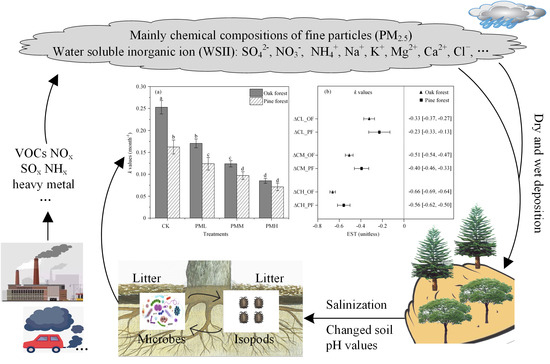
The Impact of Water-Soluble Inorganic Ions in Particulate Matter (PM2.5) on Litter Decomposition in Chinese Subtropical Forests
Abstract
:1. Introduction
2. Methods and Materials
2.1. Study Site
2.2. Collection of Soil, Leaf Litter, and Isopods
2.3. Experiment Design
2.4. Preparation of Simulated WSII-PM2.5 Solutions
2.5. Soil Physiochemical Properties and Litter Decomposition Parameter
2.6. Statistical Analyses
3. Results
3.1. Soil pH, Carbon and Nitrogen Contents
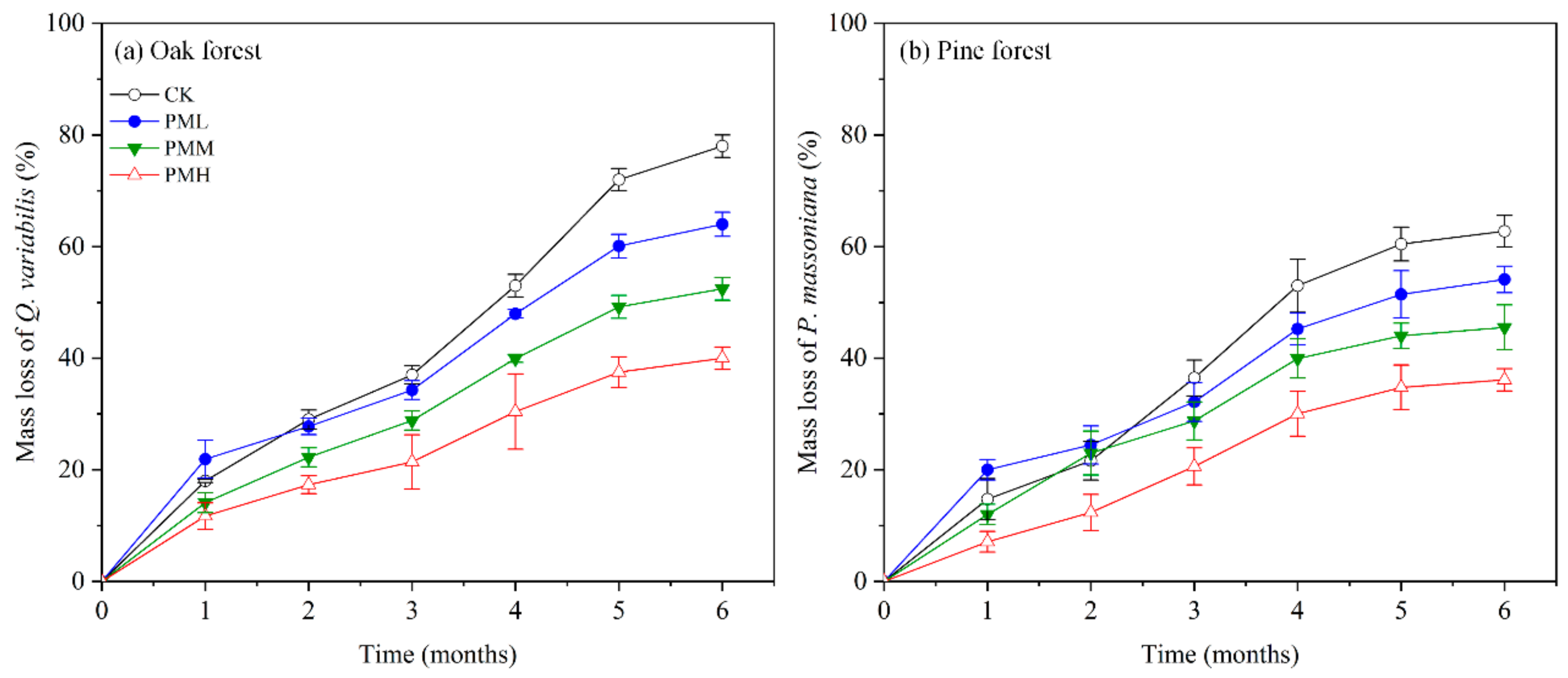
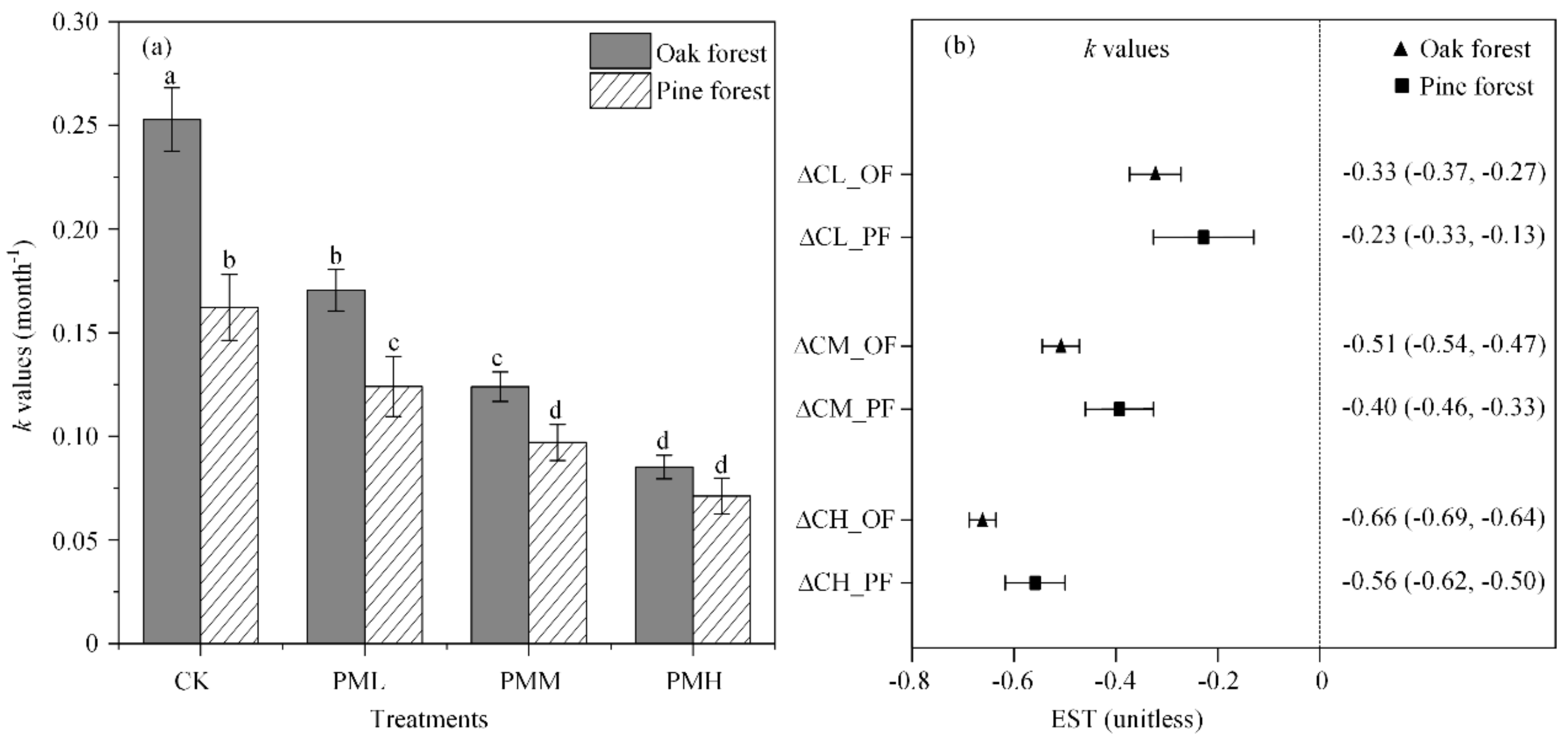
3.2. Leaf Litter Accumulative Mass Loss and Decomposition Rates
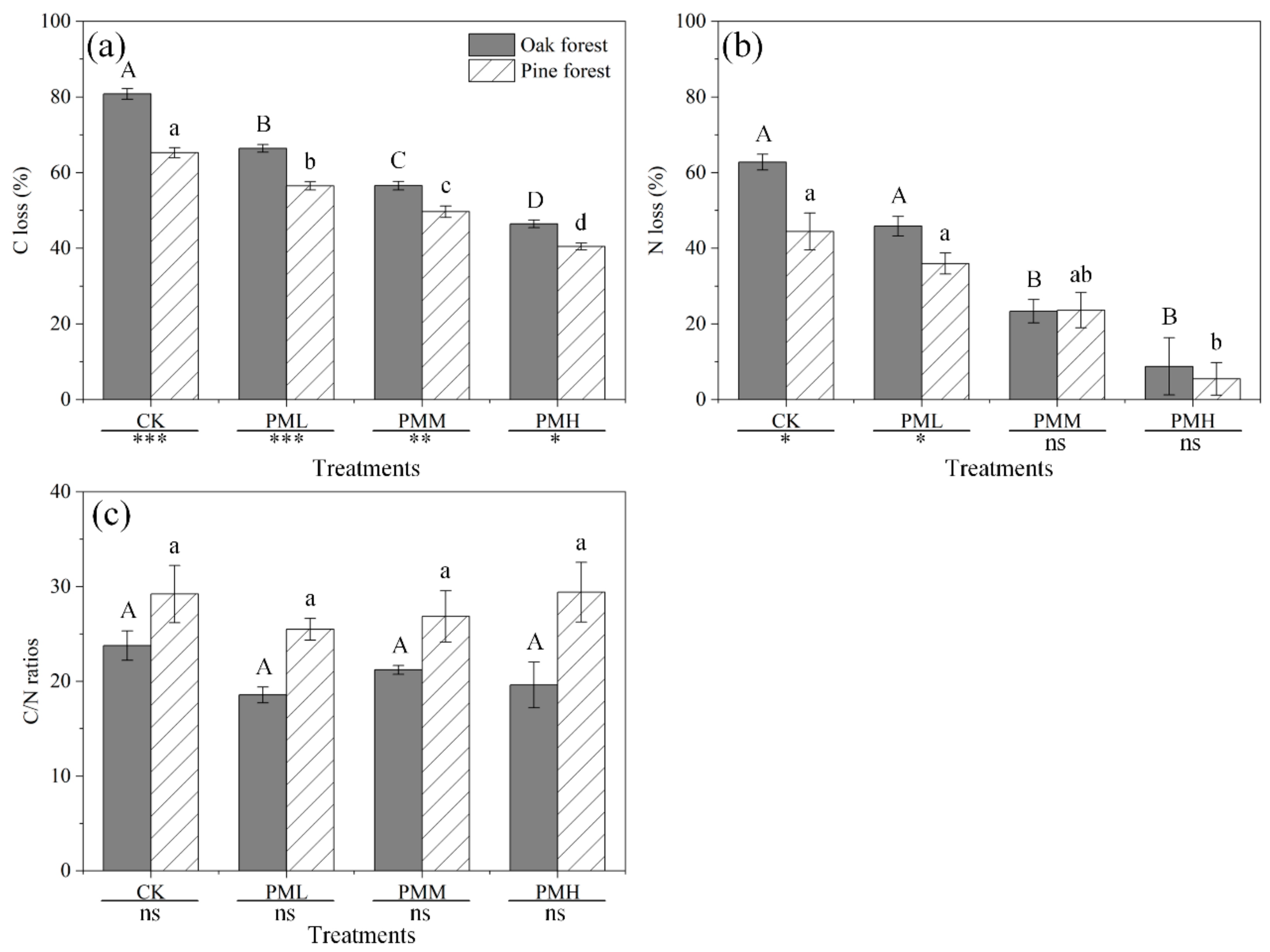
3.3. Leaf Litter Carbon and Nitrogen Loss
3.4. Soil Microbial Biomass and Survival of Isopods
3.5. Soil Extracellular Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Ma, Y.; Tigabu, M.; Guo, X.; Zheng, W.; Guo, L.; Guo, F. Water-Soluble Inorganic Ions in Fine Particulate Emission During Forest Fires in Chinese Boreal and Subtropical Forests: An Indoor Experiment. Forests 2019, 10, 994. [Google Scholar] [CrossRef] [Green Version]
- Shang, Y.; Zhu, T.; Lenz, A.G.; Frankenberger, B.; Tian, F.; Chen, C.Y.; Stoeger, T. Reduced in vitro toxicity of fine particulate matter collected during the 2008 summer Olympic Games in Beijing: The roles of chemical and biological components. Toxicol. In Vitro 2013, 27, 2084–2093. [Google Scholar] [CrossRef]
- Wei, Y.J.; Han, I.K.; Shao, M.; Hu, M.; Zhang, J.F.; Tang, X.Y. PM2.5 Constituents and Oxidative DNA Damage in Humans. Environ. Sci. Technol. 2009, 43, 4757–4762. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Lin, Z.Q.; Jia, R.H.; Li, G.J.; Xi, Z.G.; Wang, D.Y. Transgenerational effects of traffic-related fine particulate matter (PM2.5) on nematode Caenorhabditis elegans. J. Hazard. Mater. 2014, 274, 106–114. [Google Scholar] [CrossRef]
- Hartono, D.; Lioe, B.; Zhang, Y.X.; Li, B.L.; Yu, J.Z. Impacts of particulate matter (PM2.5) on the behavior of freshwater snail Parafossarulus striatulus. Sci. Rep. 2017, 7, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.T.; Zhang, Y.X. Effects of particulate matter (PM2.5) and associated acidity on ecosystem functioning: Response of leaf litter breakdown. Environ. Sci. Pollut. Res. 2018, 25, 30720–30727. [Google Scholar] [CrossRef] [PubMed]
