Responses to Climate Change of Maximum Latewood Density from Larix speciosa Cheng et Law and Abies delavayi Franch. in the Northwest of Yunnan Province, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Tree-Ring Data
2.3. Climate Data
2.4. Chronology Development
2.5. Climate-Growth Response Analysis
3. Results
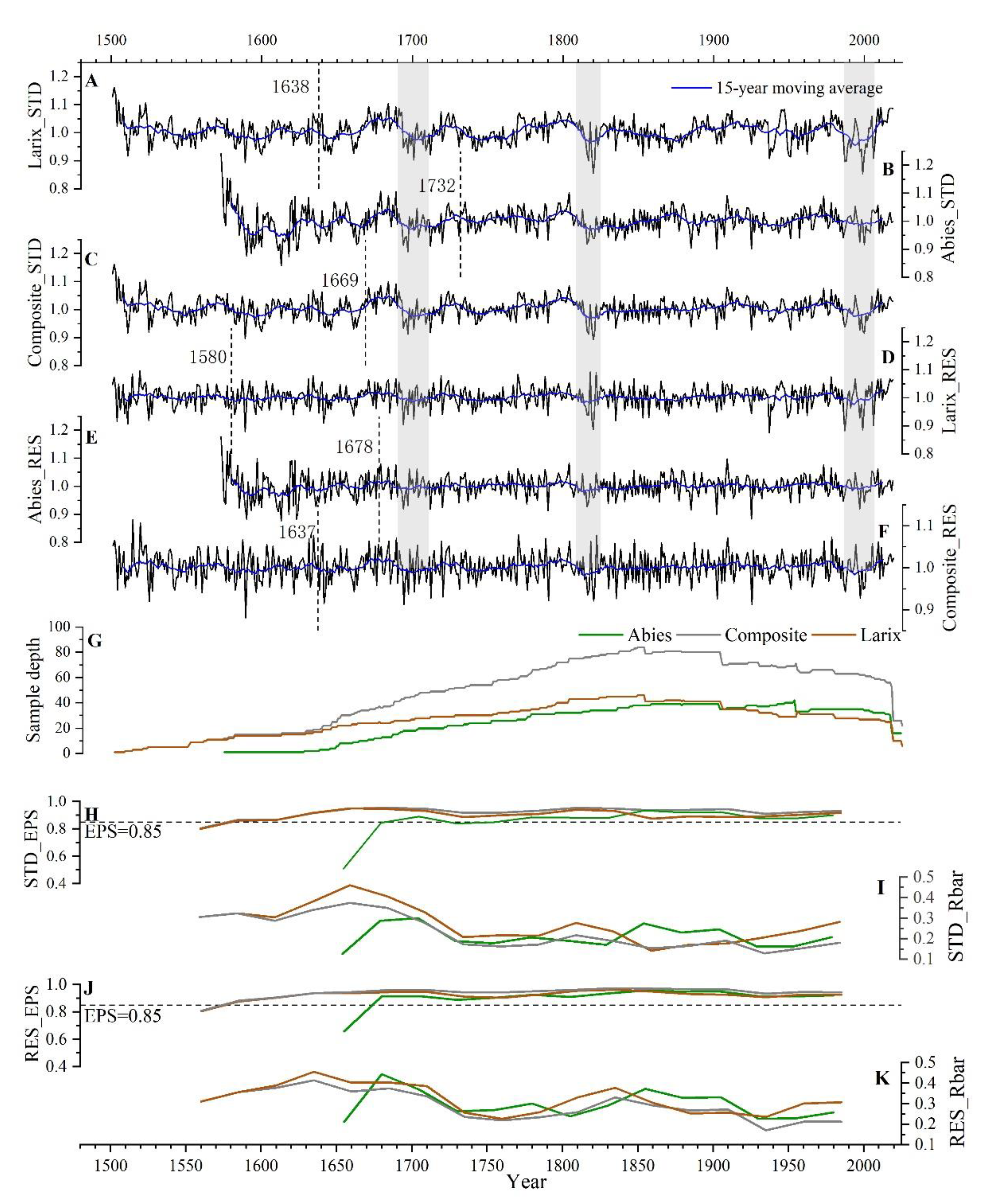
3.1. Statistical Parameters of Chronologies
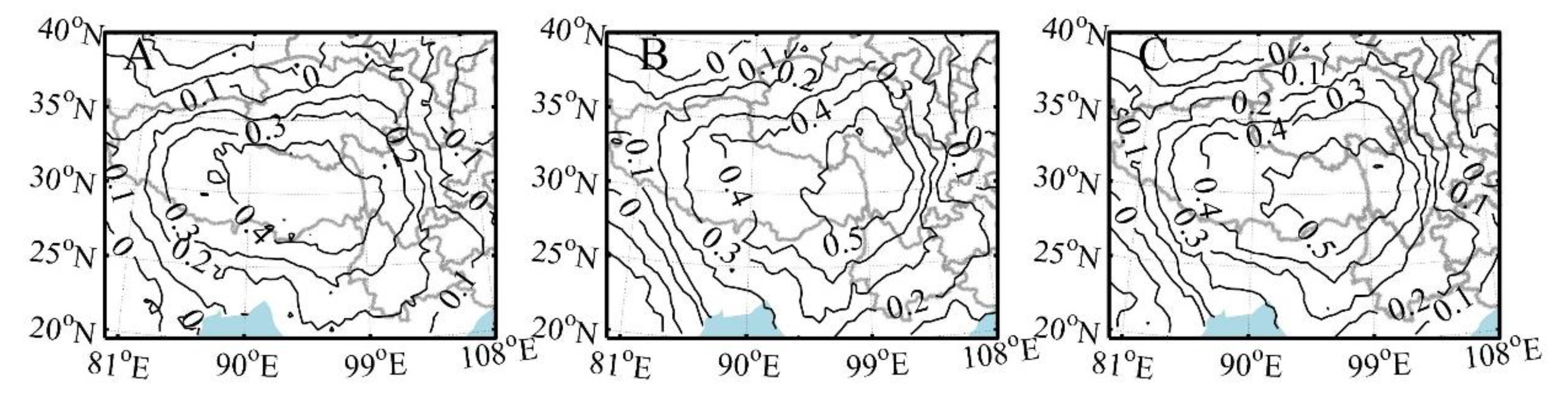
3.2. Responses of Abies and Larix MXD to Climate
3.3. Statistic Model of Climate-Growth Relationships for MXD
4. Discussion
4.1. Tree Growth-Climate Relationship
4.2. Potentials and Limitations of MXD for Different Species for Climate Reconstruction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grudd, H. Torneträsk tree-ring width and density ad 500–2004: A test of climatic sensitivity and a new 1500-year reconstruction of north Fennoscandian summers. Clim. Dyn. 2008, 31, 843–857. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Linderholm, H.W.; Gunnarson, B.E.; Björklund, J.; Chen, D. 1200 years of warm-season temperature variability in central Scandinavia inferred from tree-ring density. Clim. Past 2016, 12, 1297–1312. [Google Scholar] [CrossRef] [Green Version]
- Klippel, L.; Krusic, P.J.; Konter, O.; George, S.S.; Trouet, V.; Esper, J. A 1200+ year reconstruction of temperature extremes for the northeastern Mediterranean region. Int. J. Clim. 2018, 39, 2336–2350. [Google Scholar] [CrossRef] [Green Version]
- Esper, J.; George, S.S.; Anchukaitis, K.; D’Arrigo, R.; Ljungqvist, F.C.; Luterbacher, J.; Schneider, L.; Stoffel, M.; Wilson, R.; Büntgen, U. Large-scale, millennial-length temperature reconstructions from tree-rings. Dendrochronologia 2018, 50, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Matskovsky, V.V.; Helama, S. Testing long-term summer temperature reconstruction based on maximum density chronologies obtained by reanalysis of tree-ring data sets from northernmost Sweden and Finland. Clim. Past 2014, 10, 1473–1487. [Google Scholar] [CrossRef] [Green Version]
- Esper, J.; Schweingruber, F.H.; Winiger, M. 1300 years of climatic history for Western Central Asia inferred from tree-rings. Holocene 2002, 12, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Cook, E.R.; Anchukaitis, K.J.; Buckley, B.M.; D’Arrigo, R.D.; Jacoby, G.C.; Wright, W.E. Asian Monsoon Failure and Megadrought During the Last Millennium. Science 2010, 328, 486–489. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Wang, S.; Zhu, H.; Xu, Y.; Liang, E.; Yin, Z.-Y.; Xu, X.; Xiao, Y. A 3585-year ring-width dating chronology of qilian juniper from the northeastern qinghai-tibetan plateau. IAWA J. 2009, 30, 379–394. [Google Scholar] [CrossRef] [Green Version]
