Effects of Spatial Variability and Drainage on Extracellular Enzyme Activity in Coastal Freshwater Forested Wetlands of Eastern North Carolina, USA
Abstract
:1. Introduction
2. Methods
2.1. Site Description
2.2. Soil Sample Collection and Processing
2.3. Soil pH, Volumetric Water Content, Eh, and Percent Total Roots
2.4. Soil Organic Carbon and Soil Microbial Biomass
2.5. Extracellular Enzyme Activity and Specific Enzyme Activity Calculations
2.6. Statistical Analysis
3. Results
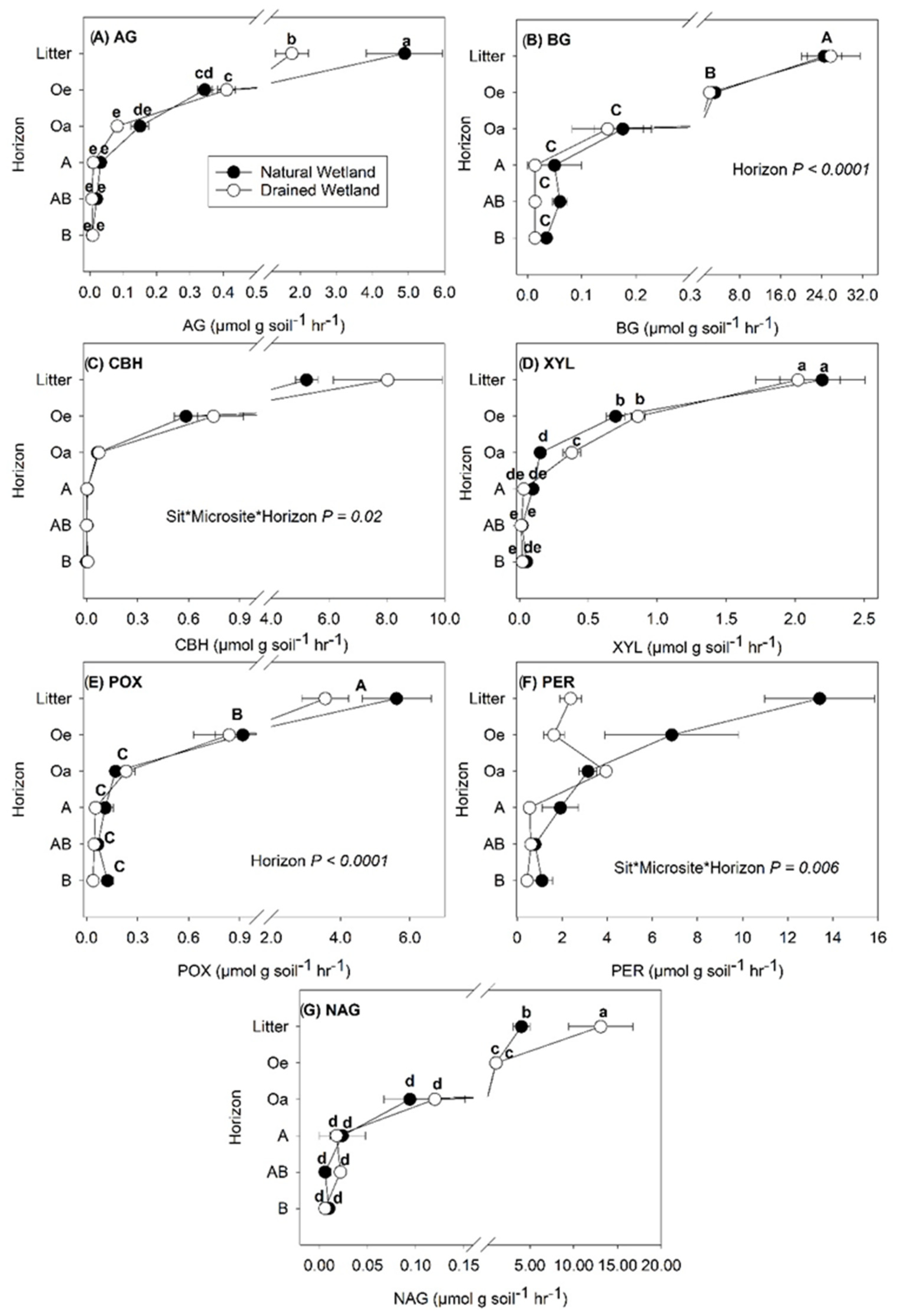
3.1. The Enzyme Activity in Different Soil Horizons and Microsites
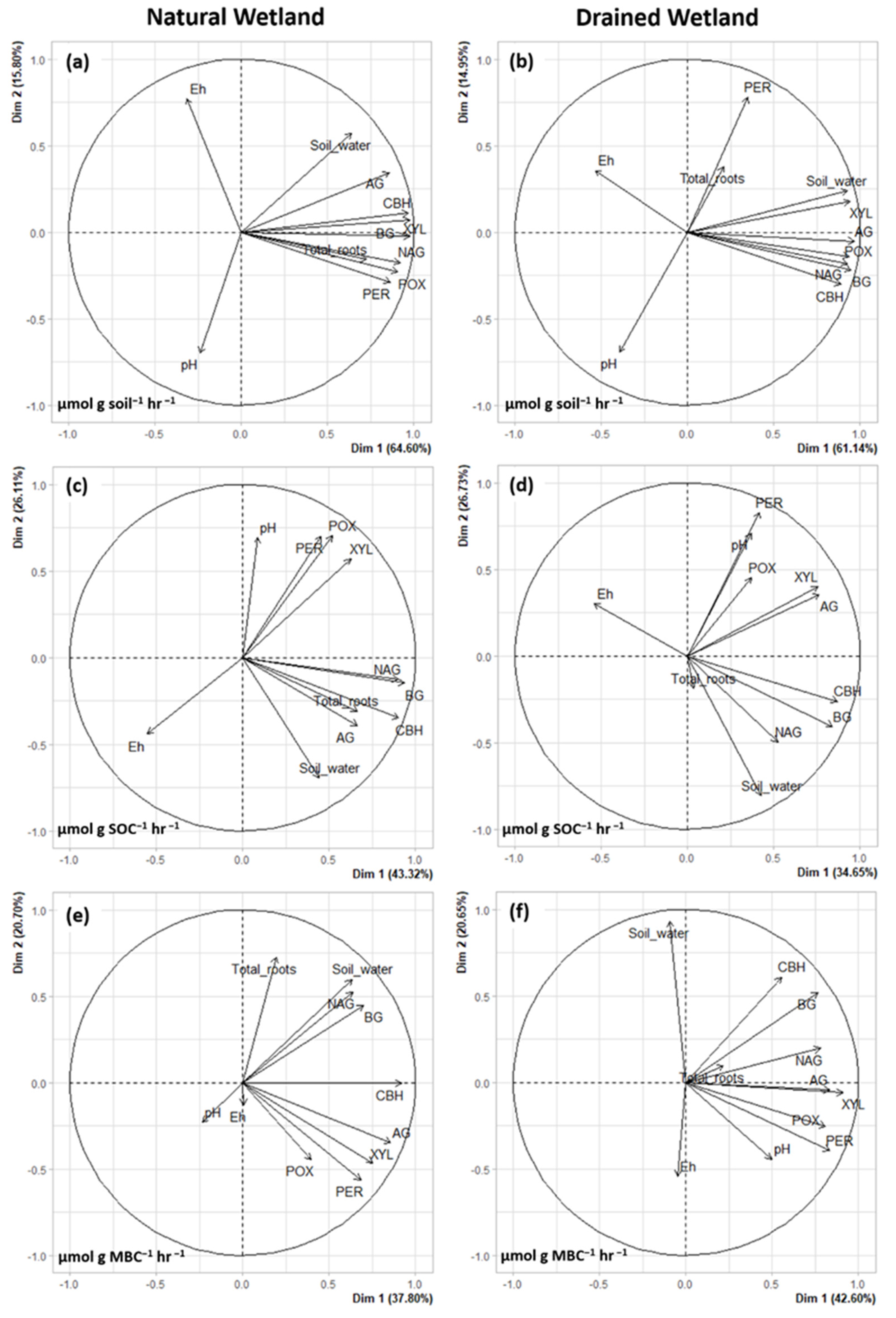
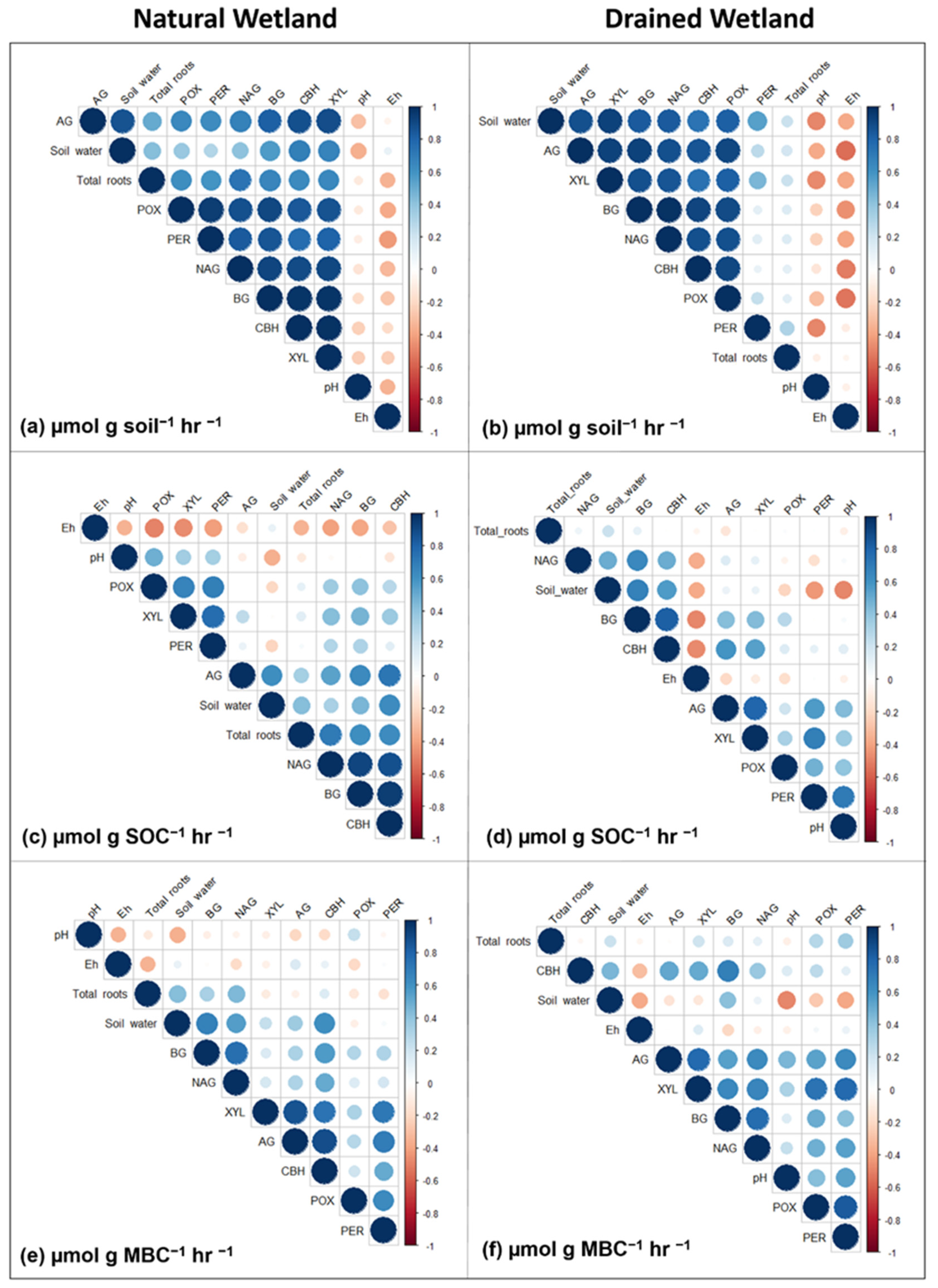
3.2. Environmental Effects on Enzyme Activity
4. Discussion