- Callen, M.S.; Iturmendi, A.; Lopez, J.M. Source apportionment of atmospheric PM2.5-bound polycyclic aromatic hydrocarbons by a PMF receptor model. Assessment of potential risk for human health. Environ. Pollut. 2014, 195, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.L.; Meng, F.; Wang, J.K.; Chen, Y.B.; Xiao, W.; Han, H.F. Numerical simulation of the spatial distribution and deposition of PM_(2.5) in East China coastal area in 2010. J. Saf. Environ. 2015, 15, 305–310. [Google Scholar]
- Gao, J.J.; Tian, H.Z.; Cheng, K.; Lu, L.; Zheng, M.; Wang, S.X.; Hao, J.M.; Wang, K.; Hua, S.B.; Zhu, C.Y.; et al. The variation of chemical characteristics of PM2.5 and PM10 and formation causes during two haze pollution events in urban Beijing, China. Atmos. Environ. 2015, 107, 1–8. [Google Scholar] [CrossRef]
- Han, T.T.; Liu, X.G.; Zhang, Y.H.; Qu, Y.; Zeng, L.M.; Hu, M.; Zhu, T. Role of secondary aerosols in haze formation in summer in the Megacity Beijing. J. Environ. Sci.-China 2015, 31, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Zhuang, G.S.; Tang, A.H.; Wang, Y.; An, Z.S. Chemical characteristics of PM2.5 and PM10 in haze-fog episodes in Beijing. Environ. Sci. Technol. 2006, 40, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, W.; Cai, T.Q.; Fang, D.Q.; Wang, Y.Q.; Song, J.; Hu, M.; Zhang, Y.X. Concentrations and chemical compositions of fine particles (PM2.5) during haze and non-haze days in Beijing. Atmos. Res. 2016, 174, 62–69. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, C.Y.; Jia, Y.Y.; Wang, W.W.; Ma, X.; Du, J.J.; Pu, G.Z.; Tian, X.J. Effects of sulfuric, nitric, and mixed acid rain on litter decomposition, soil microbial biomass, and enzyme activities in subtropical forests of China. Appl. Soil Ecol. 2014, 79, 1–9. [Google Scholar] [CrossRef]
- Wang, C.Y.; Guo, P.; Han, G.M.; Feng, X.G.; Zhang, P.; Tian, X.J. Effect of simulated acid rain on the litter decomposition of Quercus acutissima and Pinus massoniana in forest soil microcosms and the relationship with soil enzyme activities. Sci. Total Environ. 2010, 408, 2706–2713. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Bingham, F.T. Influence of Salinity on Soil Enzyme-Activities. Soil Sci. Soc. Am. J. 1982, 46, 1173–1177. [Google Scholar] [CrossRef]
- Rath, K.M.; Rousk, J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil Biol. Biochem. 2015, 81, 108–123. [Google Scholar] [CrossRef]