- Cook, E.R.; Seager, R.; Kushnir, Y.; Briffa, K.R.; Büntgen, U.; Frank, D.; Krusic, P.J.; Tegel, W.; Van Der Schrier, G.; Andreu-Hayles, L.; et al. Old World megadroughts and pluvials during the Common Era. Sci. Adv. 2015, 1, e1500561. [Google Scholar] [CrossRef] [Green Version]
- Grudd, H.; Briffa, K.; Karlén, W.; Bartholin, T.S.; Jones, P.D.; Kromer, B. A 7400-year tree-ring chronology in northern Swedish Lapland: Natural climatic variability expressed on annual to millennial timescales. Holocene 2002, 12, 657–665. [Google Scholar] [CrossRef]
- Helama, S.; Seppä, H.; Bjune, A.E.; Birks, H.J.B. Fusing pollen-stratigraphic and dendroclimatic proxy data to reconstruct summer temperature variability during the past 7.5 ka in subarctic Fennoscandia. J. Paleolimnol. 2012, 48, 275–286. [Google Scholar] [CrossRef]
- Li, M.Y.; Duan, J.P.; Wang, L.; Zhu, H.F. Late summer temperature reconstruction based on tree-ring density for Sygera Mountain, southeastern Tibetan Plateau. Glob. Planet. Chang. 2018, 163, 10–17. [Google Scholar] [CrossRef]
- Duan, J.P.; Ma, Z.G.; Li, L.; Zheng, Z.Y. August–September temperature variability on the Tibetan Plateau: Past, present, and future. J. Geophys. Res. Atmos. 2019, 124, 6057–6068. [Google Scholar] [CrossRef]
- Tardif, R.; Hakim, G.J.; Perkins, W.A.; Horlick, K.A.; Erb, M.P.; Emile-Geay, J.; Anderson, D.M.; Steig, E.J.; Noone, D. Last Millennium Reanalysis with an expanded proxy database and seasonal proxy modeling. Clim. Past 2019, 15, 1251–1273. [Google Scholar] [CrossRef] [Green Version]
- Esper, J.; Klippel, L.; Krusic, P.J.; Konter, O.; Raible, C.C.; Xoplaki, E.; Luterbacher, J.; Büntgen, U. Eastern Mediterranean summer temperatures since 730 CE from Mt. Smolikas tree-ring densities. Clim. Dyn. 2020, 54, 1367–1382. [Google Scholar] [CrossRef]
- Klippel, L.; Büntgen, U.; Konter, O.; Kyncl, T.; Esper, J. Climate sensitivity of high- and low-elevation Larix decidua MXD chronologies from the Tatra Mountains. Dendrochronologia 2020, 60, 125674. [Google Scholar] [CrossRef]
- Björklund, J.; von Arx, G.; Nievergelt, D.; Wilson, R.; Van den Bulcke, J.; Günther, B.; Loader, N.J.; Rydval, M.; Fonti, P.; Scharnweber, T.; et al. Scientific merits and analytical challenges of tree-ring densitometry. Rev. Geophys. 2019, 57, 1224–1264. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.K.; Schweingruber, F.H.; Cartwright, D.; Kelly, P.M. July–August temperature at Edinburgh between 1721 and 1975 from tree-ring density and width data. Nature 1984, 308, 341–344. [Google Scholar] [CrossRef]
- Linderholm, H.W.; Björklund, J.; Seftigen, K.; Gunnarson, B.E.; Fuentes, M. Fennoscandia revisited: A spatially improved tree-ring reconstruction of summer temperatures for the last 900 years. Clim. Dyn. 2014, 45, 933–947. [Google Scholar] [CrossRef] [Green Version]
