Eucalyptus Are Unlikely to Escape Plantations and Invade Surrounding Forests Managed with Prescribed Fire in Southeastern US
Abstract
:1. Introduction
2. Materials and Methods
2.1. Greenhouse Study
2.2. Field Study
2.3. Data Analysis
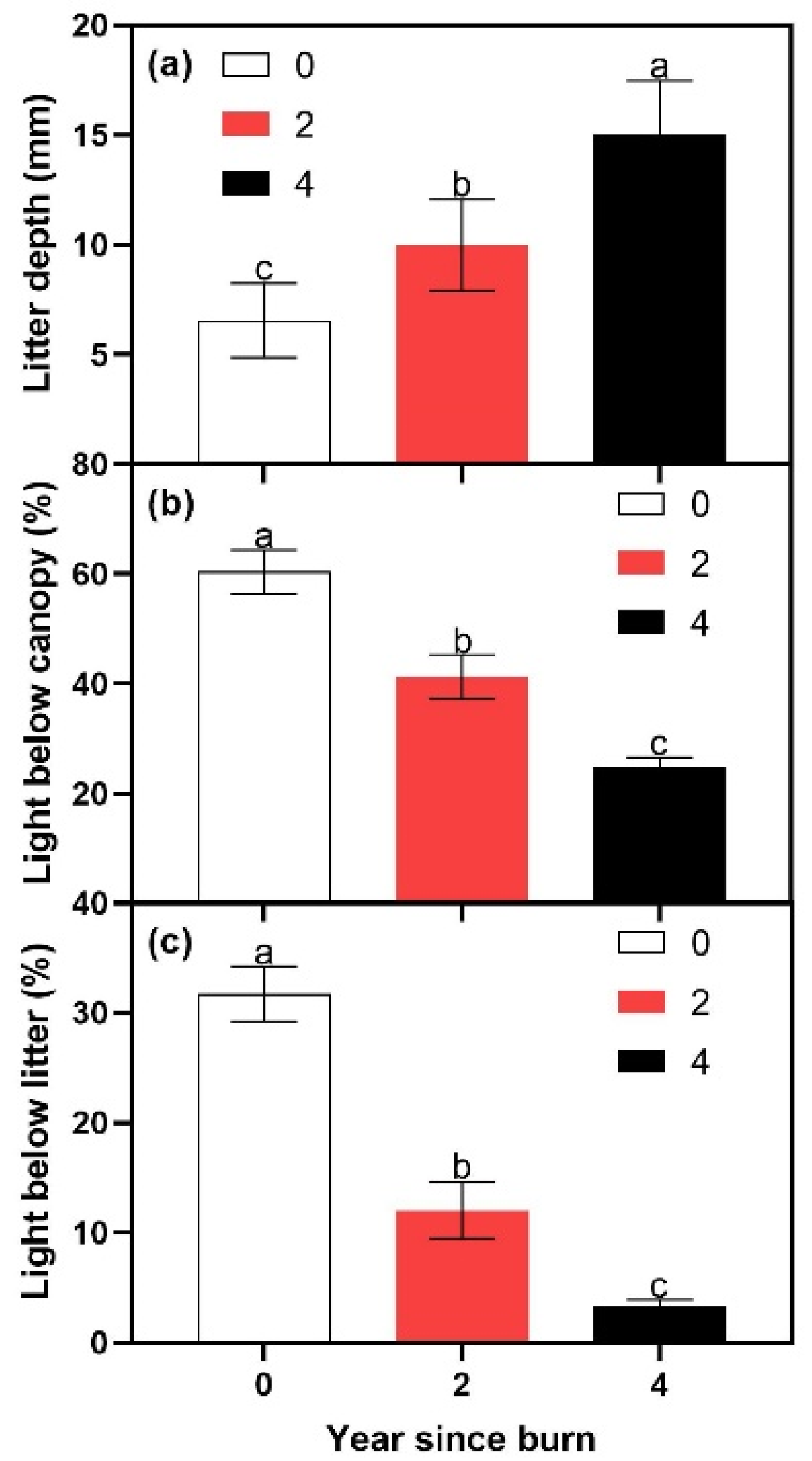
3. Results
3.1. Greenhouse Germination
3.2. Field Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bayle, G. Ecological and social impacts of Eucalyptus tree plantation on the environment. J. Biodivers. Conserv. Bioresour. Manag. 2019, 5, 93–104. [Google Scholar] [CrossRef]
- Lorentz, K.A.; Minogue, P.J. Exotic Eucalyptus plantations in the southeastern US: Risk assessment, management and policy approaches. Biol. Invasions 2015, 17, 1581–1593. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Young, T.M.; Perdue, J.H.; Dougherty, D.; Pigott, M.; Guo, Z.; Huang, X. Productivity and profitability potential for non-native Eucalyptus plantings in the southern USA. For. Policy Econ. 2018, 97, 210–222. [Google Scholar] [CrossRef]
- Albaugh, J.M.; Dye, P.J.; King, J.S. Eucalyptus and water use in South Africa. Int. J. For. Res. 2013, 2013, 852540. [Google Scholar] [CrossRef] [Green Version]
- Toledo, F.H.S.F.; Gonçalves, J.L.D.M.; Mariño, Y.A.; Ferraz, A.D.V.; Ferreira, E.V.d.O.; Moreira, G.G.; Hakamada, R.; Arthur Júnior, J.C.D. Aboveground biomass, transpiration and water use efficiency in eucalypt plantation fertilized with KCl, NaCl and phonolite rock powder. New For. 2019. [Google Scholar] [CrossRef]
- Maier, C.A.; Albaugh, T.J.; Cook, R.I.; Hall, K.; McInnis, D.; Johnsen, K.H.; Johnson, J.; Rubilar, R.A.; Vose, J.M. Comparative water use in short-rotation Eucalyptus benthamii and Pinus taeda trees in the Southern United States. For. Ecol. Manag. 2017, 397, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, R.; Treasure, T.; Wright, J.; Saloni, D.; Phillips, R.; Abt, R.; Jameel, H. Exploring the potential of Eucalyptus for energy production in the Southern United States: Financial analysis of delivered biomass. Part I. Biomass Bioenergy 2011, 35, 755–766. [Google Scholar] [CrossRef]
- Sellers, C. Eucalyptus: Its History, Growth, and Utilization; AJ Johnston: Sacramento, CA, USA, 1910. [Google Scholar]
- Stanturf, J.A.; Vance, E.D.; Fox, T.R.; Kirst, M. Eucalyptus beyond its native range: Environmental issues in exotic bioenergy plantations. Int. J. For. Res. 2013, 2013, 463030. [Google Scholar] [CrossRef]
