The Effect of Acetylation on Iron Uptake and Diffusion in Water Saturated Wood Cell Walls and Implications for Decay
Abstract
:1. Introduction
2. Materials and Methods
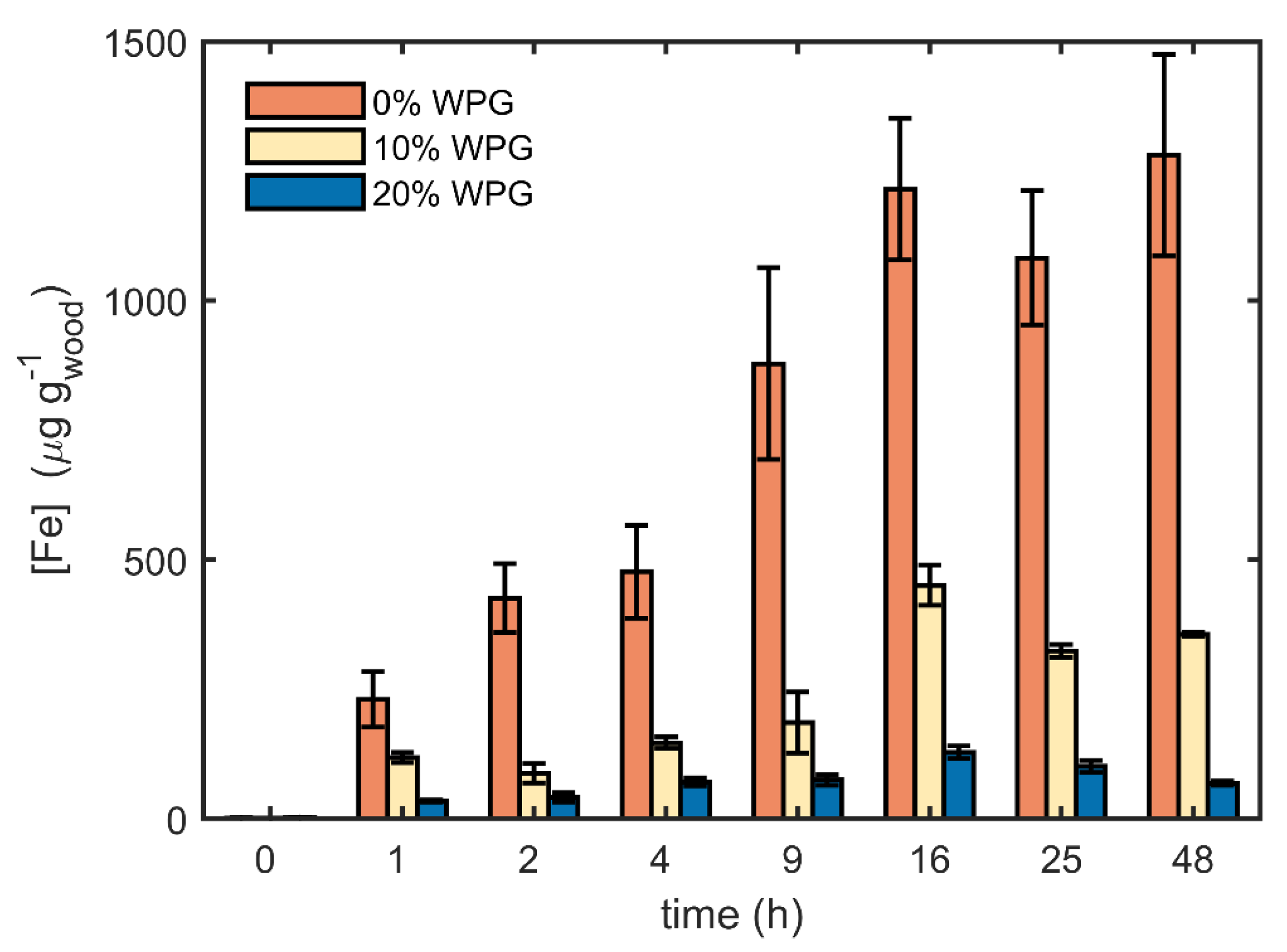
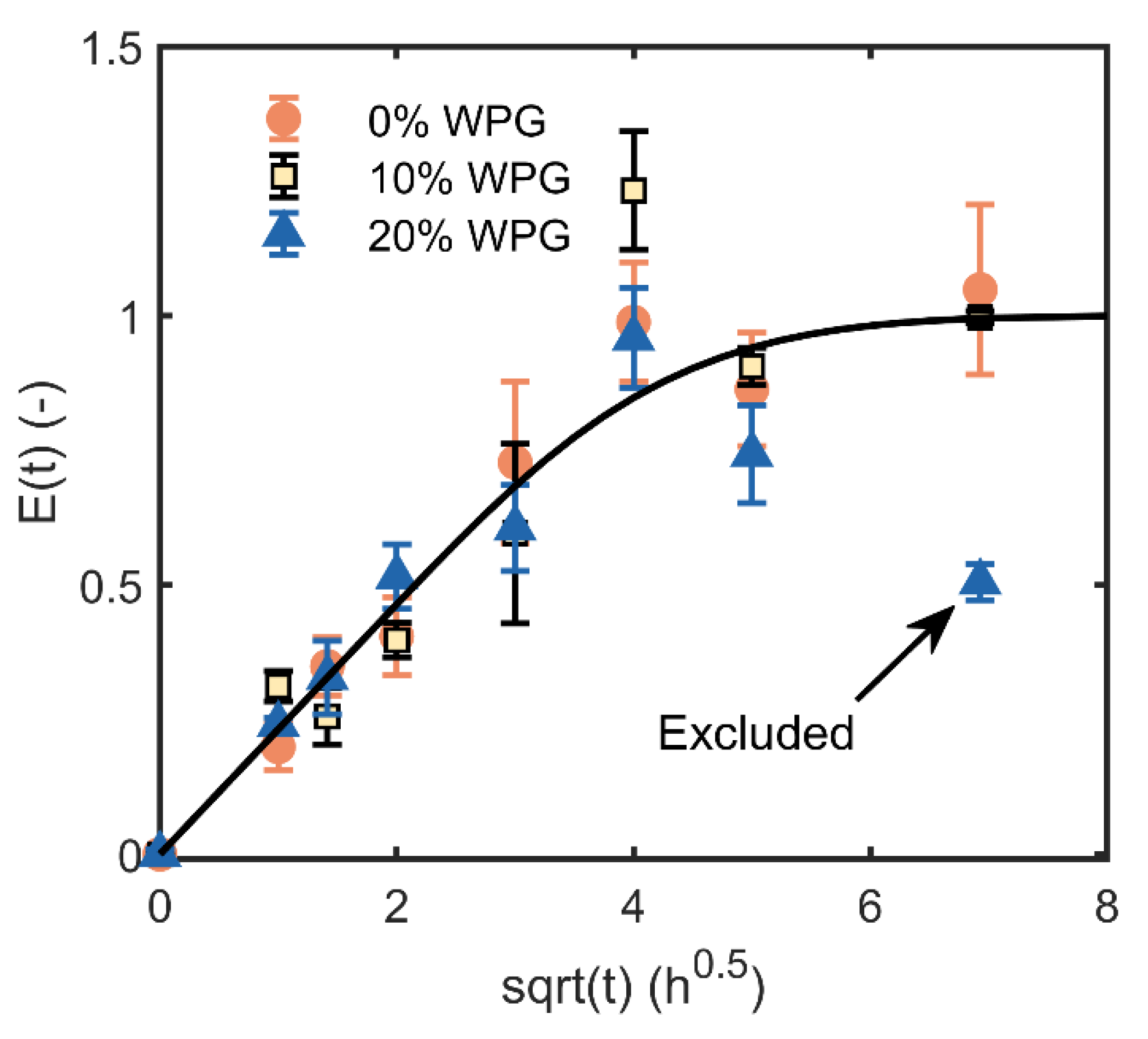
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Eaton, R.A.; Hale, M.D. Wood: Decay, Pests and Protection; Chapman and Hall Ltd.: London, UK, 1993. [Google Scholar]
- Green, F.; Highley, T.L. Mechanism of brown-rot decay: Paradigm or paradox. Int. Biodeterior. Biodegrad. 1997, 39, 113–124. [Google Scholar]
- Viitanen, H.A. Factors Affecting the Development of Mould and Brown Rot Decay in Wooden Material and Wooden Structures—Effect of Humidity, Temperature and Exposure Time. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 1996. [Google Scholar]
- Arantes, V.; Jellison, J.; Goodell, B. Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl. Microbiol. Biotechnol. 2012, 94, 323–338. [Google Scholar]
- Goodell, B.; Jellison, J.; Liu, J.; Daniel, G.; Paszczynski, A.; Fekete, F.; Krishnamurthy, S.; Jun, L.; Xu, G. Low molecular weight chelators and phenolic compounds isolated from wood decay fungi and their role in the fungal biodegradation of wood. J. Biotechnol. 1997, 53, 133–162. [Google Scholar]