- Brussaard, L.; Behan-Pelletier, V.M.; Bignell, D.E.; Brown, V.K.; Didden, W.; Folgarait, P.; Fragoso, C.; Freckman, D.W.; Gupta, V.V.S.R.; Hattori, T.; et al. Biodiversity and ecosystem functioning in soil. Ambio 1997, 26, 563–570. [Google Scholar]
- Hinojosa, M.B.; García-Ruíz, R.; Viñegla, B.; Carreira, J.A. Microbiological rates and enzyme activities as indicators of functionality in soils affected by the Aznalcóllar toxic spill. Soil Biol. Biochem. 2004, 36, 1637–1644. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Yang, Y.G.; Jin, Z.S.; Bi, X.Y.; Li, F.L.; Sun, L.; Liu, J.; Fu, Z.Y. Atmospheric Deposition-Carried Pb, Zn, and Cd from a Zinc Smelter and Their Effect on Soil Microorganisms. Pedosphere 2009, 19, 422–433. [Google Scholar] [CrossRef]
- Haines, T.A. Acidic Precipitation and Its Consequences for Aquatic Ecosystems—A Review. Trans. Am. Fish. Soc. 1981, 110, 669–707. [Google Scholar] [CrossRef]
- Guo, P.; Wang, C.Y.; Jia, Y.; Wang, Q.A.; Han, G.M.; Tian, X.J. Responses of soil microbial biomass and enzymatic activities to fertilizations of mixed inorganic and organic nitrogen at a subtropical forest in East China. Plant Soil 2011, 338, 355–366. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Meehan, T.D.; Couture, J.J.; Bennett, A.E.; Lindroth, R.L. Herbivore-mediated material fluxes in a northern deciduous forest under elevated carbon dioxide and ozone concentrations. New Phytol. 2014, 204, 397–407. [Google Scholar] [CrossRef] [Green Version]
- A’Bear, A.D.; Jones, T.H.; Boddy, L. Size matters: What have we learnt from microcosm studies of decomposer fungus-invertebrate interactions? Soil Biol. Biochem. 2014, 78, 274–283. [Google Scholar] [CrossRef]
- Martin, M.H.; Duncan, E.M.; Coughtrey, P.J. The distribution of heavy-metals in a contaminated woodland ecosystem. Environ. Pollut. B 1982, 3, 147–157. [Google Scholar] [CrossRef]
- Fanin, N.; Fromin, N.; Bertrand, I. Functional breadth and home-field advantage generate functional differences among soil microbial decomposers. Ecology 2016, 97, 1023–1037. [Google Scholar] [CrossRef]
- Decker, M.B.; Breitburg, D.L.; Marcus, N.H. Geographical differences in behavioral responses to hypoxia: Local adaptation to an anthropogenic stressor? Ecol. Appl. 2003, 13, 1104–1109. [Google Scholar] [CrossRef]
