Effects of Graphene on Larix olgensis Seedlings and Soil Properties of Haplic Cambisols in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Collection and Treatments
2.2. Seedling Culture and Graphene Addition
2.3. Plant Assays
2.3.1. Growth Observation, and Survival Rate and Biomass Measurements
2.3.2. Root Morphology
2.3.3. Physiological Parameter Measurements
2.3.4. Determination of Soil Chemical Properties and Enzyme Activities
2.4. Data Analysis
3. Results
3.1. Plant Growth Attributes
3.1.1. Growth and Biomass
3.1.2. Root Morphology
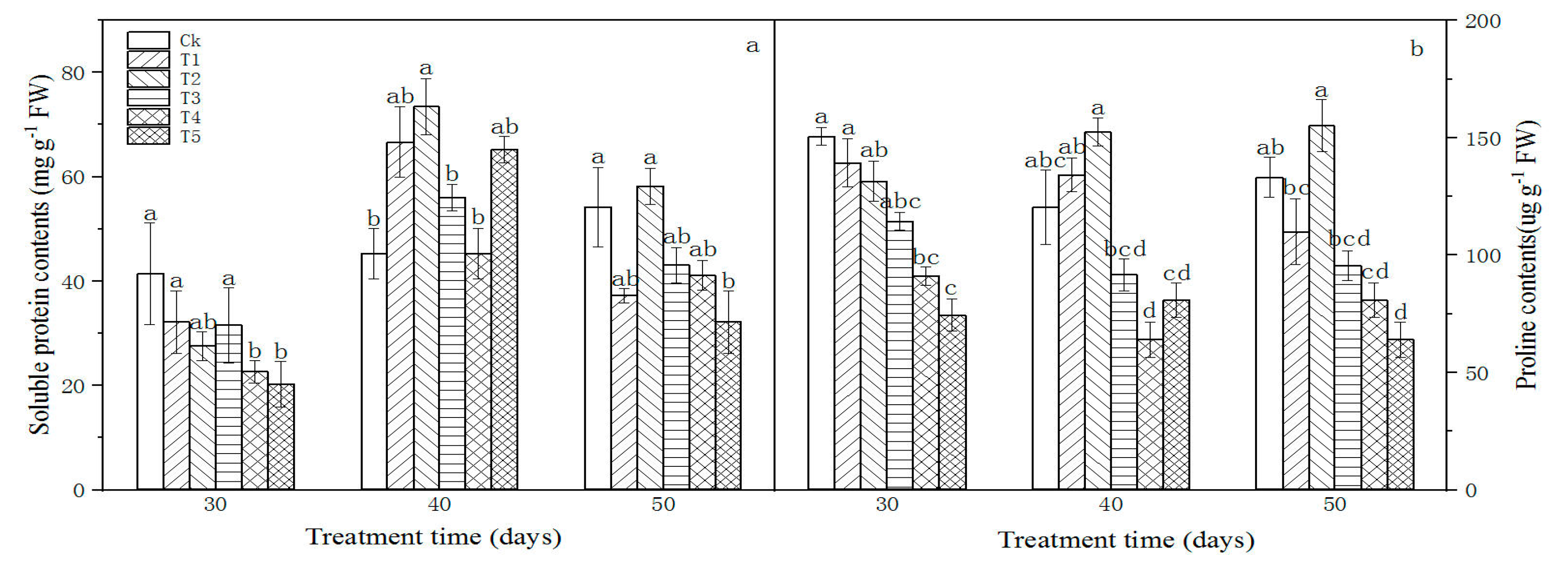
3.1.3. Physiological Responses
3.2. Soil Properties
3.2.1. Soil Chemical Properties
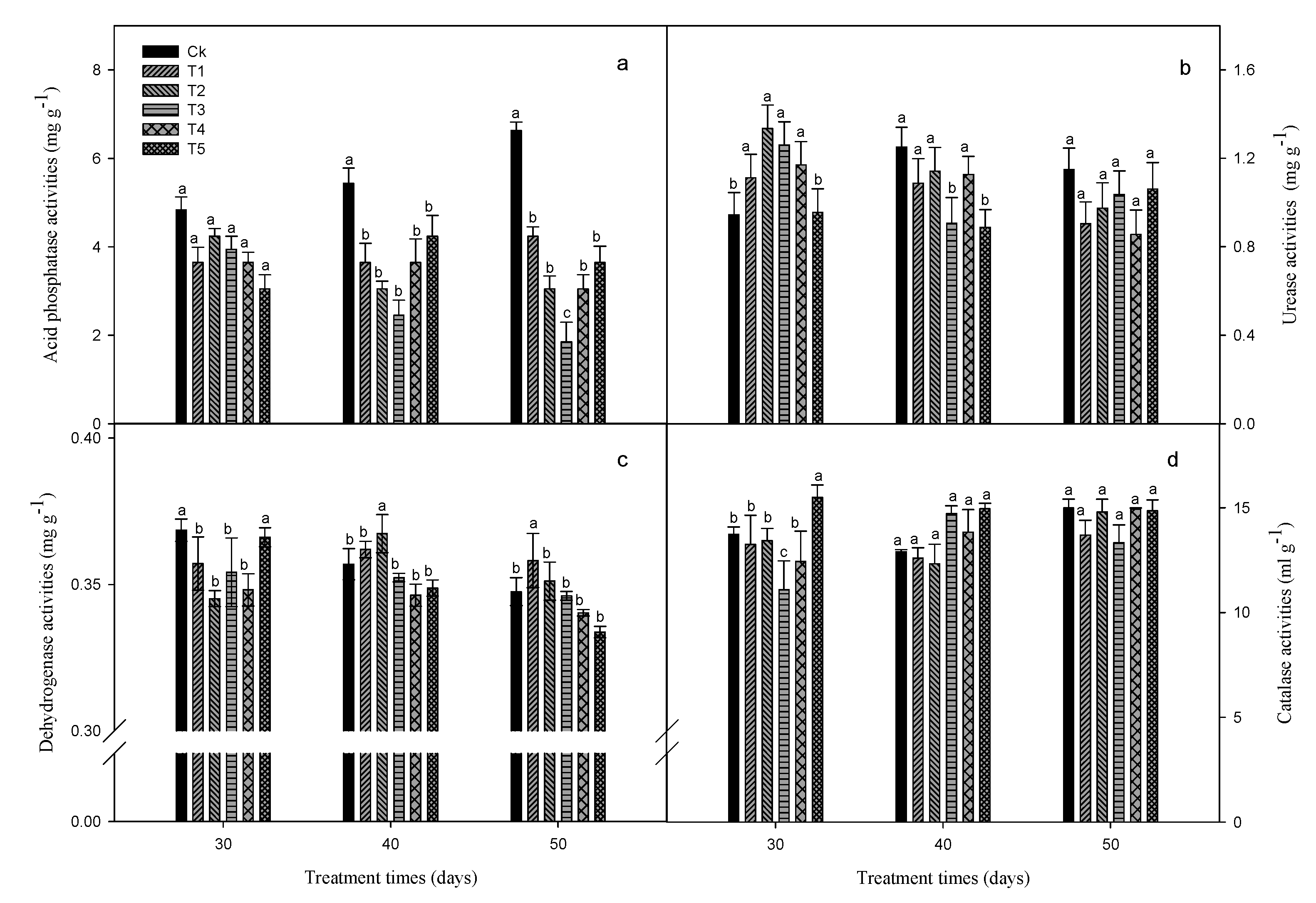
3.2.2. Soil Enzyme Activities
3.2.3. Correlation Analysis of Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.K.; Cui, Z.L. Nanotechnology and Nanomaterials; National Defense Industry Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Shen, X.C.; He, X.W.; Liang, H. The characteristic of nanoparticles and applications in bioanalysis. Chin. J. Anal. Chem. 2003, 31, 880–885. (In Chinese) [Google Scholar]
- Huang, Y.; Chen, Y.S. Functionalization of graphene and their applications. Sci. China Press 2009, 39, 887–896. (In Chinese) [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.Y.; Wang, H.L.; Casalongue, H.S.; Vinh, D.; Dai, H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. Carbon sheets an atom thick give rise to graphene dreams. Science 2009, 324, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, Z.; Zhang, Y.; Morozov, S.V.; Stormer, H.L.; Zeitler, U.; Maan, J.C.; Boebinger, G.S.; Kim, P.; Geim, A.K. Room-temperature quantum hall effect in graphene. Science 2007, 315, 1379. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Yu, F.; Ma, J.; Chen, J.H. Research Progress of application of graphene on removing heavy metals and antibiotics from water. J. Funct. Mater. 2014, 45, 23001–23009. (In Chinese) [Google Scholar]
- Paek, S.M.; Yoo, E.; Honma, I. Enhanced cyclic performance and lithium storage capacity of SnO2/graphene nanoporous electrodes with three dimensionally delaminated flexible structure. Nano Lett. 2009, 9, 72–75. [Google Scholar] [CrossRef]
- Klaine, S.J.; Alvarez, P.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Critical review–nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef]
