Abstract
Research Highlights: Biochar is the carbonaceous product of pyrolysis or the gasification of biomass that is used as soil amendment to improve soil fertility and increase soil carbon stock. Biochar has been shown to increase, decrease, or have no effect on the emissions of greenhouse gases (GHG) from soil, depending on the specific soil and biochar characteristics. However, the temperature sensitivity of these gas emissions in biochar-amended soils is still poorly investigated. Background and Objectives: A pot experiment was set up to investigate the impact of woodchips biochar on the temperature sensitivity of the main GHG (CO2, CH4, and N2O) emissions from soil. Materials and Methods: Nine pots (14 L volume) were filled with soil mixed with biochar at two application rates (0.021 kg of biochar/kg of soil and 0.042 kg of biochar/kg of soil) or with soil alone as the control (three pots per treatment). Pots were incubated in a growth chamber and the emissions of CO2, CH4, and N2O were monitored for two weeks with a cavity ring-down gas analyzer connected to three closed dynamic chambers. The temperature in the chamber increased from 10 °C to 30 °C during the first week and decreased back to 10 °C during the second week, with a daily change of 5 °C. Soil water content was kept at 20% (w/w). Results: Biochar application did not significantly affect the temperature sensitivity of CO2 and N2O emissions. However, the sensitivity of CH4 uptake from soil significantly decreased by the application of biochar, reducing the CH4 soil consumption compared to the un-amended soil, especially at high soil temperatures. Basal CO2 respiration at 10 °C was significantly higher in the highest biochar application rate compared to the control soil. Conclusions: These results confirmed that the magnitude and direction of the influence of biochar on temperature sensitivity of GHG emissions depend on the specific GHG considered. The biochar tested in this study did not affect soil N2O emission and only marginally affected CO2 emission in a wide range of soil temperatures. However, it showed a negative impact on soil CH4 uptake, particularly at a high temperature, having important implications in a future warmer climate scenario and at higher application rates.
1. Introduction
Forest management can contribute to climate change mitigation by allocating woody biomass to bioenergy production, thus displacing fossil fuel use [1]. Among the energy conversion processes that can utilize woody biomass as feedstock, pyrolysis and gasification are acknowledged to be promising technologies in terms of carbon (C) budget [2]. During pyrolysis and gasification, biomass is thermally degraded through heating (300–1200 °C) under the complete or partial exclusion of oxygen. The volatile components of biomass are therefore released in the form of syngas, that can be used to produce thermal energy, electricity, or an oily fuel, and the leftover by-product of this process is charcoal [3]. In the last two decades, this C-rich, solid material has been proposed as a soil amendment with the name of biochar [4].
Due to its chemical structure, biochar is supposed to be particularly recalcitrant to soil degradation [5], even if estimations of its mean residence time vary from decades to millennia, depending on the starting feedstock, the production conditions, and the characteristics of the amended soil [6,7,8]. Biochar has been shown to improve soil characteristics and plant productivity in agricultural and forest ecosystems [9,10,11,12,13] as well as reduce nutrient losses from soil [14,15,16]. For these reasons, biochar has been proposed in forest restoration as a replacement to other forms of organic amendments and liming agents, particularly in degraded sites [9]. Applying biochar to forest soils may therefore contribute to mitigate climate change through the increase of soil C stock, improve soil characteristics, and allow at the same time the valorization of the woody biomass gasification chain, by turning what is now considered a waste into a resource.
However, little research has been conducted on biochar in forest ecosystems compared to agricultural crops [1,13,17] and most of the information on charcoal in forest ecosystems in the literature derives from studies on wildfires [18].
To evaluate the real climate change mitigation potential of biochar, its impact on greenhouse gases (GHG) emissions from soil has to be accounted. In fact, it is estimated that soil emissions of carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) represent 35%, 47%, and 53% of the total annual global emissions of these greenhouse gases (GHG), respectively [19,20]. Biochar has been shown to affect soil GHG emissions in different ways, depending on biochar and soil characteristics. For example, biochar application decreased [21], increased [22,23], or had no effect [24,25,26] on soil CO2 emissions. Different results have also been obtained for CH4. Biochar can in fact contribute to mitigate CH4 emissions from flooded soils under anoxic conditions, while in non-flooded soils, especially if neutral or alkaline, biochar may decrease soil CH4 uptake [27]. Finally, biochar has been shown to strongly reduce soil N2O emissions in different situations, even if increases in emissions have been observed as well [28].
While different studies have examined the effect of biochar application on GHG soil emissions, only a few have evaluated the impact that specific environmental parameters exert on these emissions in biochar-amended soils. Soil temperature is known to be the most important driver of GHG fluxes from soil [20,29,30], as well as of biochar oxidation and decomposition [31]. However, the effect of temperature on GHG fluxes in biochar-amended soil has been poorly investigated. Understanding the role of temperature is fundamental to assessing the effect of biochar on GHG emissions in different climatic conditions and in the context of climate change.
The overall aim of this study was to assess the effect of biochar on temperature sensitivity and basal emission of soil GHG fluxes. In particular, soil CO2, CH4, and N2O fluxes were measured in soils amended with woodchip biochar at two application rates and in un-amended (control) soils. During the experiment, the soil moisture was kept constant at 20% (w/w) in all treatments and the temperature ranged between 10 °C and 30 °C.
We hypothesized that the application of biochar could affect the sensitivity to temperature and the basal value of CO2, N2O, and CH4 flux.