- Zeng, S.; Xie, Z.; Yu, Y.; Liu, Y. Available microelements in soils under different stands in northern subtropics of China. Acta Ecol. Sin. 2002, 22, 2141–2146. [Google Scholar]
- Chen, G. The faunastic characteristics of terrestrial Isopoda of typical zones in China (In Chinese). J. Jishou Univ. 2005, 26, 26–28. [Google Scholar]
- Zimmer, M. Is decomposition of woodland leaf litter influenced by its species richness? Soil Biol. Biochem. 2002, 34, 277–284. [Google Scholar] [CrossRef]
- Paoletti, M.G.; Hassall, M. Woodlice (Isopoda: Oniscidea): Their potential for assessing sustainability and use as bioindicators. Agric. Ecosyst. Environ. 1999, 74, 157–165. [Google Scholar] [CrossRef]
- Jia, Y.Y.; Lv, Y.N.; Kong, X.S.; Jia, X.Q.; Tian, K.; Du, J.J.; Tian, X.J. Insight into the indirect function of isopods in litter decomposition in mixed subtropical forests in China. Appl. Soil. Ecol. 2015, 86, 174–181. [Google Scholar] [CrossRef]
- Osono, T.; Takeda, H. Comparison of litter decomposing ability among diverse fungi in a cool temperate deciduous forest in Japan. Mycologia 2002, 94, 421–427. [Google Scholar] [CrossRef]
- Bailey, V.L.; Peacock, A.D.; Smith, J.L.; Bolton, H. Relationships between soil microbial biomass determined by chloroform fumigation-extraction, substrate-induced respiration, and phospholipid fatty acid analysis. Soil Biol. Biochem. 2002, 34, 1385–1389. [Google Scholar] [CrossRef]
- Olson, J.S. Energy-storage and balance of producers and decomposers in ecological-systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef] [Green Version]
- St, L.; Wold, S. Analysis of variance (ANOVA). Chemometr. Intell. Lab. 1989, 6, 259–272. [Google Scholar]
- Tukey, J.W. Multiple comparisons. J. Am. Stat. Assoc. 1953, 48, 624–625. [Google Scholar]
- El-Tarabily, K.A.; Nassar, A.H.; Sivasithamparam, K. Promotion of growth of bean (Phaseolus vulgaris L.) in a calcareous soil by a phosphate-solubilizing, rhizosphere-competent isolate of Micromonospora endolithica. Appl. Soil Ecol. 2008, 39, 161–171. [Google Scholar] [CrossRef]