- Zhang, L.M.; Xia, J.G.; Zhao, Q.H.; Liu, L.W.; Zhang, Z.J. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Srivastava, G.; Talat, M.; Raghubanshi, H.; Srivastava, O.N.; Kayastha, A.M. Cicer alphagalactosidase immobilization onto functionalized graphene nanosheets using response surface method and its applications. Food Chem. 2014, 142, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.F.; Liu, Z.B.; Zhang, X.L.; Wang, Y.; Tian, J.G.; Huang, Y.; Ma, Y.F.; Zhang, X.Y.; Chen, Y.S. A graphene hybrid material covalently functionalized with porphyrin: Synthesis and optical limiting property. Adv. Mater. 2009, 21, 1275–1279. [Google Scholar] [CrossRef]
- Pan, Y.; Sahoo, N.G.; Li, L. The application of graphene oxide in drug delivery. Expert Opin. Drug Del. 2012, 9, 1365–1376. [Google Scholar] [CrossRef]
- Dinesh, R.; Anandaraj, M.; Srinivasan, V.; Hamza, S. Engineered nanoparticles in the soil and their potential implications to microbial activity. Geoderma 2012, 173–174, 19–27. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.; Kim, E.J.; Gu, S.; Sohn, E.J.; Seo, Y.S.; An, H.J.; Chang, Y.S. Exposure of iron nanoparticles to arabidopsis thaliana enhances root elongation by triggering cell wall loosening. Environ. Sci. Technol. 2014, 48, 3477–3485. [Google Scholar] [CrossRef]
- Zhang, P. Mechanisms of graphene toxicity to plants. Ph.D. Thesis, Zhejang Gongshang University, Hangzhou, Zhejiang, China, 2015. (In Chinese). [Google Scholar]
- Chakravarty, D.; Erande, M.B.; Late, D.J. Graphene quantum dots as enhanced plant growth regulators: Effects on coriander and garlic plants. J. Sci. Food Agric. 2015, 95, 2772–2778. [Google Scholar] [CrossRef]
- Yao, J.Z.; Zhang, Z.C.; Xue, B.L.; Zhou, Y.Q.; Hu, X.F.; Xing, B.Y.; Wang, H.Y.; Bai, H.H.; Liu, X.J.; Zhou, J.G. Effect of graphene on adventitious roots’ morphology of tissue culture seedlings of Populus Davidiana. J. Shanxi Datong Univ. (Nat. Sci.) 2018, 34, 6–9. (In Chinese) [Google Scholar]
