Live Crown Ratio as an Indicator for Tree Vigor and Stability of Turkey Oak (Quercus cerris L.): A Case Study
Abstract
:1. Introduction
2. Materials and Methods
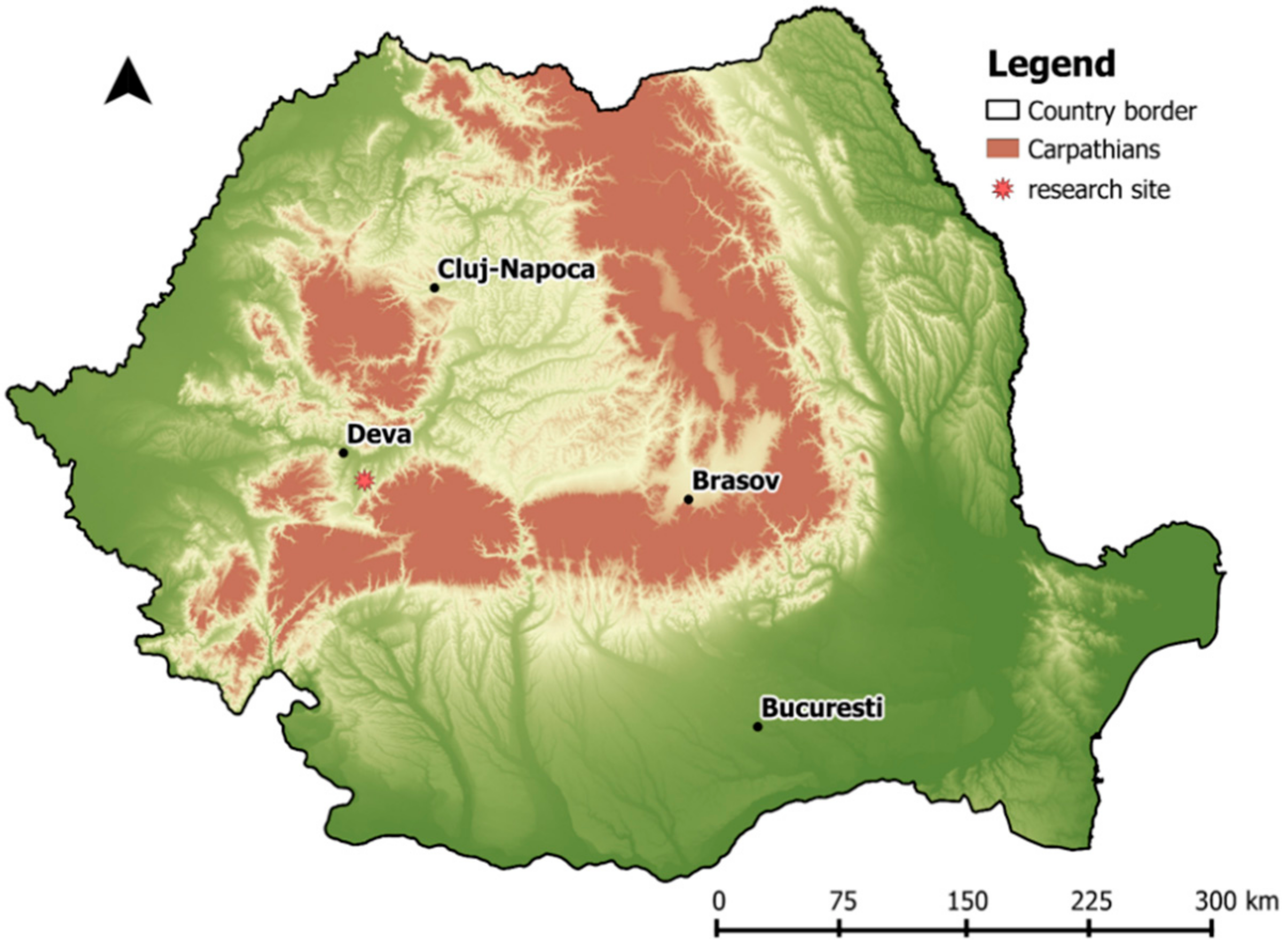
2.1. Study Site
- (1)
- stand 28A (covering 18.5 ha): a naturally regenerated (seed and sprouts) mixture of 50% sessile oak (Quercus petraea (Matt.) Liebl.), 30% Turkey oak, 10% hornbeam (Carpinus betulus L.), and 10% planted red oak (Quercus rubra L.);
- (2)
- stand 28B (covering 50 ha): a naturally regenerated (seed and sprouts) mixture of 50% sessile oak, 40% Turkey oak, and 10% planted European larch (Larix decidua Mill.).