Our experimental results partially confirmed our hypothesis. In fact, biochar application did not affect the temperature sensitivity of CO2 and N2O fluxes, while it significantly reduced that of CH4 flux. On the contrary, basal respiration significantly increased for CO2 by biochar application.
2. Materials and Methods
2.1. Experimental Set Up and Soil and Biochar Characteristics
The soil used in the experiment was sampled near Merano (Bolzano Province, Northern Italy, 46°40′0.181” N, 11°11′39.282” E; about 600 m a.s.l.). The soil was sandy-loam soil (USDA classification), with 64% sand, 29% silt, and 7% clay. The soil organic carbon (SOC) content was 2.4 ± 0.8% and soil pH was 6.4 ± 0.2. The soil water content at field capacity, calculated using the SPAW model (USDA-ARS) was 20% (v/v). The soil was sieved to a 8 mm mesh size to remove stones and coarse organic matter fragments.
The biochar used in the experiment consisted of small particles (<5 mm) and was obtained from conifer woodchips, at approximately 500 °C, through fast pyrolysis (Record Immobiliare S.r.l., Lunano, Pesaro-Urbino, Italy). Biochar was characterized by a bulk density of 0.165 g cm−3 and C:N ratio of 151. A detailed physicochemical characterization of the biochar used in this experiment is provided in Table 1.

Table 1.
Physicochemical characterization of the biochar used in the present work.
B1 and B2 treatments were prepared by mixing biochar and the sieved soil at two rates: 0.021 kg of biochar kg−1 of soil (dry weight) (B1), and 0.042 kg of biochar kg−1 of soil (dry weight) (B2), respectively. The two mixing rates corresponded to field biochar application doses of 25 ton ha−1 and 50 ton ha−1, respectively, considering a field biochar incorporation depth of 20 cm and are in line with the biochar dosages used in the majority of previous studies in forest ecosystems [9]. Biochar and soil mixtures where then homogenized with a concrete mixer.
The experiment was set up in July 2018 in a growth chamber at the Laimburg research center for Agriculture and Forestry located in Auer/Ora (BZ), Northern Italy. A total of 9 pots (45 cm × 25 cm × 21 cm, 14 L volume) were filled with soil mixed with biochar or with un-amended soil as the control. A total of 3 replicates (pots) were prepared for each treatment. The pots were stored in the growth chamber for two weeks at 10 °C temperature. The temperature in the chamber was then increased from 10 °C to 30 °C during one week (first week of experiment), and from 30 °C back to 10 °C during the following week (second week of experiment), with an overnight change of 5 °C per day. The lowest temperature (10 °C) was chosen because it is a standard temperature used internationally to compare the soil respiration of different experimental sites or treatments, the so called basal soil respiration at 10 °C (R10) [32]. The highest temperature (30 °C) was chosen because the maximum monthly temperature measured in Merano between 2011 and 2017 was on average 29.1 °C [33]. In order to isolate the effect of soil temperature, excluding any effect of soil humidity on soil GHG fluxes, soil moisture was kept constant at 20% (w/w) in all treatments. Soil water content at the beginning of the experiment was measured in each pot by collecting a soil subsample (~10 g of soil) and drying it for 24 h at 105 °C. The amount of water to be added daily to the soil was calculated as the difference between the actual weight of the pot and the theoretical weight if the soil moisture was equal to 20% (w/w).
2.2. Measurement of Soil GHG Fluxes
The emissions of GHG from the soil were measured by a gas analyzer CRDS (Picarro Inc., Santa Clara, CA, USA), connected to 3 closed dynamic chambers (eosAC Autochamber, Eosense Inc., Dartmouth, NS, Canada) operated by a multiplexer (eosMX, Eosense Inc., Dartmouth, NS, Canada). The chambers were installed on PVC (polymerizing vinyl chloride) collars (15.2 cm diameter, 7 cm height) inserted into the soil, 1 per pot, for 4 cm. Fluxes of CO2 (µmol m−2 s−1), N2O, and CH4 (nmol m−2 s−1) were measured daily from the 3 experimental treatments by manually moving one chamber (leaving the collars on the soil) on the 3 pots of each treatment. The measurements on each pot lasted for 10 min. A valve delay of 66 s was set at the beginning and at the end of each measurement to account for the time needed to draw the air from the chamber, analyze the gas concentrations, and then recirculate the air sample back to the chamber through a tubing length of 30 m. During measurements, the soil temperature (°C) was measured at a 5 cm soil depth by a RT-1 Rugged Soil Temperature Sensor (Decagon Devices, Inc., Pullman, WA, USA).
2.3. Data Analysis
After the elimination of data associated with system malfunctioning, soil CO2 flux (soil respiration) measured in the different treatments were related to soil temperature using the following exponential model:
where Fs is the soil CO2 flux, T is the soil temperature (°C) at 5 cm depth, and R10 is the basal soil respiration, i.e., the value of Fs at the reference temperature of 10 °C. The model parameters R10 and b were estimated by nonlinear regression analysis. The apparent sensitivity of CO2 flux to soil temperature was determined by the Q10 temperature coefficient as follows:
Fs = R10 eb(T−10)
Q10 =e10b
Fluxes of CH4 and N2O were related to soil temperature using a linear model:
where Fs is the soil CH4 or N2O flux, T is the soil temperature (°C) at 5 cm depth, and R10 is the basal emission at 10 °C. Parameters R10 and b (slope of the regression line) are estimated by linear regression analysis.