- Begum, P.; Ikhtiari, R.; Fugetsu, B. Graphene phytotoxicity in the seedling stage of cabbage, tomato, red spinach, and lettuce. Carbon 2011, 49, 3907–3919. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhang, R.; Fang, X.; Song, T.; Cai, X.; Liu, H.; Du, S. Toxic effects of graphene on the growth and nutritional levels of wheat (Triticum aestivum L.): Short- and long-term exposure studies. J. Hazard. Mater. 2016, 317, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Badiane, N.N.Y.; Chotte, J.L.; Patea, E.; Masse, D.; Rouland, C. Use of soil enzyme activities to monitor soil quality in natural and improved fallows in semi-arid tropical regions. Appl. Soil Ecol. 2001, 18, 229–238. [Google Scholar] [CrossRef]
- Li, L.N.; Teng, Y.; Ren, W.J.; Li, Z.G.; Luo, Y.M. Effects of graphene on soil enzyme activities and microbial communities. Soils 2016, 48, 102–108. (In Chinese) [Google Scholar]
- Yan, J.; Han, X.Z.; Ji, Z.J.; Li, Y.; Wang, E.T.; Xie, Z.H.; Chen, W.F. Abundance and diversity of sovbean-nodulating rhizobia in black soil are impacted by land use and crop management. Appl. Environ. Microb. 2014, 80, 5394–5402. (In Chinese) [Google Scholar] [CrossRef] [Green Version]
- Shrestha, B.; Martinezb, V.A.; Cox, S.B.; Green, M.J.; Li, S.; Cañas-Carrell, J.E. An evaluation of the impact of multiwalled carbon nanotubes on Soil microbial community structure and functioning. J. Hazard. Mater. 2013, 261, 188–197. [Google Scholar] [CrossRef]
- He, C.; Gao, F.; Lu, X.X.; Hou, Z.; Zhang, S. Ecotoxicological effects of multi-wall carbon nanotube on soil microorganisms. Asian J. Ecotoxicol. 2012, 7, 155–161. (In Chinese) [Google Scholar]
- Teng, Y.; Luo, Y.M.; Li, Z.G. Microbial diversity in polluted soils: An overview. Acta Pedol. Sin. 2006, 43, 1018–1026. (In Chinese) [Google Scholar]
- Tong, Z.; Bischoff, M.; Nies, L.; Applegate, B.; Turco, R.F. Impact of fullerene (C60) on a soil microbial community. Environ. Sci. Technol. 2007, 41, 2985–2991. [Google Scholar] [CrossRef]
- Jin, L. High concentrations of single-walled carbon nanotubes lower soil enzyme activity and microbial biomass. Ecotoxicol. Environ. Saf. 2013, 88, 9–15. [Google Scholar] [CrossRef]
- Lammel, T.; Boisseaux, P.; Fernández-Cruz, M.L.; Navas, J.M. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line HepG2. Part. Fibre Toxicol. 2013, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Lanphere, J.D.; Luth, C.J.; Walker, S.L. Effects of solution chemistry on the transport of graphene oxide in saturated porous media. Environ. Sci. Technol. 2013, 47, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006, World Soil Resource Reports 103; FAO: Rome, Italy, 2006; pp. 53–65. [Google Scholar]