- Rydval, M.; Gunnarson, B.E.; Loader, N.J.; Cook, E.R.; Druckenbrod, D.L.; Wilson, R. Spatial reconstruction of Scottish summer temperatures from tree rings. Int. J. Clim. 2016, 37, 1540–1556. [Google Scholar] [CrossRef] [Green Version]
- Linderholm, H.W.; Gunnarson, B.E. Were medieval warm-season temperatures in Jämtland, central Scandinavian Mountains, lower than previously estimated? Dendrochronologia 2019, 57, 125607. [Google Scholar] [CrossRef]
- Anchukaitis, K.J.; D’Arrigo, R.D.; Andreu-Hayles, L.; Frank, D.; Verstege, A.; Curtis, A.; Buckley, B.M.; Jacoby, G.C.; Cook, E.R. Tree-ring-reconstructed summer temperatures from Northwestern North America during the last nine centuries. J. Clim. 2013, 26, 3001–3012. [Google Scholar] [CrossRef] [Green Version]
- Davi, N.K.; Jacoby, G.C.; Wiles, G.C. Boreal temperature variability inferred from maximum latewood density and tree-ring width data, Wrangell Mountain region, Alaska. Quat. Res. 2003, 60, 252–262. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Mashig, E.; Frank, D.; Jacoby, G.; Wilson, R. Reconstructed warm season temperatures for Nome, Seward Peninsula, Alaska. Geophys. Res. Lett. 2004, 31, L09202. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, G.C.; D’Arrigo, R.D. Tree-ring width and density evidence of climatic and potential forest change in Alaska. Glob. Biogeochem. Cycles 1995, 9, 227–234. [Google Scholar] [CrossRef]
- D’Arrigo, R.D.; Cook, E.R.; Jacoby, G.C. Annual to decadal-scale variations in northwest Atlantic sector temperatures inferred from Labrador tree rings. Can. J. For. Res. 1996, 26, 143–148. [Google Scholar] [CrossRef]
- Briffa, K.R.; Jones, P.D.; Schweingruber, F.H. Summer temperatures across northern North America: Regional reconstructions from 1760 using tree-ring densities. J. Geophys. Res. Earth Surf. 1994, 99, 25835–25844. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.; Wei, W.; Wang, L.; Yu, S.; Zhang, R.; Fan, Z.; Shang, H.; Zhang, T.; Li, Y. Tree ring density-based summer temperature reconstruction for Zajsan Lake area, East Kazakhstan. Int. J. Clim. 2011, 32, 1089–1097. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Briffa, K.R.; Jones, P.D. Yearly maps of summer temperatures in Western Eroupe from AD 1750 to 1975 and Western North America from 1600 to 1982: Results of a radiodensitometrical study on tree rings. Vegetatio 1991, 92, 5–71. [Google Scholar] [CrossRef]
- Briffa, K.R.; Jones, P.D.; Schweingruber, F.H. Tree-ring density reconstructions of summer temperature patterns across Western North America since 1600. J. Clim. 1992, 5, 735–754. [Google Scholar] [CrossRef] [Green Version]