2.2. Methods
2.2.1. Data
2.2.2. Calculation of Height-Diameter Ratio and Live Crown Ratio
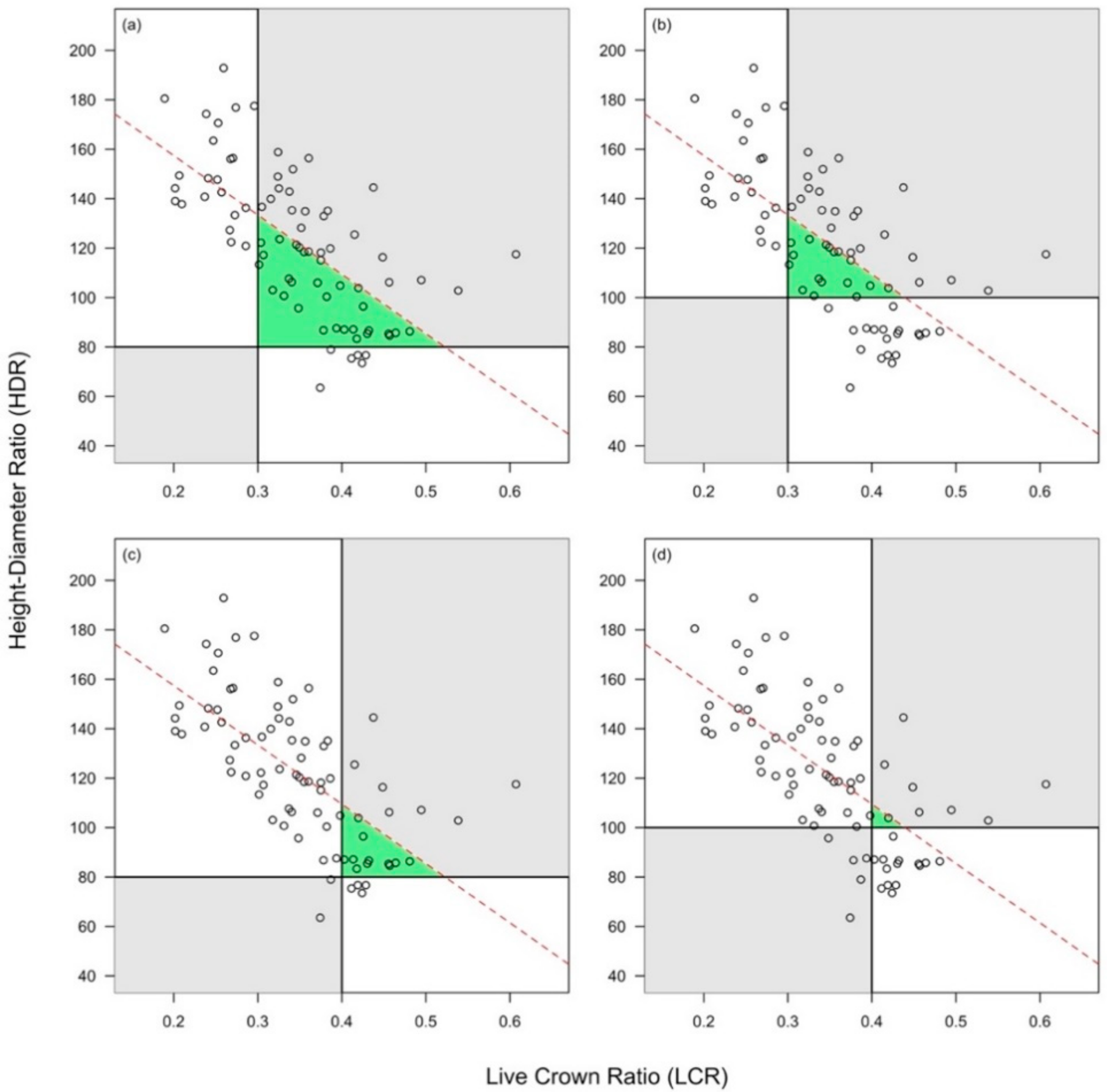
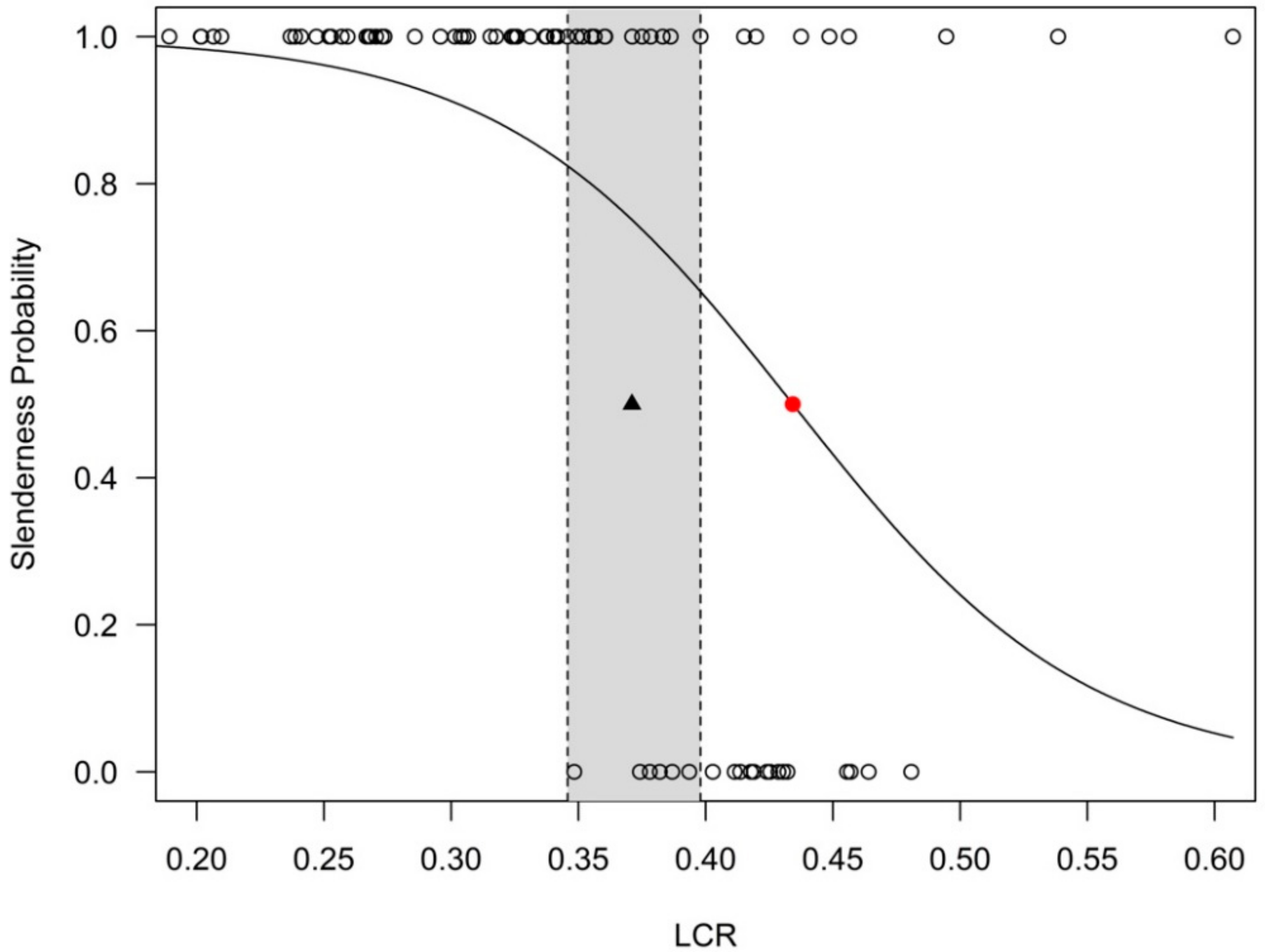
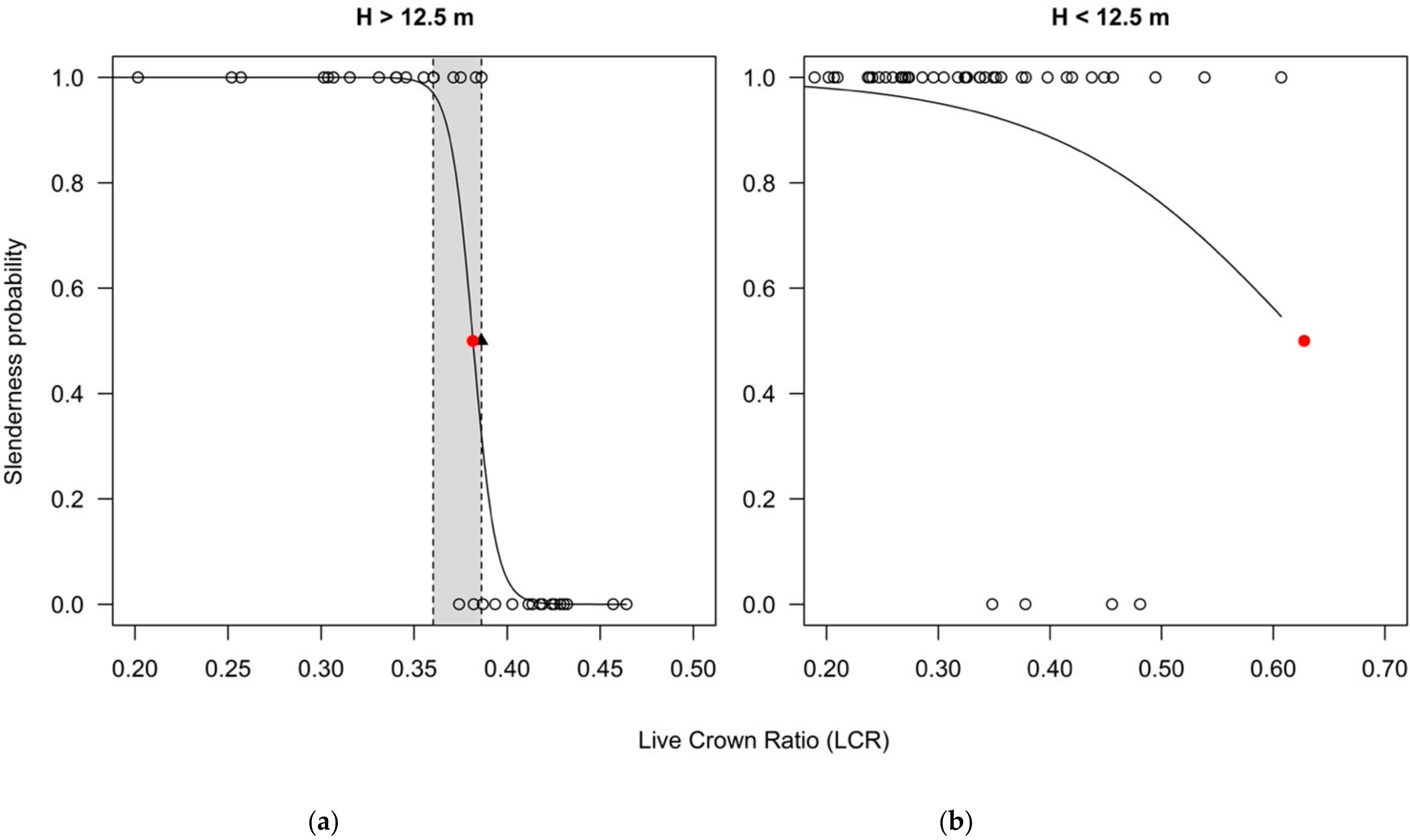
2.2.3. Determining the HDR Threshold for Slenderness
2.2.4. Statistical Analysis
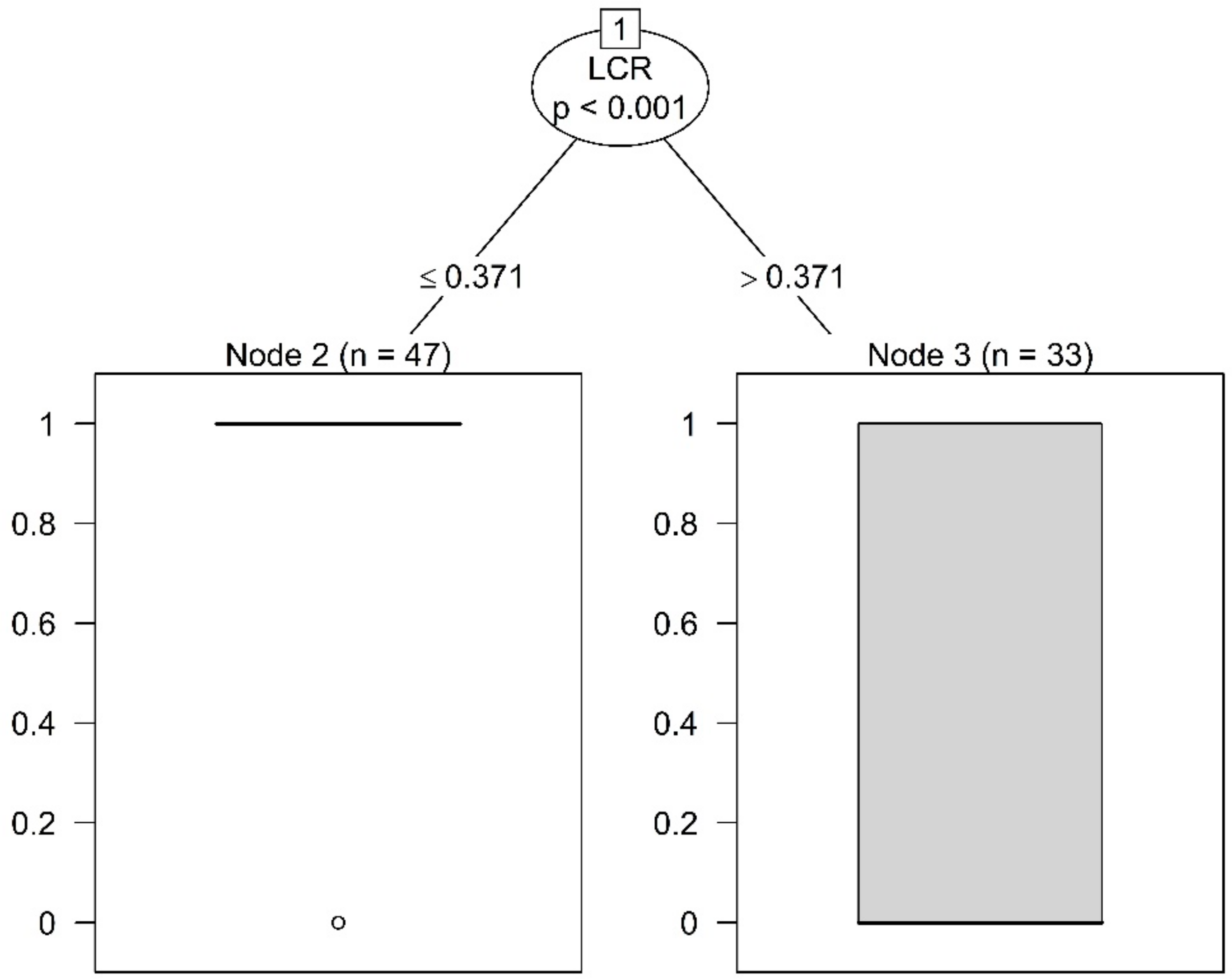
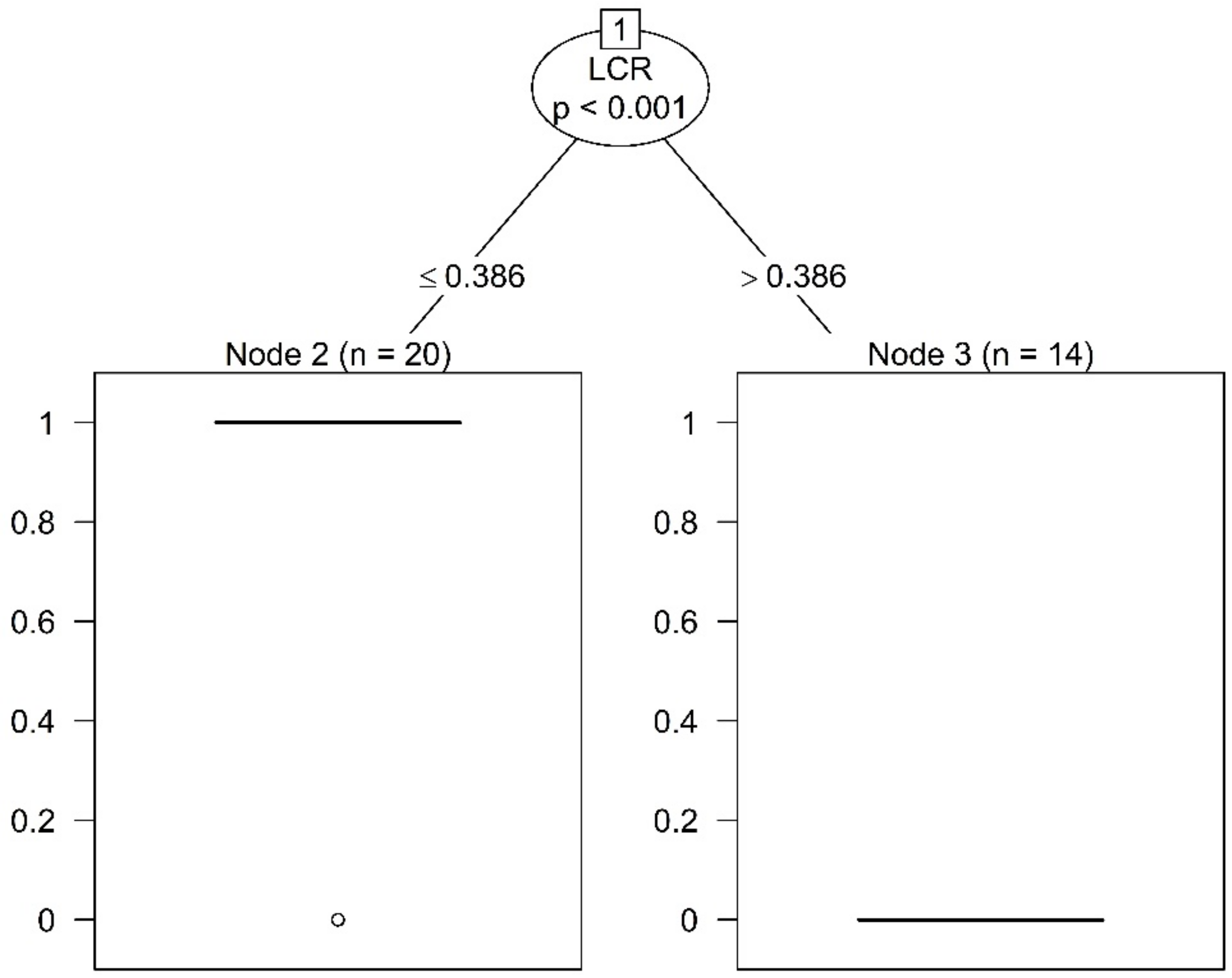
- (1)
- conditional inference tree method [38] combined with resampling by bootstrapping, to obtain not only the threshold value, but also a confidence interval. The bootstrapping approach to obtain the confidence interval of the threshold LCR is preferred to analytical approach since it is easily implemented and can deal with potential skewed distributions. Furthermore, reporting an interval instead of a single estimate value makes the results more applicable into practice [37];
- (2)
- the logistic regression model [39]. After selecting the HDR threshold (Section 2.2.3), the trees were classified as slender (HDR > 100) and not slender (HDR < 100). The logistic regression model was:
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Estimate | Std. Error | z Value | p Value | |
|---|---|---|---|---|
| Intercept | 7.580 | 1.907 | 3.975 | 7.03 × 10−5 |
| LCR | −17.456 | 4.881 | −3.577 | 0.000348 |
| Estimate | Std. Error | z Value | p Value | |
|---|---|---|---|---|
| Intercept | 62.24 | 32.09 | 1.939 | 0.0525 |