- Li, H.S.; Sun, Q.; Zhao, S.J.; Zhang, W.H. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2000; pp. 134–263. (In Chinese) [Google Scholar]
- Chen, L.X. Soil Experiment and Practice Course; Northeast Forestry University Press: Harbin, China, 2005; pp. 61–95. (In Chinese) [Google Scholar]
- Häussling, M.; Marschner, H. Organic and inorganic soil phosphates and acid phosphatase activity in the rhizosphere of 80-year-old Norway spruce [Picea abies (L.) Karst.] trees. Biol. Fert. Soils 1989, 8, 128–133. [Google Scholar]
- Baligar, V.C.; Wright, R.J.; Smedley, M.D. Acid phosphatase activity in soils of the appalachian region. Soil Sci. Soc. Am. J. 1988, 52, 1612. [Google Scholar] [CrossRef]
- Hoffmann, G.; Teicher, K. Ein kolorimetrisches verfahren zur bestimmung der ureaseaktivität in Böden. J. Plant Nutr. Soil Sci. 2010, 95, 55–63. [Google Scholar] [CrossRef]
- Johnson, J.L.; Temple, K.L. Some variables affecting the measurement of “catalase activity” in soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Hu, W.B.; Peng, C.; Luo, W.J.; Lv, M.; Li, X.M.; Li, D.; Huang, Q.; Fan, C.H. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Fan, H.L.; Hong, W.; Wu, C.Z.; Chen, C.; Li, J. Effects of water stress on the physiological indexes of carbon and nitrogen metabolization of Liriope muscari (Decne.) Bailey. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2012, 41, 454–458. (In Chinese) [Google Scholar]
- Liu, S.J. The Effects of Grephene on the Germination and Seeding Growth in Rice. Ph.D. Thesis, Yangtze University, Jingzhou, Hubei, China, 2013. (In Chinese). [Google Scholar]
- Liu, H.J.; Zhang, S.X.; Hu, X.N.; Chen, C.D. Phytotoxicity and oxidative stress effect of 1-octyl-3-methylimidazolium chloride ionic liquid on rice seedlings. Environ. Poll. 2013, 181, 242–249. [Google Scholar] [CrossRef]
- Hu, W.B.; Peng, C.; Lv, M.; Li, X.M.; Zhang, Y.J.; Chen, N.; Fan, C.H.; Huang, Q. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef]
- Fu, L.F.; Li, M.L.; Qin, H.M.; Yin, H.; Mo, C.H. Effects of decabromodiphenyl ether on enzyme activities of two type soils. Soils 2014, 46, 689–696. (In Chinese) [Google Scholar]