- Wiles, G.C.; D’Arrigo, R.D.; Jacoby, G.C. Temperature changes along the Gulf of Alaska and the Pacific Northwest coast modeled from coastal tree rings. Can. J. For. Res. 1996, 26, 474–481. [Google Scholar] [CrossRef]
- Deng, G.; Li, M. Advances of study on the relationship between tree-ring density and climate and climate reconstruction. Prog. Geogr. 2021, 40, 343–356. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Chen, J.; Duan, J. Reconstructing mean maximum temperatures of May–August from tree-ring maximum density in North Da Hinggan Mountains, China. Chin. Sci. Bull. 2012, 57, 2007–2014. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, L.L.; Chen, J. Response of tree growth to climate change and reconstruction of summer temperature based on Korean larch. J. Earth Environ. 2012, 3, 889–899. [Google Scholar] [CrossRef]
- Yu, S.; Yuan, Y.; Wei, W.; Chen, F.; Zhang, T.; Shang, H.; Zhang, R.; Qing, L. A 352-year record of summer temperature reconstruction in the western Tianshan Mountains, China, as deduced from tree-ring density. Quat. Res. 2013, 80, 158–166. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, J. Precipitation and average monthly high temperature in the Jimusare, Xinjiang as reconstructed from tree density and tree width. Acta Meteorol. Sin. 1998, 56, 77–86. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Zhu, H.; Wu, P. Reconstructing mean maximum temperature of growing season from the maximum density of the Schrenk Spruce in Yili, Xinjiang, China. Chin. Sci. Bull. 2009, 54, 2300–2308. [Google Scholar] [CrossRef]
- Yin, H.; Liu, H.; Linderholm, H.; Sun, Y. Tree ring density-based warm-season temperature reconstruction since A.D. 1610 in the eastern Tibetan Plateau. Palaeogeogr. Palaeoclim. Palaeoecol. 2015, 426, 112–120. [Google Scholar] [CrossRef]
- Fan, Z.X.; Bräuning, A.; Yang, B.; Cao, K.F. Tree ring density-based summer temperature reconstruction for the central Hengduan Mountains in southern China. Glob. Planet. Chang. 2009, 65, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Duan, J.; Chen, J.; Huang, L.; Shao, X. Temperature reconstruction from tree-ring maximum density of Balfour spruce in eastern Tibet, China. Int. J. Clim. 2009, 30, 972–979. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, Q.B. A 449 year warm season temperature reconstruction in the southeastern Tibetan Plateau and its relation to solar activity. J. Geophys. Res. Atmos. 2014, 119, 11578–11592. [Google Scholar] [CrossRef]
- Xing, P.; Zhang, Q.B.; Lv, L.X. Absence of late-summer warming trend over the past two and half centuries on the eastern Tibetan Plateau. Glob. Planet. Chang. 2014, 123, 27–35. [Google Scholar] [CrossRef]
- Liang, H.; Lyu, L.; Wahab, M. A 382-year reconstruction of August mean minimum temperature from tree-ring maximum latewood density on the southeastern Tibetan Plateau, China. Dendrochronologia 2016, 37, 1–8. [Google Scholar] [CrossRef]