Fs = R10 + b(T−10)
For each gas, the linear regression models obtained in the different experimental treatments were then compared by Analysis of Covariance (ANCOVA) to analyze the effect of biochar on the sensitivity of GHG fluxes to temperature. Equation (1) was linearized with a log-transformation of CO2 efflux data before analysis. At first the slopes of the linear regression model were compared and then only when the slopes were not significantly different, and the intercepts of the regression lines were also compared. In case ANCOVA highlighted significant differences, post-hoc individual comparisons were performed with the Tukey’s HSD test. The homogeneity of variances was checked before analysis by plotting the residual vs. fitted values. When this condition was not fulfilled, a square root transformation was applied to the data before analysis. Statistical analysis was performed using the software R (version 3.4.2) [34].
3. Results
The highest CO2 emission rates were observed in the biochar-treated soils (Figure 1). Biochar application did not significantly affect the Q10 value of CO2 fluxes, while it significantly increased R10 of CO2 when applied at the highest rate in comparison to the control (Figure 1 and Table 2).
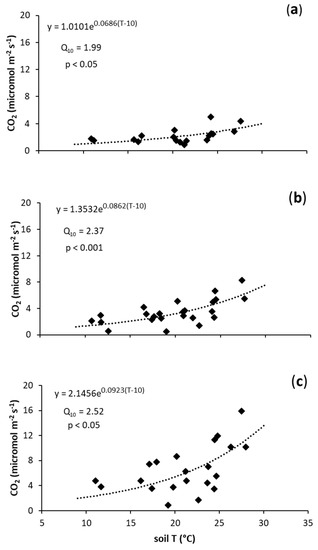
Figure 1.
Relationship between CO2 fluxes (µmol m−2 s−1) and soil temperature (°C) in: (a) N (control treatment); (b) B1 (0.021 kg of biochar/kg of soil); and (c) B2 (0.042 kg of biochar/kg of soil).

Table 2.
Results of Analysis of Covariance (ANCOVA) and the post-hoc Tukey test for a pairwise comparison of the slopes and intercepts of the linear models relating the fluxes of CO2, CH4, and N2O to soil temperature (T, °C) in the treatments N (control), B1 (0.021 kg of biochar/kg of soil), and B2 (0.042 kg of biochar/kg of soil). Different letters indicate significant differences between model parameters determined for each soil treatment (p < 0.05) in the table.
A negative CH4 flux, i.e., a net CH4 consumption in the soil, was observed in all treatments (Figure 2). The temperature sensitivity of soil CH4 uptake significantly decreased following biochar application, showing a reduction in CH4 uptake in biochar-amended soil in comparison to the control (Table 2). This effect was dependent on the biochar application rate and was particularly evident in the B2 treatment (Figure 2, Table 2). At this application rate, CH4 flux was not significantly affected by soil temperature (slope: −0.0087) and its flux was always close to zero (Figure 2, Table 2).
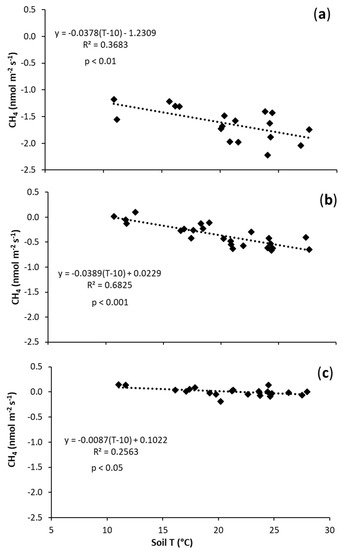
Figure 2.
Relationship between CH4 fluxes (nmol m−2 s−1) and soil temperature (°C) in: (a) N (control treatment); (b) B1 (0.021 kg of biochar/kg of soil); and (c) B2 (0.042 kg of biochar/kg of soil).
The highest N2O emissions from the soil were observed in the B1 treatment in comparison with control and B2, but the sensitivity of N2O emissions from the soil, as well as the N2O basal emission, were not significantly affected by the application of biochar (Figure 3, Table 2).
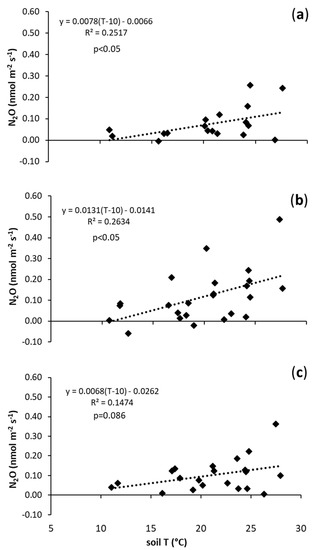
Figure 3.
Relationship between N2O fluxes (nmol m−2 s−1) and soil temperature (°C) in: (a) N (control treatment); (b) B1 (0.021 kg of biochar/kg of soil); and (c) B2 (0.042 kg of biochar/kg of soil).
4. Discussion
Soil temperature is known to be the most important driver of GHG fluxes from soil [20,29,30], as well as of biochar oxidation and decomposition [31]. The temperature sensitivity of GHG fluxes is therefore a key parameter to predict the impact that global warming will have on the flux of GHG [35,36].