- Jellison, J.; Connolly, J.; Goodell, B.; Doyle, B.; Illman, B.; Fekete, F.; Ostrofsky, A. The role of cations in the biodegradation of wood by the brown rot fungi. Int. Biodeterior. Biodegrad. 1997, 39, 165–179. [Google Scholar]
- Jellison, J.; Smith, K.C.; Shortle, W.T. Cation Analysis of Wood Degraded by White-and Brown-rot Fungi. Document-the International Research Group on Wood Preservation (Sweden). No. 1552. 1992. Available online: https://agris.fao.org/agris-search/search.do?recordID=SE19930016252 (accessed on 16 October 2020).
- Kirker, G.; Zelinka, S.; Gleber, S.-C.; Vine, D.; Finney, L.; Chen, S.; Hong, Y.P.; Uyarte, O.; Vogt, S.; Jellison, J.; et al. Synchrotron-based X-ray fluorescence microscopy enables multiscale spatial visualization of ions involved in fungal lignocellulose deconstruction. Sci. Rep. 2017, 7, 41798. [Google Scholar] [CrossRef]
- Ringman, R.; Pilgård, A.; Brischke, C.; Windeisen, E.; Richter, K. Incipient brown rot decay in modified wood: Patterns of mass loss, structural integrity, moisture and acetyl content in high resolution. Int. Wood Prod. J. 2017, 8, 172–182. [Google Scholar]
- Winandy, J.E.; Morrell, J.J. Relationship between incipient decay, strength, and chemical composition of Douglas-fir heartwood. Wood Fiber Sci. 1993, 25, 278–288. [Google Scholar]
- Curling, S.F.; Clausen, C.A.; Winandy, J.E. Relationships between mechanical properties, weight loss, and chemical composition of wood during incipient brownrot decay. Forest Prod. J. 2002, 52, 34–39. [Google Scholar]
- Forster, S. The Decay Resistance of Chemically Modified Softwood. Ph.D. Thesis, University of Wales, Bangor, UK, 1998. [Google Scholar]
- Hill, C.A.; Curling, S.F.; Kwon, J.H.; Marty, V. Decay resistance of acetylated and hexanoylated hardwood and softwood species exposed to Coniophora puteana. Holzforschung 2009, 63, 619–625. [Google Scholar]
- Meyer, L.; Brischke, C.; Treu, A.; Larsson-Brelid, P. Critical moisture conditions for fungal decay of modified wood by basidiomycetes as detected by pile tests. Holzforschung 2016, 70, 331–339. [Google Scholar]
- Hill, C.A. Wood Modification: Chemical, Thermal and other Processes; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 5, Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/0470021748 (accessed on 13 January 2006).