- Duan, J.; Li, L.; Ma, Z.; Chen, L. Post-industrial late summer warming recorded in tree-ring density in the eastern Tibetan Plateau. Int. J. Clim. 2019, 40, 795–804. [Google Scholar] [CrossRef]
- Yin, H.; Li, M.Y.; Huang, L. Summer mean temperature reconstruction based on tree-ring density over the past 440 years on the eastern Tibetan Plateau. Quat. Int. 2020, 571, 81–88. [Google Scholar] [CrossRef]
- Duan, J.P.; Wang, L.L.; Li, L.; Chen, K.L. Temperature variability since A.D. 1837 inferred from tree-ring maximum density of Abies fabric in Gongga Mountains, China. Chin. Sci. Bull. 2010, 55, 3015–3022. [Google Scholar] [CrossRef]
- Zhang, P.; Björklund, J.; Linderholm, H. The influence of elevational differences in absolute maximum density values on regional climate reconstructions. Trees 2015, 29, 1259–1271. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Okuhara, I.; Tokumitsu, Y.; Yasue, K. Responses to climate by tree-ring widths and maximum latewood densities of two Abies species at upper and lower altitudinal distribution limits in central Japan. Trees 2011, 25, 745–753. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, T.; Jiang, S.; Zhang, R.; Qin, L.; Shang, H.; Zhang, H. Tree-ring minimum density chronologies of Picea schrenkiana along an elevation gradient in the Tien Shan Mountains, China. Geogr. Ann. Ser. A Phys. Geogr. 2020, 102, 209–221. [Google Scholar] [CrossRef]
- Liang, E.; Shao, X.; Eckstein, D.; Huang, L.; Liu, X. Topography- and species-dependent growth responses of Sabina przewalskii and Picea crassifolia to climate on the northeast Tibetan Plateau. For. Ecol. Manag. 2006, 236, 268–277. [Google Scholar] [CrossRef]
- Di Filippo, A.; Baliva, M.; Brunetti, M.; Di Fiore, L. Long-term tree-ring response to drought and frost in two Pinus halepensis populations growing under contrasting environmental conditions in Peninsular Italy. Forests 2021, 12, 305. [Google Scholar] [CrossRef]
- Martínez-Sancho, E.; Slámová, L.; Morganti, S.; Grefen, C.; Carvalho, B.; Dauphin, B.; Rellstab, C.; Gugerli, F.; Opgenoorth, L.; Heer, K.; et al. The GenTree Dendroecological Collection, tree-ring and wood density data from seven tree species across Europe. Sci. Data 2020, 7, 1. [Google Scholar] [CrossRef]
- Pompa-García, M.; Hevia, A.; Camarero, J.J. Minimum and maximum wood density as proxies of water availability in two Mexican pine species coexisting in a seasonally dry area. Trees 2021, 35, 597–607. [Google Scholar] [CrossRef]
- Wang, L.L.; Shao, X.M.; Lei, H.; Liang, E.Y. Tree-ring characteristics of Larix Gmelinii and Pinus Sylvestris Var. Mongolica and their response to climate in Mohe, China. Chin. J. Plant Ecol. 2005, 29, 380–385. [Google Scholar] [CrossRef]