- Wear, D.N.; Ernest, D.I.V.; Abt, R.C.; Singh, N. Projecting potential adoption of genetically engineered freeze-tolerant Eucalyptus in the United States. For. Sci. 2014, 61, 466–480. [Google Scholar] [CrossRef] [Green Version]
- Hall, K.B.; Stape, J.; Bullock, B.P.; Frederick, D.; Wright, J.; Scolforo, H.F.; Cook, R. A Growth and yield model for Eucalyptus benthamii in the southeastern United States. For. Sci. 2019, 66, 25–37. [Google Scholar] [CrossRef]
- Arnold, R.; Li, B.; Luo, J.; Bai, F.; Baker, T. Selection of cold-tolerant Eucalyptus species and provenances for inland frost-susceptible, humid subtropical regions of southern China. Aust. For. 2015, 78, 180–193. [Google Scholar] [CrossRef]
- Zalesny, R., Jr.; Cunningham, M.; Hall, R.; Mirck, J.; Rockwood, D.; Stanturf, J.; Volk, T. Woody biomass from short rotation energy crops. In Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass; ACS Publications: Washington, DC, USA, 2011; pp. 27–63. [Google Scholar]
- Raghu, S.; Anderson, R.C.; Daehler, C.C.; Davis, A.S.; Wiedenmann, R.N.; Simberloff, D.; Mack, R.N. Ecology. Adding biofuels to the invasive species fire? Science 2006, 313, 1742. [Google Scholar] [CrossRef] [PubMed]
- Mooney, H.A. Invasive Alien Species: A New Synthesis; Island Press: Washington, DC, USA, 2005; Volume 63. [Google Scholar]
- Becerra, P.I. Invasión de árboles alóctonos en una cuenca pre-andina de Chile central. Gayana. Botánica 2006, 63, 161–174. [Google Scholar] [CrossRef] [Green Version]
- Forsyth, G.G.; Richardson, D.M.; Brown, P.J.; Van Wilgen, B.W. A rapid assessment of the invasive status of Eucalyptus species in two South African provinces: Working for water. S. Afr. J. Sci. 2004, 100, 75–77. [Google Scholar]
- Larcombe, M.J.; Silva, J.S.; Vaillancourt, R.E.; Potts, B.M. Assessing the invasive potential of Eucalyptus globulus in Australia: Quantification of wildling establishment from plantations. Biol. Invasions 2013, 15, 2763–2781. [Google Scholar] [CrossRef]
- Becerra, P.I.; Catford, J.A.; Luce McLeod, M.; Andonian, K.; Aschehoug, E.T.; Montesinos, D.; Callaway, R.M. Inhibitory effects of Eucalyptus globulus on understorey plant growth and species richness are greater in non-native regions. Glob. Ecol. Biogeogr. 2018, 27, 68–76. [Google Scholar] [CrossRef]