- Hines, J.; Megonigal, J.P.; Denno, R.F. Nutrient subsidies to belowground microbes impact aboveground food web interactions. Ecology 2006, 87, 1542–1555. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.Q.; Zhang, J.E.; Ouyang, Y.; Lin, L.; Quan, G.M.; Zhao, B.L.; Yu, J.Y. Effects of simulated acid rain on microbial characteristics in a lateritic red soil. Environ. Sci. Pollut. Res. 2015, 22, 18260–18266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.E.; Yu, J.Y.; Ouyang, Y.; Xu, H.Q. Impact of Simulated Acid Rain on Trace Metals and Aluminum Leaching in Latosol from Guangdong Province, China. Soil Sediment Contam. 2014, 23, 725–735. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ayres, E.; Wall, D.H.; Li, G.; Bardgett, R.D.; Wu, T.H.; Garey, J.R. Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties. Glob. Ecol. Biogeogr. 2014, 23, 968–978. [Google Scholar] [CrossRef]
- Qi, Z.H.; Song, Y.Y.; Ding, Q.Q.; Liao, X.L.; Li, R.J.; Liu, G.G.; Tsang, S.; Cai, Z.W. Water soluble and insoluble components of PM2.5 and their functional cardiotoxicities on neonatal rat cardiomyocytes in vitro. Ecotox. Environ. Saf. 2019, 168, 378–387. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Li, Y.B.; Shi, Z.X.; Wu, J.; Yang, X.Z.; Feng, L.; Ren, L.H.; Duan, J.C.; Sun, Z.W. Metabolic impact induced by total, water soluble and insoluble components of PM2.5 acute exposure in mice. Chemosphere 2018, 207, 337–346. [Google Scholar] [CrossRef]
- Zou, Y.J.; Jin, C.Y.; Su, Y.; Li, J.R.; Zhu, B.S. Water soluble and insoluble components of urban PM2.5 and their cytotoxic effects on epithelial cells (A549) in vitro. Environ. Pollut. 2016, 212, 627–635. [Google Scholar] [CrossRef]
- Sekowska, A.; Kung, H.F.; Danchin, A. Sulfur metabolism in Escherichia coli and related bacteria: Facts and fiction. J. Mol. Microb. Biotech. 2000, 2, 145–177. [Google Scholar]
- Gualtieri, M.; Longhin, E.; Mattioli, M.; Mantecca, P.; Tinaglia, V.; Mangano, E.; Proverbio, M.C.; Bestetti, G.; Camatini, M.; Battaglia, C. Gene expression profiling of A549 cells exposed to Milan PM2.5. Toxicol. Lett. 2012, 209, 136–145. [Google Scholar] [CrossRef]
- Wang, D.B.; Pakbin, P.; Shafer, M.M.; Antkiewicz, D.; Schauer, J.J.; Sioutas, C. Macrophage reactive oxygen species activity of water-soluble and water-insoluble fractions of ambient coarse, PM2.5 and ultrafine particulate matter (PM) in Los Angeles. Atmos. Environ. 2013, 77, 301–310. [Google Scholar] [CrossRef]
- Wang, Q.; Kwak, J.H.; Choi, W.J.; Chang, S.X. Decomposition of trembling aspen leaf litter under long-term nitrogen and sulfur deposition: Effects of litter chemistry and forest floor microbial properties. For. Ecol. Manag. 2018, 412, 53–61. [Google Scholar] [CrossRef]