| LCR | −163.11 | 84.15 | −1.938 | 0.0526 |
| Estimate | Std. Error | z Value | p Value | |
|---|---|---|---|---|
| Intercept | 5.685 | 2.203 | 2.581 | 0.00986 |
| LCR | −9.058 | 5.338 | −1.697 | 0.08974 |
References
- Blennow, K.; Olofsson, E. The probability of wind damage in forestry under a changed wind climate. Clim. Change 2008, 87, 347–360. [Google Scholar] [CrossRef]
- Donat, M.G.; Leckebusch, G.C.; Pinto, J.G.; Ulbrich, U. European storminess and associated circulation weather types: Future changes deduced from a multi-model ensemble of GCM simulations. Clim. Res. 2010, 42, 27–43. [Google Scholar] [CrossRef]
- Moriondo, M.; Good, P.; Durao, R.; Bindi, M.; Giannakopoulos, C.; Corte-Real, J. Potential impact of climate change on fire risk in the Mediterranean area. Clim. Res. 2006, 31, 85–95. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Brang, P.; Spathelf, P.; Larsen, J.B.; Bauhus, J.; Bončína, A.; Chauvin, C.; Drössler, L.; García-Güemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of close-to-nature silviculture for adapting temperate European forests to climate change. Forestry 2014, 87, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Waring, R.H.; Thies, W.G.; Muscato, D. Stem growth per unit of leaf area: A measure of tree vigor. For. Sci. 1980, 26, 112–117. [Google Scholar]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics: Updated Edition; John Wiley and Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Ashton, M.S.; Kelthy, M.J. The Practice of Silviculture: Applied Forest Ecology, 10th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Daniel, T.W.; Helms, J.A.; Baker, F.S. Principles of Silviculture; McGraw-Hill: New York, NY, USA, 1979. [Google Scholar]
- Kozlowski, T.T. Growth and Development of Trees. Volume I: Seed Germination, Ontogeny, and Shoot Growth; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Smith, D.M.; Larson, B.G.; Kelty, M.J.; Ashton, P.M.S. The Practice of Silviculture: Applied Forest Ecology, 9th ed.; John Wiley and Sons Ltd.: New York, NY, USA, 1997. [Google Scholar]
- Larsson, S.; Oren, R.; Waring, R.H.; Barrett, J.W. Attacks of mountain pine beetle as related to tree vigor of ponderosa pine. For. Sci. 1983, 29, 395–402. [Google Scholar]
- Mitchell, R.G.; Waring, R.H.; Pitman, G.B. Thinning lodgepole pine increases tree vigor and resistance to mountain pine beetle. For. Sci. 1983, 29, 204–211. [Google Scholar]
- Waring, R.H.; Pitman, G.B. Modifying lodgepole pine stands to change susceptibility to mountain pine beetle attack. Ecology 1985, 66, 889–897. [Google Scholar] [CrossRef]
- Wenk, M.; Apel, K.H. Die Regenerationsfähigkeit von durch Fraß des Kiefernspinners (Dendrolimus pini L.) und der Nonne (Lymantria monacha L.) geschädigten Kiefernbeständen in Brandenburg. Eberswalder Forstl. Schr. 2007, 32, 280–287. [Google Scholar]