In our experiment, we observed a positive exponential relationship between CO2 fluxes and the temperature in biochar-amended soils. This relation was typically observed in forest and plantation ecosystems [25,26,30,37,38]. The absence of a significant modification of the Q10 after the application of biochar (Figure 1, Table 2) was coherent with what was observed in previous studies with different biochars and application rates [26,37,39]. However, this result is inconsistent with other studies that reported a decrease [40,41,42] or an increase [25,43] to the temperature sensitivity of CO2 emissions. These contrasting results derives from the complexity of the factors involved. In fact, the Q10 of biochar was expected to be higher than the less recalcitrant native soil organic matter (SOM) [44], but the sensitivity of CO2 fluxes in biochar-amended soils also depends on the impact that biochar has on the Q10 of the native soil’s organic matter [45]. Moreover, results can also change according to the incubation temperature range and soil type. More specifically, a smaller sensitivity was expected in soils with high clay, Fe, and Al oxides content as well as with an acidic pH [46].
In the present study, basal soil respiration increased significantly in the soil that was amended with biochar at a higher application rate. A R10 increase was found for heterotrophic respiration in different environments such as apple orchards [26] and the desert [47] after the application of biochar produced from wood and cotton straw, respectively. A significant increase in R10 for total soil respiration was also observed in soils amended with poultry litter biochar [48].
These results have been attributed to an increase of microbial biomass and/or activity [26,48]. The stimulation of soil microbes can derive from the decomposition of the labile fraction of biochar, consisting of bio-oils and condensation products [49,50,51,52,53]. However, the degradation of the more recalcitrant C compounds cannot be excluded [54]. This mechanism can be the main driver of the increased CO2 efflux observed after biochar application in both agricultural and forest ecosystems [17,26]. Moreover, we cannot exclude that the increased CO2 fluxes derive from an increased decomposition of the native soil’s organic matter, the so-called priming effect (PE). In fact, a positive PE has been observed in several short term studies [55,56], especially in sandy soils [52], while in the long term, a protection of native organic matter from decomposition (negative PE) is generally observed in biochar-amended soils [23,57]. A boost in soil microbiota activity can also be due to a shift in soil properties such as soil aeration [58]. Biochar is characterized by a high porosity, which may have increased soil gas permeability and oxygen availability for soil microbes. Moreover, even if the soil water content was kept constant during the incubation experiment, the presence of biochar may have affected the availability of soil water by soil microorganisms. Biochar can in fact alter soil water potential [59,60], which is known to impact soil microbial population and activity [61].
Non-flooded soils in an oxic condition usually show a CH4-sink capacity [62], as CH4 is oxidized by soil methanotrophic bacteria, and the rate of CH4 oxidation depends on soil temperature [63]. This trend was also observed in the present study, as CH4 consumption increased linearly with soil temperature (Figure 2). The decreased sensitivity to soil temperature of CH4 flux in biochar-treated soil means that biochar decreased CH4 consumption at a higher soil temperature, while at a lower soil temperature this effect was less pronounced. In their meta-analysis, Jeffery et al. [27] showed that the application of biochar from woody feedstock, produced at temperatures between 400 °C and 600 °C, decreases soil CH4 uptake in non-flooded soils, especially in neutral or alkaline soil pH. Results of the present study are in line with these findings as our biochar was produced from wood chips at approximately 500 °C, and the soil water content was kept at 20%. Non-flooded upland soils contribute to approximately 15% of global CH4 oxidation [64], therefore biochar application may decrease net CH4 oxidation, reducing the climate change mitigation potential of these soils. However, few studies examined the sensitivity of CH4 soil flux to temperature. Our study showed that the reduction of soil CH4 uptake induced by biochar increased with soil temperature. This effect could therefore more pronounced under warmer climatic conditions and may worsen within the context of global warming. Moreover, the reduction of sensitivity to temperature for CH4 was much more evident in the B2 treatment, which suggests not using high biochar application rates in order to preserve the soil CH4 uptake capacity. However, a significant increase in soil CH4 uptake [37,65] and sensitivity to temperature [37] was observed in some experiments in non-flooded soils, contradicting the results of the present work and showing that the relation between biochar, soil, and CH4 emissions is complex and hard to predict.
The mechanism behind the reduction of CH4 oxidation might be a modification of the methanogenic/methanotrophic bacteria ratio in biochar-amended soils [66], and the release of chemicals with a toxic effect on the methanotrophic bacteria population, such as ethylene [67]. In addition, even if the soil water content was kept constant, biochar may have altered soil water potential and water availability for soil bacteria.
Soil N2O emissions in the present work increased linearly with the temperature in all soil treatments and the application of biochar did not affect temperature sensitivity or basal soil N2O emissions (Figure 3, Table 2). These results confirm a previous study by [68] but are in contrast with other studies, reporting a significant reduction of N2O flux sensitivity to temperature, both in subtropical [37] and continental climate [69]. In a meta-analysis by Cayuela et al. [28], an average reduction of 54% in N2O emissions has been reported in biochar-treated soils. In this case, the variability observed in the experimental results has been shown to depend on different characteristics of biochar (feedstock used, pyrolysis conditions, and C/N ratio) and soil. In particular, when biochar is applied to drained soils with a coarse texture, reduction in N2O emissions has not been observed [28,70]. In our experiment, soil moisture was kept at a relatively low value, not exceeding the field capacity of the sandy-loam textured soil. In these conditions, it was likely that N2O emission was not promoted and the effect of biochar was consequently not relevant.