- Fan, Z.X.; Bräuning, A.; Cao, K.F.; Zhu, S.D. Growth-climate responses of high-elevation conifers in the central Hengduan Mountains, southwestern China. For. Ecol. Manag. 2009, 258, 306–313. [Google Scholar] [CrossRef]
- Ou, G.L.; Peng, M.C.; He, Z.R.; Wang, C.Y.; Wang, H.B.; Xiang, L. Quantitative classification of plant communities on the Northern part of Gaoligong Mountains by Twinspan. Plant Divers. 2008, 30, 679–687. [Google Scholar] [CrossRef]
- Fan, Z.X.; Bräuning, A.; Tian, Q.H.; Yang, B.; Cao, K.F. Tree ring recorded May–August temperature variations since A.D. 1585 in the Gaoligong Mountains, southeastern Tibetan Plateau. Palaeogeogr. Palaeoclim. Palaeoecol. 2010, 296, 94–102. [Google Scholar] [CrossRef]
- Zhao, F.; Fan, Z.; Su, T.; Li, S.; Tang, H.; Spicer, T.E.; Zhou, Z. Tree-ring δ18O inferred spring drought variability over the past 200 years in the Hengduan Mountains, Southwest China. Palaeogeogr. Palaeoclim. Palaeoecol. 2019, 518, 22–33. [Google Scholar] [CrossRef]
- Li, M.Y.; Wang, L.L.; Fan, Z.X.; Shen, C.C. Tree-ring density inferred late summer temperature variability over the past three centuries in the Gaoligong Mountains, southeastern Tibetan Plateau. Palaeogeogr. Palaeoclim. Palaeoecol. 2015, 422, 57–64. [Google Scholar] [CrossRef]
- Yang, R.Q.; Zhao, F.; Fan, Z.X.; Panthi, S.; Fu, P.-L.; Bräuning, A.; Grießinger, J.; Li, Z.S. Long-term growth trends of Abies delavayi and its physiological responses to a warming climate in the Cangshan Mountains, southwestern China. For. Ecol. Manag. 2021, 505, 119943. [Google Scholar] [CrossRef]
- Bräuning, A. Tree-ring evidence of ‘Little Ice Age’ glacier advances in southern Tibet. Holocene 2006, 16, 369–380. [Google Scholar] [CrossRef]
- Jacoby, G.C.; Ivanciu, I.S.; Ulan, L.D. A 263-year record of summer temperature for northern Quebec reconstructed from tree-ring data and evidence of a major climatic shift in the early 1800’s. Palaeogeogr. Palaeoclim. Palaeoecol. 1988, 64, 69–78. [Google Scholar] [CrossRef]
- Sun, L.; Cai, Y.; Zhou, Y.; Shi, S.; Zhao, Y.; Gunnarson, B.E.; Jaramillo, F. Radial growth responses to climate of Pinus yunnanensis at low elevations of the Hengduan Mountains, China. Forests 2020, 11, 1066. [Google Scholar] [CrossRef]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef] [Green Version]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology, 1st ed.; Springer: Dordrecht, The Netherlands, 1990; pp. 40–61. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 51–67. [Google Scholar]
- Cook, E.R. A time series analysis approach to tree ring standardization. Ph.D. Dissertation, The University of Arizona, Tucson, AZ, USA, 5 August 1985. [Google Scholar]