- Ledo, A.; Paul, K.I.; Burslem, D.F.R.P.; Ewel, J.J.; Barton, C.; Battaglia, M.; Brooksbank, K.; Carter, J.; Eid, T.H.; England, J.R.; et al. Tree size and climatic water deficit control root to shoot ratio in individual trees globally. New Phytol. 2018, 217, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Tavankar, F.; Lo Monaco, A.; Nikooy, M.; Venanzi, R.; Bonyad, A.; Picchio, R. Snow damages on trees of an uneven age in mixed broadleaf forests: Effects of topographical conditions and tree characteristics. J. For. Res. 2019, 30, 1383–1394. [Google Scholar] [CrossRef]
- Wallentin, C.; Nilsson, U. Storm and snow damage in a Norway spruce thinning experiment in southern Sweden. Forestry 2014, 87, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Petty, J.A.; Worrell, R. Stability of coniferous tree stems in relation to damage by snow. Forestry 1981, 54, 115–128. [Google Scholar] [CrossRef]
- Cremer, K.; Borough, C.; Mckinnell, F.; Carter, A. Effects of stocking and thinning on wind damage in plantations. N. Z. J. For. Sci. 1982, 12, 244–268. [Google Scholar]
- Petty, J.A.; Swain, C. Factors influencing stem breakage of conifers in high winds. Forestry 1985, 58, 75–84. [Google Scholar] [CrossRef]