4.1. The Enzyme Activity as Affected by Soil Horizons and Microsites
4.2. Environmental Effects on Enzyme Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinsabaugh, R.S. Enzymic analysis of microbial pattern and process. Biol. Fertil. Soils 1994, 17, 69–74. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Moorhead, D.L. Resource allocation to extracellular enzyme production: A model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Burns, R.G. Ecology of extracellular enzyme activities and organic matter degradation in soil: A complex community-driven process. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 2011. [Google Scholar]
- Freeman, C.; Liska, G.; Ostle, N.; Lock, M.; Reynolds, B.; Hudson, J. Microbial activity and enzymic decomposition processes following peatland water table drawdown. Plant Soil 1996, 180, 121–127. [Google Scholar] [CrossRef]
- Freeman, C.; Ostle, N.J.; Fenner, N.; Kang, H. A regulatory role for phenol oxidase during decomposition in peatlands. Soil Biol. Biochem. 2004, 36, 1663–1667. [Google Scholar] [CrossRef]
- Minick, K.J.; Kelley, A.M.; Miao, G.; Li, X.; Noormets, A.; Mitra, B.; King, J.S. Microtopography alters hydrology, phenol oxidase activity and nutrient availability in organic soils of a coastal freshwater forested wetland. Wetlands 2019, 39, 263–273. [Google Scholar] [CrossRef]
- Angle, J.C.; Morin, T.H.; Solden, L.M.; Narrowe, A.B.; Smith, G.J.; Borton, M.A.; Rey-Sanchez, C.; Daly, R.A.; Mirfenderesgi, G.; Hoyt, D.W.; et al. Methanogenesis in oxygenated soils is a substantial fraction of wetland methane emissions. Nat. Commun. 2017, 8, 1567. [Google Scholar] [CrossRef] [Green Version]
- Teh, Y.A.; Silver, W.L.; Conrad, M.E. Oxygen effects on methane production and oxidation in humid tropical forest soils. Glob. Change Biol. 2005, 11, 1283–1297. [Google Scholar] [CrossRef]
- Minick, K.J.; Mitra, B.; Noormets, A.; King, J.S. Saltwater reduces potential CO2 and CH4 production in peat soils from a coastal freshwater forested wetland. Biogeosciences 2019, 16, 4671–4686. [Google Scholar] [CrossRef] [Green Version]
- Dahl, T.E. Status and Trends of Wetlands in the Conterminous United States 2004–2009; U.S. Department of Interior, Fish and Wildlife Service: Washington, DC, USA, 2011.