- Cook, E.R.; Briffa, K.; Meko, D.M.; Graybill, D.A.; Funkhouser, G. The ‘segment length curse’ in long tree-ring chronology development for palaeoclimatic studies. Holocene 1995, 5, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Li, M.Q.; Huang, L.; Yin, Z.Y.; Shao, X.M. Temperature reconstruction and volcanic eruption signal from tree-ring width and maximum latewood density over the past 304 years in the southeastern Tibetan Plateau. Int. J. Biometeorol. 2017, 61, 2021–2032. [Google Scholar] [CrossRef]
- Meng, X.L.; Rosenthal, R.; Rubin, D.B. Comparing correlated correlation coefficients. Psychol. Bull. 1992, 111, 172–175. [Google Scholar] [CrossRef]
- Björklund, J.; Seftigen, K.; Fonti, P.; Nievergelt, D.; von Arx, G. Dendroclimatic potential of dendroanatomy in temperature-sensitive Pinus sylvestris. Dendrochronologia 2020, 60, 125673. [Google Scholar] [CrossRef]
- Heeter, K.J.; Harley, G.L.; Maxwell, J.T.; McGee, J.H.; Matheus, T.J. Late summer temperature variability for the Southern Rocky Mountains (USA) since 1735 CE: Applying blue light intensity to low-latitude Picea engelmannii Parry ex Engelm. Clim. Chang. 2020, 162, 965–988. [Google Scholar] [CrossRef]
- Björklund, J.; Seftigen, K.; Schweingruber, F.; Fonti, P.; Arx, G.; Bryukhanova, M.V.; Cuny, H.E.; Carrer, M.; Castagneri, D.; Frank, D.C. Cell size and wall dimensions drive distinct variability of earlywood and latewood density in Northern Hemisphere conifers. New Phytol. 2017, 216, 728–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Liu, X. Plant Physiology, 2nd ed.; Science Press: Beijing, China, 2011; p. 128. [Google Scholar]
- Büntgen, U.; Krusic, P.J.; Verstege, A.; Barreda, G.S.; Wagner, S.; Camarero, J.J.; Ljungqvist, F.C.; Zorita, E.; Oppenheimer, C.; Konter, O.; et al. New Tree-ring evidence from the Pyrenees reveals Western Mediterranean climate variability since Medieval Times. J. Clim. 2017, 30, 5295–5318. [Google Scholar] [CrossRef]
- Nagavciuc, V.; Roibu, C.-C.; Ionita, M.; Mursa, A.; Cotos, M.-G.; Popa, I. Different climate response of three tree ring proxies of Pinus sylvestris from the Eastern Carpathians, Romania. Dendrochronologia 2019, 54, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Camarero, J.J.; Rozas, V.; Olano, J.M. Minimum wood density of Juniperus thurifera is a robust proxy of spring water availability in a continental Mediterranean climate. J. Biogeogr. 2014, 41, 1105–1114. [Google Scholar] [CrossRef]
- Carrer, M.; Nola, P.; Eduard, J.L.; Motta, R.; Urbinati, C. Regional variability of climate-growth relationships in Pinus cembra high elevation forests in the Alps. J. Ecol. 2007, 95, 1072–1083. [Google Scholar] [CrossRef]