In previous studies, an observed reduction of N2O emissions from soils was explained by a toxic effect on soil microbes involved in N2O production of Polycyclic aromatic hydrocarbons (PAHs) [71,72]. The PAHs content in the biochar used in this study was very low (Table 1) and therefore a toxic effect on soil biota was unlikely.
A decrease of soil N2O emissions has also been associated with a shift in the soil’s physical properties, such as a reduction in soil compaction [73]. This mechanism cannot have occurred in our experiment, as it was set up in controlled conditions and the soil was not subjected to compaction.
The reduction of N2O emissions observed in previous studies has also been attributed to the sorption of reactive N on biochar surfaces and the reduction of its availability for N2O emitting reactions. However, this mechanism is observed in case of biochar production at temperatures higher than 600 °C [74,75]. The absence of the biochar effect on the sensitivity to temperature of N2O emission would suggest that biochar will not affect the emission of this powerful greenhouse gas in warmer climatic conditions.
It has to be considered that the short experimental duration of this study might limit the validity of the results to the first period after the application of biochar [76,77]. Therefore, these results may not be representative of the effect of biochar on long-term GHG emissions from soil in field application.
5. Conclusions
Before concluding if biochar application to soil is a forest management practice that is able to mitigate climate change, an evaluation of its effect on soil GHG emission is fundamental. The results of the present work show that biochar addition to soil did not significantly affect the sensitivity of CO2 and N2O fluxes, while it slightly increased the CO2 basal soil respiration in case of a high application rate, indicating that biochar application would not affect the emission of these gases in warmer climatic conditions. However, the significant decrease of the temperature sensitivity of soil CH4 uptake indicated that biochar can induce an important reduction of the soil CH4 sink potential, in particular in a warmer environment, and this effect can become more relevant in a global warming scenario. Moreover, the reduction of sensitivity to temperature for CH4 was much more evident in the case of the higher application rate, suggesting that high biochar dosages should be avoided in order to preserve the soil CH4 uptake capacity.
The observed effects seem to depend on specific biochar characteristics (temperature of production, low content of PAHs) and soil characteristics (sandy-loam, drained soil). However, long-term field studies are advisable in order to guarantee a thorough understanding of the impact of biochar on GHG emissions from soil.
Author Contributions
Conceptualization, G.T.; Methodology, G.T., M.V., and I.C.; validation, M.V., G.T., I.C., and P.P.; Formal analysis, I.C. and M.V.; Investigation, I.C., A.S.; Resources, I.C., P.P., A.S., and M.V.; Data Curation, I.C., A.S.; Writing—original draft preparation, I.C. and M.V.; Writing—review and editing, I.C., M.V., P.P., and G.T.; Visualization, I.C. and M.V.; Supervision, G.T.; Project administration, G.T.; Funding acquisition, G.T.
Funding
This research was funded by the Wood-Up project (Optimization of WOOD gasification chain in South Tyrol to produce bioenergy and other high-value green products to enhance soil fertility and mitigate climate change, EFRE-FESR 2014–2020, project number 1028), funded by the European Regional Development Fund of the European Union and the Autonomous Province of Bolzano/Bozen.
Acknowledgments
The authors would like to thank the Laimburg Research Center for the availability of the climatic chamber, and Christian Ceccon and Christian Grümer from the Free University of Bolzano for their support in soil sample preparation and analysis.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Cowie, A.; Barton, C.; Singh, B.; Ximenes, F.; Stone, C. Climate Change Impacts and Research Priorities for the Forestry Sector. In DPI Priority Actions for Climate Change Workshop; NSW Department of Primary Industries: Orange, NSW, Australia, 2007. [Google Scholar]
- Lehmann, J. A Handful of Carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Bridgwater, A.V. The Production of Biofuels and Renewable Chemicals by Fast Pyrolysis of Biomass. Int. J. Glob. Energy Issues 2007, 27, 160–203. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-Energy in the Black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Downie, A.; Munroe, P. Characteristics of Biochar—Physical and Structural Properties. In Biochar for Environmental Management: Science, Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 13–29. [Google Scholar]
- Lehmann, J.; Abiven, S.; Kleber, M.; Pan, G.; Singh, B.P.; Sohi, S.P.; Zimmerman, A.R. Persistence of Biochar in Soil. In Biochar for Environmental Management: Science, Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 235–282. [Google Scholar]
- Criscuoli, I.; Alberti, G.; Baronti, S.; Favilli, F.; Martinez, C.; Calzolari, C.; Pusceddu, E.; Rumpel, C.; Viola, R.; Miglietta, F. Carbon Sequestration and Fertility after Centennial Time Scale Incorporation of Charcoal into Soil. PLoS ONE 2014, 9, e91114. [Google Scholar] [CrossRef] [PubMed]