- Fuchs, W. Zur Kenntnis des genuinen Lignins, I.: Die Acetylierung des Fichtenholzes. Ber. Deutsch. Chem. Ges. (A B Ser.) 1928, 61, 948–951. [Google Scholar] [CrossRef]
- Thybring, E.E. The decay resistance of modified wood influenced by moisture exclusion and swelling reduction. Int. Biodeterior. Biodegrad. 2013, 82, 87–95. [Google Scholar] [CrossRef]
- Stamm, A.J.; Baechler, R. Decay resistance and dimensional stability of five modified woods. Forest Prod. J. 1960, 10, 22–26. [Google Scholar]
- Zelinka, S.L.; Ringman, R.; Pilgård, A.; Thybring, E.E.; Jakes, J.E.; Richter, K. The role of chemical transport in the brown-rot decay resistance of modified wood. Int. Wood Prod. J. 2016, 7, 66–70. [Google Scholar] [CrossRef]
- Ringman, R.; Pilgard, A.; Brischke, C.; Richter, K. Mode of action of brown rot decay resistance in modified wood: A review. Holzforschung 2014, 68, 239–246. [Google Scholar] [CrossRef]
- Jakes, J.E.; Plaza, N.; Stone, D.S.; Hunt, C.G.; Glass, S.V.; Zelinka, S.L. Mechanism of transport through wood cell wall polymers. J. Forest Prod. Ind. 2013, 2, 10–13. [Google Scholar]
- Jakes, J.E.; Hunt, C.G.; Zelinka, S.L.; Ciesielski, P.N.; Plaza, N.Z. Effects of Moisture on Diffusion in Unmodified Wood Cell Walls: A Phenomenological Polymer Science Approach. Forests 2019, 10, 1084. [Google Scholar] [CrossRef] [Green Version]
- Jakes, J.E. Mechanism for Diffusion through Secondary Cell Walls in Lignocellulosic Biomass. J. Phys. Chem. B 2019, 123, 4333–4339. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Passarini, L.; Colon Quintana, J.L.; Glass, S.V.; Jakes, J.E.; Wiedenhoeft, A.C. Cell wall domain and moisture content influence southern pine electrical conductivity. Wood Fiber Sci. 2016, 48, 54–61. [Google Scholar]
- Zelinka, S.L.; Glass, S.; Stone, D. A Percolation Model for Electrical Conduction in Wood with Implications for Wood-Water Relations. Wood Fiber Sci. 2008, 40, 544–552. [Google Scholar]
- Zelinka, S.L.; Rammer, D.R.; Stone, D.S. Impedance spectroscopy and circuit modeling of Southern pine above 20% moisture content. Holzforschung 2008, 62, 737–744. [Google Scholar] [CrossRef]
- Hunt, C.G.; Zelinka, S.L.; Frihart, C.R.; Lorenz, L.; Yelle, D.; Gleber, S.-C.; Vogt, S.; Jakes, J.E. Acetylation increases relative humidity threshold for ion transport in wood cell walls–A means to understanding decay resistance. Int. Biodeterior. Biodegrad. 2018, 133, 230–237. [Google Scholar] [CrossRef]
- Carll, C.; Highley, T.L. Decay of wood and wood-based products above ground in buildings. J. Test. Eval. 1999, 27, 150–158. [Google Scholar]
- Brischke, C.; Alfredsen, G. Wood-water relationships and their role for wood susceptibility to fungal decay. Appl. Microbiol. Biotechnol. 2020, 104, 3781–3795. [Google Scholar] [CrossRef]
- Fredriksson, M.; Thybring, E.E. On sorption hysteresis in wood: Separating hysteresis in cell wall water and capillary water in the full moisture range. PLoS ONE 2019, 14, e0225111. [Google Scholar] [CrossRef]
- Tiemann, H.D. Effect of Moisture upon the Strength and Stiffness of Wood; U.S. Department of Agriculture Forest Service—Bulletin 70; Government Printing Office: Washington, DC, USA, 1906.