- Laine, J.; Minkkinen, K. Effect of forest drainage on the carbon balance of a mire: A case study. Scand. J. For. Res. 1996, 1, 307–312. [Google Scholar] [CrossRef]
- Trettin, C.C.; Jurgensen, M.F. Carbon cycling in wetland forest soils. In The Potential of U.S. Forest Soils to Sequester Carbon and Mitigate the Greenhouse Effect; Kimble, J.M., Heath, L.S., Birdsey, R.A., Lal, R., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 311–331. [Google Scholar]
- Minkkinen, K.; Laine, J. Long-term effect of forest drainage on the peat carbon stores of pine mires in Finland. Can. J. For. Res. 1998, 28, 1267–1275. [Google Scholar] [CrossRef]
- Toberman, H.; Freeman, C.; Artz, R.; Evans, C.; Fenner, N. Impeded drainage stimulates extracellular phenol oxidase activity in riparian peat cores. Soil Use Manag. 2008, 24, 357–365. [Google Scholar] [CrossRef]
- Toberman, H.; Laiho, R.; Evans, C.D.; Artz, R.R.E.; Fenner, N.; Straková, P.; Freeman, C. Long-term drainage for forestry inhibits extracellular phenol oxidase activity in Finnish boreal mire peat. Eur. J. Soil Sci. 2010, 61, 950–957. [Google Scholar] [CrossRef]
- Minick, K.J.; Mitra, B.; Li, X.; Noormets, A.; King, J.S. Water table drawdown alters soil and microbial carbon pool size and isotope composition in coastal freshwater forested wetlands. Front. For. Glob. Change 2019, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Qualls, R.G.; Haines, B.L. The influence of humic substances on the aerobic decomposition of submerged leaf litter. Hydrobiologia 1990, 206, 133–138. [Google Scholar] [CrossRef]
- Freeman, C.; Evans, C.; Monteith, D.; Reynolds, B.; Fenner, N. An enzymic ‘latch’ on a global carbon store. Nature 2001, 409, 149–150. [Google Scholar] [CrossRef]
- Wang, H.; Richardson, C.J.; Ho, M. Dual controls on carbon loss during drought in peatlands. Nat. Clim. Change 2015, 5, 584–587. [Google Scholar] [CrossRef]
- Allison, S.D. Soil minerals and humic acids alter enzyme stability: Implications for ecosystem processes. Biogeochemistry 2006, 81, 361–373. [Google Scholar] [CrossRef]
- Belyea, L.R.; Clymo, R.S. Feedback control of the rate of peat formation. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 1315–1321. [Google Scholar] [CrossRef] [Green Version]
- Jauhiainen, J.; Takahashi, H.; Heikkinen, J.E.P.; Martikainen, P.J.; Vasander, H. Carbon fluxes from a tropical peat swamp forest floor. Glob. Change Biol. 2005, 11, 1788–1797. [Google Scholar] [CrossRef]
- Li, X.; Minick, K.J.; Luff, J.; Noormets, A.; Miao, G.; Mitra, B.; Sun, G.; Domec, J.C.; McNulty, S.; King, J.S. Effects of Microtopography on Absorptive and Transport Fine Root Biomass, Necromass, Production, Mortality and Decomposition in a Coastal Freshwater Forested Wetland, Southeastern USA. Ecosystems 2020, 23, 1294–1308. [Google Scholar] [CrossRef]
- Miao, G.; Noormets, A.; Domec, J.-C.; Fuentes, M.; Trettin, C.C.; Sun, G.; McNulty, S.; King, J.S. Hydrology and microtopography control carbon dynamics in wetlands: Implications in partitioning ecosystem respiration in a coastal plain forested wetland. Agric. For. Meteorol. 2017, 247, 343–355. [Google Scholar] [CrossRef]
- Jones, R.H.; Lockaby, B.G.; Somers, G.L. Effects of microtopography and disturbance on fine-root dynamics in wetland forests of low-order stream floodplains. Am. Midl. Nat. 1996, 136, 57–71. [Google Scholar] [CrossRef]
- Duberstein, J.A.; Conner, W.H. Use of hummocks and hollows by trees in tidal freshwater forested wetlands along the Savannah River. For. Ecol. Manag. 2009, 258, 1613–1618. [Google Scholar] [CrossRef]
- Minick, K.J.; Leggett, Z.H.; Sucre, E.B.; Fox, T.R.; Strahm, B.D. Soil and aggregate-associated carbon in a young loblolly pine plantation: Influence of bioenergy intercropping. Soil Sci. 2017, 182, 233–240. [Google Scholar] [CrossRef]