- Gurwick, N.P.; Moore, L.A.; Kelly, C.; Elias, P. A Systematic Review of Biochar Research, with a Focus on Its Stability in Situ and Its Promise as a Climate Mitigation Strategy. PLoS ONE 2013, 8, e75932. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.C.; Gale, N. Biochar and Forest Restoration: A Review and Meta-Analysis of Tree Growth Responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Biederman, L.; Harpole, W.S. Biochar and Its Effects on Plant Productivity and Nutrient Cycling: A Meta-Analysis. GCB Bioenergy 2012, 5, 202–214. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s Effect on Crop Productivity and the Dependence on Experimental Conditions—A Meta-Analysis of Literature Data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Criscuoli, I.; Baronti, S.; Alberti, G.; Rumpel, C.; Giordan, M.; Camin, F.; Ziller, L.; Martinez, C.; Pusceddu, E.; Miglietta, F. Anthropogenic Charcoal-Rich Soils of the XIX Century Reveal That Biochar Leads to Enhanced Fertility and Fodder Quality of Alpine Grasslands. Plant Soil 2017, 411, 499–516. [Google Scholar] [CrossRef]
- Li, Y.; Hu, S.; Chen, J.; Mueller, K.; Li, Y.; Fu, W.; Lin, Z.; Wang, H. Effects of Biochar Application in Forest Ecosystems on Soil Properties and Greenhouse Gas Emissions: A Review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Ventura, M.; Sorrenti, G.; Panzacchi, P.; George, E.; Tonon, G. Biochar Reduces Short-Term Nitrate Leaching from a Horizon in an Apple Orchard. J. Environ. Qual. 2013, 42, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Major, J.; Steiner, C.; Downie, A.; Lehmann, J. Biochar Effects on Nutrient Leaching. In Biochar for Environmental Management: Science, Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge, Earthscam: New York, NY, USA, 2015; pp. 271–287. [Google Scholar]
- Sorrenti, G.; Ventura, M.; Toselli, M. Effect of Biochar on Nutrient Retention and Nectarine Tree Performance: A Three-Year Field Trial. J. Plant Nutr. Soil Sci. 2016, 179, 1–11. [Google Scholar] [CrossRef]
- Johnson, M.S.; Webster, C.; Jassal, R.S.; Hawthorne, I.; Black, T.A. Biochar Influences on Soil CO2 and CH4 Fluxes in Response to Wetting and Drying Cycles for a Forest Soil. Sci. Rep. 2017, 7, 6480. [Google Scholar] [CrossRef] [PubMed]
- Stavi, I. Biochar Use in Forestry and Tree-Based Agro-Ecosystems for Increasing Climate Change Mitigation and Adaptation. Int. J. Sustain. Dev. World Ecol. 2013, 20, 166–181. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.M.B.; Miller, H.L. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse Gas Emissions from Soils—A Review. Chemie der Erde—Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Case, S.D.; McNamara, N.P.; Reay, D.S.; Whitaker, J. Can Biochar Reduce Soil Greenhouse Gas Emissions from a Miscanthus Bioenergy Crop? GCB Bioenergy 2014, 6, 76–89. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Laird, D.A.; Ahmedna, M.A.; Niandou, M.A.S. Short-Term CO2 Mineralization after Additions of Biochar and Switchgrass to a Typic Kandiudult. Geoderma 2010, 154, 281–288. [Google Scholar] [CrossRef]
- Ventura, M.; Alberti, G.; Panzacchi, P.; Delle Vedove, G.; Miglietta, F.; Tonon, G. Biochar Mineralization and Priming Effect on SOM Decomposition. Results from a Field Trial in a Short Rotation Coppice in Italy. In EGU General Assembly Conference Abstracts; EGU General Assembly: Vienna, Austria, 2016; Volume 18, p. 9109. [Google Scholar]
- Scheer, C.; Grace, P.R.; Rowlings, D.W.; Kimber, S.; van Zwieten, L. Effect of Biochar Amendment on the Soil-Atmosphere Exchange of Greenhouse Gases from an Intensive Subtropical Pasture in Northern New South Wales, Australia. Plant Soil 2011, 345, 47–58. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Chang, S.X.; Zhang, J.; Jiang, P.; Zhou, G.; Shen, Z. Contrasting Effects of Bamboo Leaf and Its Biochar on Soil CO2 Efflux and Labile Organic Carbon in an Intensively Managed Chinese Chestnut Plantation. Biol. Fertil. Soils 2014, 50, 1109–1119. [Google Scholar] [CrossRef]
- Ventura, M.; Zhang, C.; Baldi, E.; Fornasier, F.; Sorrenti, G.; Panzacchi, P.; Tonon, G. Effect of Biochar Addition on Soil Respiration Partitioning and Root Dynamics in an Apple Orchard. Eur. J. Soil Sci. 2014, 65, 186–195. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; Kammann, C.; Abalos, D. Biochar Effects on Methane Emissions from Soils: A Meta-Analysis. Soil Biol. Biochem. 2016, 101, 251–258. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s Role in Mitigating Soil Nitrous Oxide Emissions: A Review and Meta-Analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Reichstein, M.; Beer, C. Soil Respiration across Scales: The Importance of a Model-Data Integration Framework for Data Interpretation. J. Plant Nutr. Soil Sci. 2008, 171, 344–354. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, L.; Cheng, H.; Yue, S.; Li, S. Effects of Biochar Application on CO2 Emissions from a Cultivated Soil under Semiarid Climate Conditions in Northwest China. Sustainability 2017, 9, 1482. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Lehmann, J.; Hockaday, W.C.; Joseph, S.; Masiello, C.A. Temperature Sensitivity of Black Carbon Decomposition and Oxidation. Environ. Sci. Technol. 2010, 44, 3324–3331. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.; Taylor, A. On the Temperature Dependence of Soil Respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Provincia Autonoma di Bolzano. Valori Medi Delle Temperature Massime e Minime—Merano/Quarazze cod. 23200MS. Available online: http://www.provincia.bz.it/meteo/download/23200MS-TS-MeranoQuarazze-MeranGratsch.pdf (accessed on 8 July 2019).