- Nykänen, M.L.; Peltola, H.; Quine, C.; Kellomäki, S.; Broadgate, M. Factors affecting snow damage of trees with particular reference to European conditions. Silva Fenn. 1997, 31, 193–213. [Google Scholar] [CrossRef] [Green Version]
- Peltola, H.; Nykänen, M.L.; Kellomäki, S. Model computations on the critical combination of snow loading and windspeed for snow damage of Scots pine, Norway spruce and Birch sp. at stand edge. For. Ecol. Manag. 1997, 95, 229–241. [Google Scholar] [CrossRef]
- Cameron, A.D. Importance of early selective thinning in the development of long-term stand stability and improved log quality: A review. Forestry 2002, 75, 25–35. [Google Scholar] [CrossRef]
- Sellier, D.; Fourcaud, T. Crown structure and wood properties:Influence on tree sway and response to high winds. Am. J. Bot. 2009, 96, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Kitenberga, M.; Šņepsts, G.; Vuguls, J.; Elferts, D.; Jaunslaviete, I.; Jansons, Ā. Tree-and stand-scale factors shape the probability of wind damage to birch in hemiboreal forests. Silva Fenn. 2021, 55, 1–15. [Google Scholar] [CrossRef]
- Wonn, H.T.; O’Hara, K.L. Height:diameter ratios and stability relationships for four northern Rocky Mountain tree species. West. J. Appl. For. 2001, 16, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Waring, R.H.; Schroeder, P.E.; Oren, R. Application of the pipe model theory to predict canopy leaf area. Can. J. For. Res. 1982, 12, 556–560. [Google Scholar] [CrossRef]