- Engelund, E.; Thygesen, L.; Svensson, S.; Hill, C.S. A critical discussion of the physics of wood–water interactions. Wood Sci. Technol. 2013, 47, 141–161. [Google Scholar] [CrossRef] [Green Version]
- Zelinka, S.L.; Glass, S.V.; Jakes, J.E.; Stone, D.S. A solution thermodynamics definition of the fiber saturation point and the derivation of a wood–water phase (state) diagram. Wood Sci. Technol. 2016, 50, 443–462. [Google Scholar] [CrossRef]
- Glass, S.V.; Zelinka, S.L. Moisture Relations and Physical Properties of Wood. In Wood Handbook. Wood as an Engineering Material; Ross, R.J., Ed.; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2010. [Google Scholar]
- Siau, J.F. Wood: Influence of Moisture on Physical Properties; Department of Wood Science and Forest Products, Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 1995. [Google Scholar]
- Skaar, C. Wood-Water Relations; Springer: New York, NY, USA, 1988. [Google Scholar]
- Stamm, A.J. Review of nine methods for determining the fiber saturation points of wood and wood products. Wood Sci. 1971, 4, 114–128. [Google Scholar]
- Griffin, D. Water potential and wood-decay fungi. Ann. Rev. Phytopathol. 1977, 15, 319–329. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Mai, C. Mode of action of brown rot decay resistance of acetylated wood: Resistance to Fenton’s reagent. Wood Sci. Technol. 2016, 50, 413–426. [Google Scholar] [CrossRef]
- Passarini, L.; Zelinka, S.L.; Glass, S.V.; Hunt, C.G. Effect of weight percent gain and experimental method on fiber saturation point of acetylated wood determined by differential scanning calorimetry. Wood Sci. Technol. 2017, 51, 1291–1305. [Google Scholar] [CrossRef]
- Suzuki, M.R.; Hunt, C.G.; Houtman, C.J.; Dalebroux, Z.D.; Hammel, K.E. Fungal hydroquinones contribute to brown rot of wood. Environ. Microbiol. 2006, 8, 2214–2223. [Google Scholar]
- Wei, D.; Houtman, C.J.; Kapich, A.N.; Hunt, C.G.; Cullen, D.; Hammel, K.E. Laccase and its role in production of extracellular reactive oxygen species during wood decay by the brown rot basidiomycete Postia placenta. Appl. Environ. Microbiol. 2010, 76, 2091–2097. [Google Scholar] [PubMed] [Green Version]
- Korripally, P.; Timokhin, V.I.; Houtman, C.J.; Mozuch, M.D.; Hammel, K.E. Evidence from Serpula lacrymans that 2,5-dimethoxyhydroquinone is a lignocellulolytic agent of divergent brown rot basidiomycetes. Appl. Environ. Microbiol. 2013, 79, 2377–2383. [Google Scholar]
- Byrne, R.; Luo, Y.-R.; Young, R. Iron hydrolysis and solubility revisited: Observations and comments on iron hydrolysis characterizations. Marine Chem. 2000, 70, 23–35. [Google Scholar]
- Smith, R.; Martell, A.; Motekaitis, R. Critically selected stability constants of metal complexes. In NIST Standard Reference Database; National Institute of Standards: Gaithersburg, MD, USA, 2004; Volume 46, p. 8. [Google Scholar]
- Deneux, M.; Meilleur, R.; Benoit, R. Chélates du fer (III) avec des anions dicarboxylates. Can. J. Chem. 1968, 46, 1383–1388. [Google Scholar]
- Anon. AWPA A21: Standard Method for the Analysis of Wood and Wood Treating Solutions by Inductively Coupled Plasma Emission Spectrometry; American Wood Protection Association: Granbury, TX, USA, 2007. [Google Scholar]
- Hill, C.A.; Forster, S.; Farahani, M.; Hale, M.; Ormondroyd, G.; Williams, G. An investigation of cell wall micropore blocking as a possible mechanism for the decay resistance of anhydride modified wood. Int. Biodeterior. Biodegrad. 2005, 55, 69–76. [Google Scholar]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; Wiley: Hoboken, NJ, USA, 2005; p. 672. [Google Scholar]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975; p. 414. [Google Scholar]
- Jakes, J.E.; Zelinka, S.L.; Hunt, C.G.; Ciesielski, P.; Frihart, C.R.; Yelle, D.; Passarini, L.; Gleber, S.-C.; Vine, D.; Vogt, S. Measurement of moisture-dependent ion diffusion constants in wood cell wall layers using time-lapse micro X-ray fluorescence microscopy. Nat. Sci. Rep. 2020, 10, 9919. [Google Scholar] [CrossRef]