- Zukswert, J.M.; Prescott, C.E. Relationships among leaf functional traits, litter traits, and mass loss during early phases of leaf litter decomposition in 12 woody plant species. Oecologia 2017, 185, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Schreeg, L.A.; Mack, M.C.; Turner, B.L. Nutrient-specific solubility patterns of leaf litter across 41 lowland tropical woody species. Ecology 2013, 94, 94–105. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wu, Q.L.; Tang, M.; Wang, D.Y. The in vivo underlying mechanism for recovery response formation in nano-titanium dioxide exposed Caenorhabditis elegans after transfer to the normal condition. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 89–98. [Google Scholar] [CrossRef]
- Crowther, T.W.; Stanton, D.W.G.; Thomas, S.M.; A’Bear, A.D.; Hiscox, J.; Jones, T.H.; Voriskova, J.; Baldrian, P.; Boddy, L. Top-down control of soil fungal community composition by a globally distributed keystone consumer. Ecology 2013, 94, 2518–2528. [Google Scholar] [CrossRef]
- Janouskova, M.; Kohout, P.; Moradi, J.; Doubkova, P.; Frouz, J.; Vosolsobe, S.; Rydlova, J. Microarthropods influence the composition of rhizospheric fungal communities by stimulating specific taxa. Soil Biol. Biochem. 2018, 122, 120–130. [Google Scholar] [CrossRef]
- Schweiger, A.H.; Beierkuhnlein, C. The ecological legacy of 20th century acidification carried on by ecosystem engineers. Appl. Veg. Sci. 2017, 20, 215–224. [Google Scholar] [CrossRef]
- Rusek, J.; Marshall, V.G. Impacts of airborne pollutants on soil fauna. Annu. Rev. Ecol. Syst. 2000, 31, 395–423. [Google Scholar] [CrossRef]


| Species (unit: mg/L) | Treatments | |||
|---|---|---|---|---|
| CK | PML | PMM | PMH | |
| NH4NO3 | 5.12 | 23.82 | 60.83 | 33.44 |
| (NH4)2SO4 | 36.05 | 65.78 | 108.46 | 247.76 |
| KCl | 1.45 | 0.48 | 4.68 | 2.58 |
| KNO3 | 1.58 | 4.61 | 1.74 | 8.70 |
| NaCl | - | - | - | 6.84 |
| Na2SO4 | 2.13 | 2.10 | 2.75 | - |
| Ca (NO3)2 | 10.33 | 3.69 | 6.39 | 64.33 |
| Mg (NO3)2 | 2.81 | 0.42 | 1.11 | 45.63 |
| H2SO4 | - | 16.68 | 37.10 | 57.92 |
| Leaf Litter Traits | Species | |
| Oak | Pine | |
| Total C (%) | 49.12 ± 0.07 b | 50.78 ± 0.10 a |
| Total N (%) | 1.00 ± 0.01 a | 0.64 ± 0.02 b |