- Alabama Cooperative Extension Service. Thinning Pine Stands for Top Returns. Forest Management Fact Sheet (Circular ANR-396); Alabama Cooperative Extension Service: La Fayette, AL, USA, 1983. [Google Scholar]
- Schmidt, W.C.; Seidel, K.W. Western larch and space: Thinning to optimize growth. In Proceedings-Future Forests of the Mountain West: A Stand Culture Symposium; Gen. Tech. Rep. INT-243; US Department of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1988. [Google Scholar]
- de Rigo, D.; Enescu, C.M.; Houston Durrant, T.; Caudullo, G. Quercus cerris in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publishing Office European: Luxembourg, 2016; pp. 148–149. [Google Scholar]
- NFI Forest Resources Assessment in Romania, Cycle II. Available online: http://roifn.ro/site/rezultate-ifn-2/ (accessed on 15 September 2021).
- Șofletea, N.; Curtu, L. Dendrologie; Editura Universităţii Transilvania din Brasov: Brasov, Romania, 2007. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- FMP. Forest Management Plan for the Production Unit No. I Deva, Forest District Simeria; FMP: Bucharest, Romania, 2012. [Google Scholar]
- Müller, J.; Bütler, R. A review of habitat thresholds for dead wood: A baseline for management recommendations in European forests. Eur. J. For. Res. 2010, 129, 981–992. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; Zeileis, A. Ctree: Conditional inference trees. Compr. R Arch. Netw. 2015, 8, 1–34. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Fehrmann, L.; Kleinn, C. General considerations about the use of allometric equations for biomass estimation on the example of Norway spruce in central Europe. For. Ecol. Manag. 2006, 236, 412–421. [Google Scholar] [CrossRef]
- Praciak, A.; Pasiecznik, N.; Sheil, D.; Van Heist, M.; Sassen, M.; Correia, C.S.; Dixon, C.; Fyson, G.; Rushford, K.; Teeling, C. The CABI Encyclopedia of Forest Trees; CABI: Oxford, UK, 2013. [Google Scholar]
- Savill, P.S. The Silviculture of Trees Used in British Forestry, 3rd ed.; CABI: Wallingford, UK, 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stăncioiu, P.T.; Șerbescu, A.A.; Dutcă, I. Live Crown Ratio as an Indicator for Tree Vigor and Stability of Turkey Oak (Quercus cerris L.): A Case Study. Forests 2021, 12, 1763. https://doi.org/10.3390/f12121763
Stăncioiu PT, Șerbescu AA, Dutcă I. Live Crown Ratio as an Indicator for Tree Vigor and Stability of Turkey Oak (Quercus cerris L.): A Case Study. Forests. 2021; 12(12):1763. https://doi.org/10.3390/f12121763
Chicago/Turabian StyleStăncioiu, Petru Tudor, Alexandru Alin Șerbescu, and Ioan Dutcă. 2021. "Live Crown Ratio as an Indicator for Tree Vigor and Stability of Turkey Oak (Quercus cerris L.): A Case Study" Forests 12, no. 12: 1763. https://doi.org/10.3390/f12121763