- Sevanto, S.; Suni, T.; Pumpanen, J.; Grönholm, T.; Kolari, P.; Nikinmaa, E.; Hari, P.; Vesala, T. Wintertime photosynthesis and water uptake in a boreal forest. Tree Physiol. 2006, 26, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Blanco, N.E.; Hermida-Carrera, C.; Lehotai, N.; Hurry, V.; Strand, Å. Two dominant boreal conifers use contrasting mechanisms to reactivate photosynthesis in the spring. Nat. Commun. 2020, 11, 128. [Google Scholar] [CrossRef]
- Larysch, E.; Stangler, D.F.; Nazari, M.; Seifert, T.; Kahle, H.-P. Xylem Phenology and Growth Response of European Beech, Silver Fir and Scots Pine along an Elevational Gradient during the Extreme Drought Year 2018. Forests 2021, 12, 75. [Google Scholar] [CrossRef]





| Sites | Latitude/Longitude | Elevation, m a.s.l. | Species | Time Span | C/T1 | C/T2 |
|---|---|---|---|---|---|---|
| Sampling site | 98.477° E/27.786° N | 3200–3300 | L. speciosa | 1422–2019 | 96/50 | 51/26 |
| A. delavayi | 1573–2019 | 108/55 | 51/27 | |||
| Gongshan | 98.667° E/27.750° N | 1583.3 | 1958–2019 | |||
| Deqin | 98.917° E/28.483° N | 3319.0 | 1958–2019 | |||
| Weixi | 99.283° E/27.167° N | 2326.1 | 1958–2019 | |||
| Grid set1 | 98.250° E/27.750° N | 1951–2019 | ||||
| Grid set2 | 98.750° E/27.750° N | 1951–2019 |
| Chronology | LarixSTD | AbiesSTD | LarixRES | AbiesRES | CompositeSTD | CompositeRES |
|---|---|---|---|---|---|---|
| Time span | 1501–2019 A.D. | 1573–2019 A.D. | 1501–2019 A.D. | 1573–2019 A.D. | 1501–2019 A.D. | 1501–2019 A.D. |
| Mean length | 215 | 216 | 215 | 216 | 216 | 216 |
| Trees/Cores | 26/51 | 27/51 | 26/51 | 27/51 | 53/102 | 53/102 |
| AC1 | 0.459 | 0.293 | −0.095 | −0.212 | 0.354 | −0.176 |
| Rbt | 0.230 | 0.191 | 0.302 | 0.296 | 0.133 | 0.223 |
| Mean EPS | 0.853 | 0.888 | 0.892 | 0.933 | 0.877 | 0.929 |
| Year/CEPS>0.85 | 1638/18 | 1732/23 | 1580/13 | 1678/13 | 1669/35 | 1637/20 |
| SNR | 5.813 | 7.935 | 8.227 | 13.929 | 7.147 | 13.061 |
| MS | 0.036 | 0.042 | 0.044 | 0.050 | 0.035 | 0.042 |
| PC1 | 29.3% | 24.4% | 35.4% | 33.1% | 17.2% | 25.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, G.; Li, M.; Hao, Z.; Shao, X. Responses to Climate Change of Maximum Latewood Density from Larix speciosa Cheng et Law and Abies delavayi Franch. in the Northwest of Yunnan Province, China. Forests 2022, 13, 720. https://doi.org/10.3390/f13050720
Deng G, Li M, Hao Z, Shao X. Responses to Climate Change of Maximum Latewood Density from Larix speciosa Cheng et Law and Abies delavayi Franch. in the Northwest of Yunnan Province, China. Forests. 2022; 13(5):720. https://doi.org/10.3390/f13050720
Chicago/Turabian StyleDeng, Guofu, Mingqi Li, Zhixin Hao, and Xuemei Shao. 2022. "Responses to Climate Change of Maximum Latewood Density from Larix speciosa Cheng et Law and Abies delavayi Franch. in the Northwest of Yunnan Province, China" Forests 13, no. 5: 720. https://doi.org/10.3390/f13050720