- Guan, S.Y. Soil Enzyme and Its Research Methods; Agriculture Press: Beijing, China, 1986. (In Chinese) [Google Scholar]
- Zhang, C.; Chen, X.F.; Zhou, B.; Zhang, C.L.; Li, J.J.; Zhang, J.; Dai, J. Effects of vermicompost on microbial characteristics and enzyme activities in soil. Soils 2014, 46, 70–75. (In Chinese) [Google Scholar]
- Ahmed, F.; Rodrigues, D.F. Investigation of acute effects of graphene oxide on wastewater microbial community: A case study. J. Hazard. Mater. 2013, 256, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Qi, X.C.; Shen, T.L.; Li, C.L. Effect of silver nanoparticles and graphene on soil microorganisms and enzyme activities. Acta Sci. Circum. 2017, 37, 3149–3157. (In Chinese) [Google Scholar]









| Soil Horizon | pH (H2O) | CEC a (cmol kg−1)b | Organic Matter (g kg−1) | Hydrolytic N (mg kg−1) | Available P c (mg kg−1) | Available K (mg kg−1) | Soil Texture |
|---|---|---|---|---|---|---|---|
| A1 | 5.32 | 40.20 | 87.94 | 462.45 | 45.92 | 145.31 | loam |
| Treatment | Ck | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| Graphene concentration (mg·L−1) | 0 | 25 | 50 | 100 | 250 | 500 |
| OM | HN | AP | AK | Urea | Dehy | Cata | |
|---|---|---|---|---|---|---|---|
| HN | 0.651 ** | ||||||
| AP | 0.138 | 0.243 | |||||
| AK | 0.320 * | 0.355 ** | 0.377 * | ||||
| Urea | 0.115 | 0.216 * | −0.023 | −0.036 | |||
| Dehy | 0.122 | 0.212 | 0.307 * | 0.152 | −0.035 | ||
| Cata | −0.197 | −0.193 | −0.255 | −0.207 | 0.021 | −0.118 | |
| Phos | 0.563 ** | 0.550 ** | 0.181 | 0.347 * | 0.112 | 0.100 | −0.107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Cao, K.; Duan, C.; Luo, N.; Cui, X. Effects of Graphene on Larix olgensis Seedlings and Soil Properties of Haplic Cambisols in Northeast China. Forests 2020, 11, 258. https://doi.org/10.3390/f11030258
Song J, Cao K, Duan C, Luo N, Cui X. Effects of Graphene on Larix olgensis Seedlings and Soil Properties of Haplic Cambisols in Northeast China. Forests. 2020; 11(3):258. https://doi.org/10.3390/f11030258
Chicago/Turabian StyleSong, Jinfeng, Kai Cao, Chengwei Duan, Na Luo, and Xiaoyang Cui. 2020. "Effects of Graphene on Larix olgensis Seedlings and Soil Properties of Haplic Cambisols in Northeast China" Forests 11, no. 3: 258. https://doi.org/10.3390/f11030258