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar]
- Luo, Y.; Wan, S.; Hui, D.; Wallace, L.L. Acclimatization of Soil Respiration to Warming in a Tall Grass Prairie. Nature 2001, 413, 622–625. [Google Scholar] [CrossRef]
- Zhou, T.; Shi, P.; Hui, D.; Luo, Y. Global Pattern of Temperature Sensitivity of Soil Heterotrophic Respiration (Q10) and Its Implications for Carbon-Climate Feedback. J. Geophys. Res. 2009, 114, 1–9. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Wang, H.; Singh, B.P.; Hu, S.; Luo, Y.; Li, J.; Xiao, Y.; Cai, X.; Li, Y. Responses of Soil Greenhouse Gas Emissions to Different Application Rates of Biochar in a Subtropical Chinese Chestnut Plantation. Agric. For. Meteorol. 2019, 271, 168–179. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chang, S.X.; Yang, Y.; Fu, S.; Jiang, P.; Luo, Y.; Yang, M.; Chen, Z.; Hu, S.; et al. Biochar Reduces Soil Heterotrophic Respiration in a Subtropical Plantation through Increasing Soil Organic Carbon Recalcitrancy and Decreasing Carbon-Degrading Microbial Activity. Soil Biol. Biochem. 2018, 122, 173–185. [Google Scholar] [CrossRef]
- Bamminger, C.; Poll, C.; Marhan, S. Offsetting Global Warming-Induced Elevated Greenhouse Gas Emissions from an Arable Soil by Biochar Application. Glob. Chang. Biol. 2018, 24, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Zhuang, S.; Cui, J.; Li, J.; Li, B.; Wu, J.; Fang, C. Biochar Decreased the Temperature Sensitivity of Soil Carbon Decomposition in a Paddy Field. Agric. Ecosyst. Environ. 2017, 249, 156–164. [Google Scholar] [CrossRef]
- Chen, J.; Sun, X.; Zheng, J.; Zhang, X.; Liu, X.; Bian, R.; Li, L.; Chneg, K.; Zheng, J.; Pan, G. Biochar Amendment Changes Temperature Sensitivity of Soil Respiration and Composition of Microbial Communities 3 Years after Incorporation in an Organic Carbon-Poor Dry Cropland Soil. Biol. Fertil. Soils 2018, 54, 175–188. [Google Scholar] [CrossRef]
- He, X.; Du, Z.; Wang, Y.; Lu, N.; Zhang, Q. Sensitivity of Soil Respiration to Soil Temperature Decreased under Deep Biochar Amended Soils in Temperate Croplands. Appl. Soil Ecol. 2016, 108, 204–210. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; Zhang, T.; Du, Z.; He, Y.; Wang, X.; Shao, J.; Cao, Y.; Xue, S.; Wang, H.; et al. Biochar Increased Soil Respiration in Temperate Forests but Had No Effects in Subtropical Forests. For. Ecol. Manage. 2017, 405, 339–349. [Google Scholar] [CrossRef]
- Conant, R.; Steinweg, J.; Haddix, M.; Paul, E.; Plante, A.; Six, J. Experimental Warming Shows That Decomposition Temperature Sensitivity Increases with Soil Organic Matter Recalcitrance. Ecology 2008, 89, 2384–2391. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.P.; Matta, P.; Cowie, A.L.; Van Zwieten, L. Temperature Sensitivity and Priming of Organic Matter with Different Stabilities in a Vertisol with Aged Biochar. Soil Biol. Biochem. 2017, 115, 346–356. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.P.; Singh, B. Temperature Sensitivity of Biochar and Native Carbon Mineralisation in Biochar-Amended Soils. Agric. Ecosyst. Environ. 2014, 191, 158–167. [Google Scholar] [CrossRef]
- Liao, N.; Li, Q.; Zhang, W.; Zhou, G.; Ma, L.; Min, W.; Ye, J.; Hou, Z. Effects of Biochar on Soil Microbial Community Composition and Activity in Drip-Irrigated Desert Soil. Eur. J. Soil Biol. 2016, 72, 27–34. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Klimkowicz-Pawlas, A.; Gondek, K. Influence of Poultry Litter and Poultry Litter Biochar on Soil Microbial Respiration and Nitrifying Bacteria Activity. Waste Biomass Valorization 2018, 9, 379–389. [Google Scholar] [CrossRef]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of Charcoal Quantity on Microbial Biomass and Activity in Temperate Soils. Soil Sci. Soc. Am. J. 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and Microbial Oxidation of Laboratory-Produced Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Bogomolova, I.; Glaser, B. Biochar Stability in Soil: Decomposition during Eight Years and Transformation as Assessed by Compound-Specific 14C Analysis. Soil Biol. Biochem. 2014, 70, 229–236. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar Stability in Soil: Meta-Analysis of Decomposition and Priming Effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Ventura, M.; Alberti, G.; Panzacchi, P.; Delle Vedove, G.; Miglietta, F.; Tonon, G. Biochar Mineralization and Priming Effect in a Poplar Short Rotation Coppice from a 3-Year Field Experiment. Biol. Fertil. Soils 2019, 55, 67–78. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar Induced Soil Microbial Community Change: Implications for Biogeochemical Cycling of Carbon, Nitrogen and Phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and Negative Carbon Mineralization Priming Effects among a Variety of Biochar-Amended Soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Maestrini, B.; Nannipieri, P.; Abiven, S. A Meta-Analysis on Pyrogenic Organic Matter Induced Priming Effect. GCB Bioenergy 2015, 7, 577–590. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Parkin, T.B. Impact of Six Lignocellulosic Biochars on C and N Dynamics of Two Contrasting Soils. GCB Bioenergy 2017, 9, 1279–1291. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, C.; Meng, H. Temperature Sensitivity and Basal Rate of Soil Respiration and Their Determinants in Temperate Forests of North China. PLoS ONE 2013, 8, e81793. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of Biochar Amendments on the Quality of a Typical Midwestern Agricultural Soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Amonette, J.E.; Ippolito, J.A.; Lima, I.M.; Gaskin, J.; Das, K.C.; Steiner, C.; Ahmedna, M.; et al. Biochars Impact on Soil-Moisture Storage in an Ultisol and Two Aridisols. Soil Sci. 2012, 177, 310–320. [Google Scholar] [CrossRef]
- Sinegani, A.A.S.; Maghsoudi, J. The Effects of Water Potential on Some Microbial Populations and Decrease Kinetic of Organic Carbon in Soil Treated with Cow Manure under Laboratory Conditions. J. Appl. Sci. Environ. Manag. 2011, 15, 179–188. [Google Scholar] [CrossRef][Green Version]
- Suwanwaree, P.; Robertson, G.P. Methane Oxidation in Forest, Successional, and No-till Agricultural Ecosystems: Effects of Nitrogen and Soil Disturbance. Soil Sci. Soc. Am. J. 2005, 69, 1722–1729. [Google Scholar] [CrossRef]
- Luo, G.J.; Kiese, R.; Wolf, B.; Butterbach-Bahl, K. Effects of Soil Temperature and Moisture on Methane Uptake and Nitrous Oxide Emissions across Three Different Ecosystem Types. Biogeosciences 2013, 10, 3205–3219. [Google Scholar] [CrossRef]
- Powlson, D.S.; Goulding, K.W.T.; Willison, T.W.; Webster, C.P.; Hütsch, B.W. The Effect of Agriculture on Methane Oxidation in Soil. Nutr. Cycl. Agroeco. 1997, 49, 59–70. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar Addition to Agricultural Soil Increased CH4 Uptake and Water Holding Capacity—Results from a Short-Term Pilot Field Study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, Y.; Yu, Y.; Xie, Z.; Lin, X. Mechanisms of Biochar Decreasing Methane Emission from Chinese Paddy Soils. Soil Biol. Biochem. 2012, 46, 80–88. [Google Scholar] [CrossRef]
- Spokas, K.A.; Baker, J.M.; Reicosky, D.C. Ethylene: Potential Key for Biochar Amendment Impacts. Plant Soil 2010, 333, 443–452. [Google Scholar] [CrossRef]
- Deem, L.M.; Yu, J.; Crow, S.E.; Deenik, J.; Penton, C.R. Biochar Increases Temperature Sensitivity of Soil Respiration and N2O Flux. 2016. Available online: https://biochar-us.org/presentation/biochar-increases-temperature-sensitivity-soil-respiration-and-n2o-flux (accessed on 8 July 2019).
- Chang, J.; Clay, D.E.; Clay, S.A.; Chintala, R.; Miller, J.M.; Schumacher, T. Biochar Reduced Nitrous Oxide and Carbon Dioxide Emissions from Soil with Different Water and Temperature Cycles. Agron. Soils Environ. Qual. 2016, 108, 2214–2221. [Google Scholar] [CrossRef]
- Curtin, D.; Campbell, C.A.; Jalil, A. Effects of Acidity on Mineralization: PH-Dependence of Organic Matter Mineralization in Weakly Acidic Soils. Soil Biol. Biochem. 1998, 30, 57–64. [Google Scholar] [CrossRef]
- Maliszewska-Kordybach, B.; Klimkowicz-Pawlas, A.; Smreczak, B.; Janusauskaite, D. Ecotoxic Effect of Phenanthrene on Nitrifying Bacteria in Soils of Different Properties. J. Environ. Qual. 2007, 36, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.X.; Deng, H.; Qiao, M.; Yao, H.Y.; Zhu, Y.G. Effect of Long-Term Wastewater Irrigation on Potential Denitrification and Denitrifying Communities in Soils at the Watershed Scale. Environ. Sci. Technol. 2013, 47, 3105–3113. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.; Cruse, R.; Fleming, P.; Parkin, T.; Meek, D. Impact of Biochar on Manure Carbon Stabilization and Greenhouse Gas Emissions. Soil Sci. Soc. Am. J. 2011, 75, 871–879. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Singh, B.P.; Hatton, B.; Balwant, S.; Cowie, A.L.; Kathuria, A. Influence of Biochars on Nitrous Oxide Emission and Nitrogen Leaching from Two Contrasting Soils. J. Environ. Qual. 2010, 39, 1224–1235. [Google Scholar] [CrossRef]
- Fidel, R.; Laird, D.; Parkin, T. Effect of Biochar on Soil Greenhouse Gas Emissions at the Laboratory and Field Scales. Soil Syst. 2019, 3, 8. [Google Scholar] [CrossRef]
- Spokas, K.A. Impact of Biochar Field Aging on Laboratory Greenhouse Gas Production Potentials. GCB Bioenergy 2013, 5, 165–176. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).