- Rejmánek, M.; Simberloff, D. “Eucalypts”, in Encyclopedia of Biological Invasions; Simberloff, D., Rejmanek, M., Eds.; University of California Press: Berkeley, CA, USA, 2011. [Google Scholar]
- Van Lill, W.S.; Kruger, F.J.; Van Wyk, D.B. The effect of afforestation with Eucalyptus grandis Hill ex Maiden and Pinus patula Schlecht. et Cham. on streamflow from experimental catchments at Mokobulaan, Transvaal. J. Hydrol. 1980, 48, 107–118. [Google Scholar] [CrossRef]
- Scott, D.F.; Lesch, W. Streamflow responses to afforestation with Eucalyptus grandis and Pinus patula and to felling in the Mokobulaan experimental catchments, South Africa. J. Hydrol. 1997, 199, 360–377. [Google Scholar] [CrossRef]
- Gordon, D.R.; Tancig, K.J.; Onderdonk, D.A.; Gantz, C.A. Assessing the invasive potential of biofuel species proposed for Florida and the United States using the Australian weed risk assessment. Biomass Bioenergy 2011, 35, 74–79. [Google Scholar] [CrossRef]
- Gordon, D.R.; Flory, S.L.; Cooper, A.L.; Morris, S.K. Assessing the invasion risk of Eucalyptus in the United States using the Australian weed risk assessment. Int. J. For. Res. 2012, 2012, 203768. [Google Scholar] [CrossRef] [Green Version]
- Calviño-Cancela, M.; Rubido-Bará, M. Invasive potential of Eucalyptus globulus: Seed dispersal, seedling recruitment and survival in habitats surrounding plantations. For. Ecol. Manag. 2013, 305, 129–137. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.U.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evolut. 2011, 26, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, P.H.M.D.; Bouillet, J.-P.; de Paula, R.C. Assessing the invasive potential of commercial Eucalyptus species in Brazil: Germination and early establishment. For. Ecol. Manag. 2016, 374, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Callaham, M.A.; Stanturf, J.A.; Hammond, W.J.; Rockwood, D.L.; Wenk, E.S.; O’Brien, J.J. Survey to evaluate escape of Eucalyptus spp. seedlings from plantations in Southeastern USA. Int. J. For. Res. 2013, 2013, 946374. [Google Scholar] [CrossRef]
- Bigelow, D.; Borchers, A. Major Uses of Land in the United States, 2012; Economic Information Bulletin, n. 178; U.S. Department of Agriculture: Washington, DC, USA, 2017.