- Arantes, V.; Qian, Y.; Milagres, A.M.; Jellison, J.; Goodell, B. Effect of pH and oxalic acid on the reduction of Fe3+ by a biomimetic chelator and on Fe3+ desorption/adsorption onto wood: Implications for brown-rot decay. Int. Biodeterior. Biodegrad. 2009, 63, 478–483. [Google Scholar]
- Su, P.; Granholm, K.; Pranovich, A.; Harju, L.; Holmbom, B.; Ivaska, A. Sorption of metal ions from aqueous solution to spruce bark. Wood Sci. Technol. 2013, 47, 1083–1097. [Google Scholar]
- Su, P.; Granholm, K.; Pranovich, A.; Harju, L.; Holmbom, B.; Ivaska, A. Metal ion sorption to birch and spruce wood. BioResources 2012, 7, 2141–2155. [Google Scholar] [CrossRef] [Green Version]
- Su, P.; Granholm, K.; Harju, L.; Ivaska, A. Binding affinities of different metal ions to unbleached hardwood kraft pulp. Holzforschung 2011, 65, 619–622. [Google Scholar] [CrossRef]
- Pu, Q.; Sarkanen, K. Donnan equilibria in wood-alkali interactions. Part I. Quantitative determination of carboxyl-, carboxyl ester and phenolic hydroxyl groups. J. Wood Chem. Technol. 1989, 9, 293–312. [Google Scholar] [CrossRef]
- Freudenberg, U.; Zimmermann, R.; Schmidt, K.; Behrens, S.H.; Werner, C. Charging and swelling of cellulose films. J. Colloid Interface Sci. 2007, 309, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Hyde, S.M.; Wood, P.M. A mechanism for production of hydroxyl radicals by the brown-rot fungus Coniophora puteana: Fe (III) reduction by cellobiose dehydrogenase and Fe (II) oxidation at a distance from the hyphae. Microbiology 1997, 143, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Chen, Z.; Wang, X.; Zhang, S. Ligand effects on nitrate reduction by zero-valent iron: Role of surface complexation. Water Res. 2017, 114, 218–227. [Google Scholar] [CrossRef]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef]
- Stamm, A.J. The fiber-saturation point of wood as obtained from electrical conductivity measurements. Ind. Eng. Chem. Anal. Ed. 1929, 1, 94–97. [Google Scholar] [CrossRef]
- Yata, S.; Mukudai, J.; Kajita, H. Morphological Studies on the Movement of Substances into the Cell Wall of Wood. II Diffusion of copper compounds into the cell wall. Mokuzai Gakkaishi 1979, 25, 171–176. [Google Scholar]
- Simons, P.J.; Levy, J.F.; Spiro, M. Electrical migration of exogenous mineral ions through green sapwood of Pinus sylvestris L. (Scots pine). Wood Sci. Technol. 1998, 32, 411–419. [Google Scholar] [CrossRef]
- Rowell, R.M.; Simonson, R.; Hess, S.; Plackett, D.V.; Cronshaw, D.; Dunningham, E. Acetyl distribution in acetylated whole wood and reactivity of isolated wood cell-wall components to acetic anhydride. Wood Fiber Sci. 1994, 26, 11–18. [Google Scholar]
- Arantes, V.; Goodell, B. Current understanding of brown-rot fungal biodegradation mechanisms: A review. In Deterioration and Protection of Sustainable Biomaterials; Schultz, T.P., Goodell, B., Nicholas, D., Eds.; American Chemical Society Symposium: Washington, DC, USA, 2014; pp. 1–21. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelinka, S.L.; Houtman, C.J.; Hirth, K.; Lacher, S.; Lorenz, L.; Engelund Thybring, E.; Hunt, C.G. The Effect of Acetylation on Iron Uptake and Diffusion in Water Saturated Wood Cell Walls and Implications for Decay. Forests 2020, 11, 1121. https://doi.org/10.3390/f11101121
Zelinka SL, Houtman CJ, Hirth K, Lacher S, Lorenz L, Engelund Thybring E, Hunt CG. The Effect of Acetylation on Iron Uptake and Diffusion in Water Saturated Wood Cell Walls and Implications for Decay. Forests. 2020; 11(10):1121. https://doi.org/10.3390/f11101121
Chicago/Turabian StyleZelinka, Samuel L, Carl J. Houtman, Kolby Hirth, Steven Lacher, Linda Lorenz, Emil Engelund Thybring, and Christopher G. Hunt. 2020. "The Effect of Acetylation on Iron Uptake and Diffusion in Water Saturated Wood Cell Walls and Implications for Decay" Forests 11, no. 10: 1121. https://doi.org/10.3390/f11101121




