Filho, J.P.; Nova, N.A.V.; Pinto, H.S. Base temperature and heat units for leaf flushing emission and growth of Hevea brasiliensis Muell. Arg. Int. J. Biometeorol. 1993, 37, 65–67. [Google Scholar] [CrossRef]
- De Lemos Filho, J.P.; Nova, N.A.V.; Pinto, H.S. A model including photoperiod in degree days for estimating Hevea bud growth. Int. J. Biometeorol. 1997, 41, 1–4. [Google Scholar] [CrossRef]
- Ortolani, A.A.; Sentelhas, P.C.; Camargo, M.B.P.; Pezzopane, J.E.M.; de S Goncalves, P. Agrometeorological model for seasonal rubber tree yield. Ind. J. Nat. Rubber Res. 1998, 11, 8–14. [Google Scholar]
- Montény, B.A.; Barbier, J.M.; Bernos, C.M. Determination of the energy exchanges of a forest type culture: Hevea brasiliensis. In The Forest–Atmosphere Interaction; Hutchinson, B.A., Hicks, B.B., Eds.; Reidel: Dordrecht, The Netherlands, 1985; pp. 211–233. [Google Scholar]
- Liyanage, K.K.; Khan, S.; Ranjitkar, S.; Yu, H.; Xu, J.; Brooks, S.; Beckschäfer, P.; Hyde, K.D. Evaluation of key meteorological determinants of wintering and flowering patterns of five rubber clones in Xishuangbanna, Yunnan, China. Int. J. Biometeorol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Omokhafe, K.O. Interaction between flowering pattern and latex yield in Hevea brasiliensis Muell. Arg. Cropp Breed. Appl. Biotechnol. 2004, 4, 280–284. [Google Scholar] [CrossRef]
- Priyadarshan, P.M.; Sasikumar, S.; de S Goncalves, P. Phenological changes in Hevea brasiliensis under differential geo-climates. The Planter 2001, 77, 447–459. [Google Scholar]
- Zhai, D.-L.; Yu, H.; Chen, S.-C.; Ranjitkar, S.; Xu, J. Responses of rubber leaf phenology to climatic variations in Southwest China. Int. J. Biometeorol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Whaley, W.G. Rubber-The Primary Sources for American Production. Econ. Bot. 1948, 2, 198–216. [Google Scholar] [CrossRef]
- Maeght, J.-L.; Gonkhamdee, S.; Clément, C.; Isarangkool Na Ayutthaya, S.; Stokes, A.; Pierret, A. Seasonal Patterns of Fine Root Production and Turnover in a Mature Rubber Tree (Hevea brasiliensis Müll. Arg.) Stand- Differentiation with Soil Depth and Implications for Soil Carbon Stocks. Front. Plant Sci. 2015, 6, 1022. [Google Scholar] [CrossRef] [PubMed]
- Fernando, T.H.P.S.; Jayasinghe, C.K.; Wijesundera, R.L.C.; Siriwardane, D. Some factors affecting in vitro production, germination and viability of conidia of Corynespora cassiicola from Hevea brasiliensis. J. Natl. Sci. Found. Sri Lanka 2012, 40, 241. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Lu, H.; Wang, P.; Luo, Q.; Liu, W.; Li, H. Vertical patterns of soil water acquisition by non-native rubber trees (Hevea brasiliensis) in Xishuangbanna, southwest China. Ecohydrology 2014, 7, 1234–1244. [Google Scholar] [CrossRef]
- Groom, Q.J.; Adriaens, T.; Desmet, P.; Simpson, A.; De Wever, A.; Bazos, I.; Cardoso, A.C.; Charles, L.; Christopoulou, A.; Gazda, A.; et al. Seven Recommendations to Make Your Invasive Alien Species Data More Useful. Front. Appl. Math. Stat. 2017, 3, 1–8. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golbon, R.; Cotter, M.; Mahbod, M.; Sauerborn, J. Global Assessment of Climate-Driven Susceptibility to South American Leaf Blight of Rubber Using Emerging Hot Spot Analysis and Gridded Historical Daily Data. Forests 2019, 10, 203. https://doi.org/10.3390/f10030203
Golbon R, Cotter M, Mahbod M, Sauerborn J. Global Assessment of Climate-Driven Susceptibility to South American Leaf Blight of Rubber Using Emerging Hot Spot Analysis and Gridded Historical Daily Data. Forests. 2019; 10(3):203. https://doi.org/10.3390/f10030203
Chicago/Turabian StyleGolbon, Reza, Marc Cotter, Mehdi Mahbod, and Joachim Sauerborn. 2019. "Global Assessment of Climate-Driven Susceptibility to South American Leaf Blight of Rubber Using Emerging Hot Spot Analysis and Gridded Historical Daily Data" Forests 10, no. 3: 203. https://doi.org/10.3390/f10030203