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, M.; Starr, G.; Mack, M.C.; Martin, T.A.; Gholz, H.L. Effects of a prescribed fire on understory vegetation, carbon pools, and soil nutrients in a longleaf pine-slash pine forest in Florida. Nat. Areas J. 2010, 30, 82–94. [Google Scholar] [CrossRef]
- Willson, K.G.; Barefoot, C.R.; Hart, J.L.; Schweitzer, C.J.; Dey, D.C. Temporal patterns of ground flora response to fire in thinned Pinus–Quercus stands. Can. J. For. Res. 2018, 48, 1171–1183. [Google Scholar] [CrossRef] [Green Version]
- Calviño-Cancela, M.; Lorenzo, P.; González, L. Fire increases Eucalyptus globulus seedling recruitment in forested habitats: Effects of litter, shade and burnt soil on seedling emergence and survival. For. Ecol. Manag. 2018, 409, 826–834. [Google Scholar] [CrossRef]
- Catry, F.X.; Moreira, F.; Deus, E.; Silva, J.S.; Águas, A. Assessing the extent and the environmental drivers of Eucalyptus globulus wildling establishment in Portugal: Results from a countrywide survey. Biol. Invasions 2015, 17, 3163–3181. [Google Scholar] [CrossRef]
- Águas, A.; Ferreira, A.; Maia, P.; Fernandes, P.M.; Roxo, L.; Keizer, J.; Silva, J.S.; Rego, F.C.; Moreira, F. Natural establishment of Eucalyptus globulus Labill. in burnt stands in Portugal. For. Ecol. Manag. 2014, 323, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Águas, A.; Incerti, G.; Saracino, A.; Lanzotti, V.; Silva, J.S.; Rego, F.C.; Mazzoleni, S.; Bonanomi, G. Fire effects on litter chemistry and early development of Eucalyptus globulus. Plant Soil 2018, 422, 495–514. [Google Scholar] [CrossRef]
- Boland, D.J.; Brooker, M.I.H.; Chippendale, G.; Hall, N.; Hyland, B.; Johnston, R.D.; Kleinig, D.; McDonald, M.; Turner, J. Forest trees of Australia; CSIRO Publishing: Clayton, Australia, 2006. [Google Scholar]
- White, D.L.; Gaines, K.F. The Savannah River Site: Site description, land use and management history. Stud. Avian Biol. 2000, 21, 8–17. [Google Scholar]
- Kilgo, J.; Blake, J.I. Ecology and Management of a Forested Landscape: Fifty Years on the Savannah River Site; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Parresol, B.R.; Shea, D.; Ottmar, R. Creating a fuels baseline and establishing fire frequency relationships to develop a landscape management strategy at the Savannah River Site. In Proceedings of the Fuels Management-How to Measure Success: Conference Proceedings, Portland, OR, USA, 28–30 March 2006; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006; pp. 351–366. [Google Scholar]
- Aubrey, D.P.; Blake, J.I.; Zarnoch, S.J. From Farms to Forests: Landscape Carbon Balance after 50 Years of Afforestation, Harvesting, and Prescribed Fire. Forests 2019, 10, 760. [Google Scholar] [CrossRef] [Green Version]
- Waldrop, T.A.; Goodrick, S.L. Introduction to Prescribed Fires in Southern Ecosystems; Science Update SRS-054; US Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2012; Volume 54, pp. 1–80. [Google Scholar]
- Zar, J. Biostatistical Analysis; Prentice-Hall International Inc.: London, UK, 1996. [Google Scholar]
- Chen, H.Y.H.; Brant, A.N.; Seedre, M.; Brassard, B.W.; Taylor, A.R. The contribution of litterfall to net primary production during secondary succession in the boreal forest. Ecosystems 2017, 20, 830–844. [Google Scholar] [CrossRef]