| C/N | 49.13 ± 0.53 b | 79.87 ± 2.03 a |
| Lignin (%) | 30.52 ± 0.00 b | 41.20 ± 0.00 a |
| Lignin/N | 30.53 ± 0.35 b | 64.81 ± 1.77 a |
| Soil Characteristics | Species | |
| Oak | Pine | |
| Total C (%) | 3.73 ± 0.17 b | 5.10 ± 0.14 a |
| Total N (%) | 0.28 ± 0.01 b | 0.34 ± 0.00 a |
| C/N | 13.15 ± 0.09 b | 14.84 ± 0.35 a |
| Soil pH | 4.89 ± 0.01 b | 5.27 ± 0.00 a |
| Soil SIR (μL CO2 h−1 g−1 Soil) | 25.25 ± 0.26 b | 35.13 ± 0.83 a |
| Chemical Characteristics | Oak Forest | Pine Forest | ||||||
|---|---|---|---|---|---|---|---|---|
| CK | PML | PMM | PMH | CK | PML | PMM | PMH | |
| Litter | ||||||||
| Total C (%) | 42.70 ± 0.20 f | 43.85 ± 0.16 e | 44.86 ± 0.27 d | 45.79 ± 0.11 c | 46.96 ± 0.03 b | 47.28 ± 0.18 b | 47.33 ± 0.11 b | 48.12 ± 0.02 a |
| Total N (%) | 1.50 ± 0.01 c | 1.52 ± 0.00 c | 1.61 ± 0.01 b | 1.69 ± 0.01 a | 0.89 ± 0.01 d | 0.89 ± 0.03 d | 0.94 ± 0.01 d | 0.95 ± 0.01 d |
| C/N | 28.42 ± 0.23 b | 28.90 ± 0.06 b | 27.87 ± 0.24 b | 27.06 ± 0.20 b | 52.95 ± 0.78 a | 53.34 ± 2.07 a | 50.38 ± 0.66 a | 50.94 ± 0.64 a |
| Soil | ||||||||
| pH | 4.38 ± 0.01 c | 4.28 ± 0.01 d | 4.22 ± 0.01 e | 4.19 ± 0.00 e | 4.63 ± 0.06 a | 4.60 ± 0.03 a | 4.55 ± 0.05 b | 4.53 ± 0.02 b |
| Total C (%) | 2.83 ± 0.02 f | 3.09 ± 0.02 e | 3.19 ± 0.01 d | 3.42 ± 0.01 c | 3.54 ± 0.01 b | 3.52 ± 0.01 b | 3.66 ± 0.01 a | 3.70 ± 0.05 a |
| Total N (%) | 0.22 ± 0.01 e | 0.24 ± 0.01 d | 0.25 ± 0.00 cd | 0.28 ± 0.01 a | 0.25 ± 0.01 cd | 0.26 ± 0.01 bc | 0.27 ± 0.00 ab | 0.28 ± 0.00 a |
| C/N | 12.88 ± 0.14 bcde | 12.86 ± 0.07 cde | 12.64 ± 0.13 de | 12.45 ± 0.11 e | 14.02 ± 0.29 a | 13.42 ± 0.14 abc | 13.42 ± 0.11 abc | 13.23 ± 0.20 bcd |
| SIR (μL CO2 h-1 g-1 Soil) | 10.15 ± 0.12 b | 7.33 ± 0.41 c | 4.25 ± 0.06 e | 2.43 ± 0.09 f | 12.18 ± 0.4 a | 9.55 ± 0.03 b | 7.50 ± 0.07 c | 5.45 ± 0.3 d |
| Variation | SIR | pH | BG | CBH1 | NR | URE | ACP | ALP | PER | PHO |
|---|---|---|---|---|---|---|---|---|---|---|
| Between subjects | ||||||||||
| Intercept | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Habitat | < 0.001 | < 0.001 | 0.247 | 0.003 | 0.458 | < 0.001 | < 0.001 | < 0.001 | 0.054 | 0.067 |
| Treatment | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.427 | 0.054 |
| Treatment × Habitat | < 0.001 | 0.006 | 0.291 | 0.200 | 0.282 | < 0.001 | < 0.001 | < 0.001 | 0.938 | 0.611 |
| Within subjects | ||||||||||