- Muqaddas, B.; Lewis, T. Temporal variations in litterfall biomass input and nutrient return under long-term prescribed burning in a wet sclerophyll forest, Queensland, Australia. Sci. Total Environ. 2020, 706, 136035. [Google Scholar] [CrossRef]
- Boyles, J.G.; Aubrey, D.P. Managing forests with prescribed fire: Implications for a cavity-dwelling bat species. For. Ecol. Manag. 2006, 222, 108–115. [Google Scholar] [CrossRef]
- Nereu, M.; Silva, J.S.; Deus, E.; Nunes, M.; Potts, B. The effect of management operations on the demography of Eucalyptus globulus seedlings. For. Ecol. Manag. 2019, 453, 117630. [Google Scholar] [CrossRef]
- Fernandes, P.; Antunes, C.; Pinho, P.; Máguas, C.; Correia, O. Natural regeneration of Pinus pinaster and Eucalyptus globulus from plantation into adjacent natural habitats. For. Ecol. Manag. 2016, 378, 91–102. [Google Scholar] [CrossRef]
- Xia, Q.; Ando, M.; Seiwa, K. Interaction of seed size with light quality and temperature regimes as germination cues in 10 temperate pioneer tree species. Funct. Ecol. 2016, 30, 866–874. [Google Scholar] [CrossRef]
- Kostel-Hughes, F.; Young, T.P.; Wehr, J.D. Effects of leaf litter depth on the emergence and seedling growth of deciduous forest tree species in relation to seed size. J. Torrey Bot. Soc. 2005, 132, 50–61. [Google Scholar] [CrossRef]
- Fernandes, P.; Máguas, C.; Correia, O.; González-Moreno, P. What drives Eucalyptus globulus natural establishment outside plantations? The relative importance of climate, plantation and site characteristics. Biol. Invasions 2018, 20, 1129–1146. [Google Scholar] [CrossRef]
- Fernandes, P.; Máguas, C.; Correia, O. Combined effects of climate, habitat, and disturbance on seedling establishment of Pinus pinaster and Eucalyptus globulus. Plant Ecol. 2017, 218, 501–515. [Google Scholar] [CrossRef]
- Alexander, H.D.; Arthur, M.A. Increasing red maple leaf litter alters decomposition rates and nitrogen cycling in historically oak-dominated forests of the eastern US. Ecosystems 2014, 17, 1371–1383. [Google Scholar] [CrossRef]
- Kowarik, I. Time lags in biological invasions with regard to the success and failure of alien species. Plant Invasions 1995, 15–38. [Google Scholar]
- Siemann, E.; Rogers, W.E. Genetic differences in growth of an invasive tree species. Ecol. Lett. 2001, 4, 514–518. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo, F.H.; McIntosh, T.; Knothe, C.; Aubrey, D.P. Eucalyptus Are Unlikely to Escape Plantations and Invade Surrounding Forests Managed with Prescribed Fire in Southeastern US. Forests 2020, 11, 694. https://doi.org/10.3390/f11060694
Toledo FH, McIntosh T, Knothe C, Aubrey DP. Eucalyptus Are Unlikely to Escape Plantations and Invade Surrounding Forests Managed with Prescribed Fire in Southeastern US. Forests. 2020; 11(6):694. https://doi.org/10.3390/f11060694
Chicago/Turabian StyleToledo, Fábio Henrique, Tyler McIntosh, Candice Knothe, and Douglas P. Aubrey. 2020. "Eucalyptus Are Unlikely to Escape Plantations and Invade Surrounding Forests Managed with Prescribed Fire in Southeastern US" Forests 11, no. 6: 694. https://doi.org/10.3390/f11060694