| Date | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Date × Habitat | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Date × Treatment | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Date × Treatment × Habitat | < 0.001 | 0.016 | < 0.001 | 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.339 | < 0.001 |
| Treatment | BG | CBH1 | NR | URE | ACP | ALP | PER | PHO |
|---|---|---|---|---|---|---|---|---|
| Oak forest | ||||||||
| CK | 1.97 ± 0.03 a | 1.81 ± 0.03 a | 5.69 ± 0.08 a | 3.27 ± 0.10 a | 10.92 ± 0.34 a | 13.63 ± 0.48 a | 0.16 ± 0.04 a | 0.20 ± 0.03 a |
| PML | 1.82 ± 0.03 b | 1.68 ± 0.02 b | 4.10 ± 0.03 ab | 2.79 ± 0.10 b | 10.71 ± 0.43 a | 13.08 ± 0.33 ab | 0.11 ± 0.02 b | 0.17 ± 0.02 b |
| PMM | 1.72 ± 0.03 bc | 1.57 ± 0.02 b | 3.85 ± 0.02 b | 2.68 ± 0.13 b | 10.30 ± 0.36 ab | 12.35 ± 0.26 bc | 0.11 ± 0.02 b | 0.16 ± 0.02 bc |
| PMH | 1.60 ± 0.03 c | 1.30 ± 0.05 c | 3.13 ± 0.01 b | 2.55 ± 0.12 b | 8.89 ± 0.35 b | 10.67 ± 0.26 c | 0.11 ± 0.01 b | 0.14 ± 0.02 c |
| Pine forest | ||||||||
| CK | 1.94 ± 0.03 a | 1.87 ± 0.04 a | 5.49 ± 0.08 a | 3.40 ± 0.08 a | 9.56 ± 0.29 ab | 13.81 ± 0.21 a | 0.21 ± 0.03 a | 0.29 ± 0.05 a |
| PML | 1.82 ± 0.02 b | 1.76 ± 0.04 ab | 4.92 ± 0.08 ab | 3.23 ± 0.07 ab | 10.06 ± 0.20 a | 12.68 ± 0.17 b | 0.20 ± 0.03 a | 0.21 ± 0.03 ab |
| PMM | 1.76 ± 0.03 bc | 1.61 ± 0.05 bc | 4.39 ± 0.03 b | 3.10 ± 0.06 bc | 9.23 ± 0.30 ab | 11.91 ± 0.36 b | 0.17 ± 0.02 ab | 0.16 ± 0.02 b |
| PMH | 1.70 ± 0.02 c | 1.48 ± 0.05 c | 3.12 ± 0.01 b | 2.83 ± 0.06 c | 8.91 ± 0.42 b | 11.47 ± 0.21 c | 0.16 ± 0.02 b | 0.14 ± 0.01 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Li, Q.; Ye, R.; Tian, K.; Tian, X. The Impact of Water-Soluble Inorganic Ions in Particulate Matter (PM2.5) on Litter Decomposition in Chinese Subtropical Forests. Forests 2020, 11, 238. https://doi.org/10.3390/f11020238
Ji Y, Li Q, Ye R, Tian K, Tian X. The Impact of Water-Soluble Inorganic Ions in Particulate Matter (PM2.5) on Litter Decomposition in Chinese Subtropical Forests. Forests. 2020; 11(2):238. https://doi.org/10.3390/f11020238
Chicago/Turabian StyleJi, Yanli, Qiang Li, Rumeng Ye, Kai Tian, and Xingjun Tian. 2020. "The Impact of Water-Soluble Inorganic Ions in Particulate Matter (PM2.5) on Litter Decomposition in Chinese Subtropical Forests" Forests 11, no. 2: 238. https://doi.org/10.3390/f11020238