- Strickland, M.; Leggett, Z.H.; Sucre, E.B.; Bradford, M.A. Biofuel intercropping effects on soil carbon and microbial activity. Ecol. Appl. 2015, 25, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Minick, K.J.; Strahm, B.D.; Fox, T.R.; Sucre, E.B.; Leggett, Z.H. Microbial nitrogen cycling response to forest-based bioenergy production. Ecol. Appl. 2015, 25, 2366–2381. [Google Scholar] [CrossRef]
- Fox, T.R.; Allen, H.L.; Albaugh, T.J.; Rubilar, R.; Carlson, C.A. The development of pine plantation silviculture in the southern United States. J. For. 2007, 105, 337–347. [Google Scholar]
- Blume, E.; Bischoff, M.; Reichert, J.M.; Moorman, T.; Konopka, A.; Turco, R. Surface and subsurface microbial biomass, community structure and metabolic activity as a function of soil depth and season. Appl. Soil Ecol. 2002, 20, 171–181. [Google Scholar] [CrossRef]
- Stone, M.M.; DeForest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Webster, K.L.; Creed, I.F.; Malakoff, T.; Delaney, K. Potential vulnerability of deep carbon deposits of forested swamps to drought. Soil Sci. Soc. Am. J. 2014, 78, 1097–1107. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leiros, M.C.; Gil-Sotres, F. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem. 2008, 40, 2146e2155. [Google Scholar] [CrossRef] [Green Version]
- Jackson, C.R.; Liew, K.C.; Yule, C.M. Structural and functional changes with depth in microbial communities in a tropical Malaysian peat swamp forest. Microb. Ecol. 2009, 57, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Romanowicz, K.J.; Kane, E.S.; Potvin, L.R.; Daniels, A.L.; Kolka, R.K.; Lilleskov, E.A. Understanding drivers of peatland extracellular enzyme activity in the PEATcosm experiment: Mixed evidence for enzymic latch hypothesis. Plant Soil 2015, 397, 371–386. [Google Scholar] [CrossRef]
- Pinsonneault, A.J.; Moore, T.R.; Roulet, N.T. Temperature the dominant control on the enzyme-latch across a range of temperate peatland types. Soil Biol. Biochem. 2016, 97, 121–130. [Google Scholar] [CrossRef]
- DeBusk, W.F.; Reddy, K.R. Turnover of detrital organic carbon in a nutrient-impacted Everglades marsh. Soil Sci. Soc. Am. J. 1998, 62, 1460–1468. [Google Scholar] [CrossRef]
- Allen, T.; Wang, Y.; Gore, B.; Swords, J.; Newcomb, D. Coastal Wetland mapping Using Time Series SAR Imagery and LiDAR: Alligator River National Wildlife Refuge, North Carolina. In Proceedings of the Pecora 18 Symposium, Herndon, VA, USA, 14–15 November 2011; pp. 14–17. [Google Scholar]
- Aguilos, M.; Mitra, B.; Noormets, A.; Minick, K.J.; Prajapati, P.; Gavazzi, M.; Sun, G.; McNulty, S.; Li, X.; King, J.S. Long-term carbon fluxes and balance in managed and natural coastal forested wetlands of the Southeastern USA. Agric. For. Meteorol. 2020, 288–289, 108022. [Google Scholar] [CrossRef]
- Riggs, S.R. Sediment evolution and habitat function of organic-rich muds within the Albemarle estuarine system, North Carolina. Estuaries 1996, 19, 169–185. [Google Scholar] [CrossRef]
- Minick, K.J.; Strahm, B.D.; Fox, T.R.; Sucre, E.B.; Leggett, Z.H.; Zerpa, J. Switchgrass intercropping reduces soil inorganic N in a young loblolly pine plantation located in coastal North Carolina. For. Ecol. Manag. 2014, 319, 161–168. [Google Scholar] [CrossRef]
- USDA Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Official Soil Series Descriptions. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/B/BELHAVEN.html (accessed on 8 August 2019).
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass-C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Antibus, R.; Linkins, A.; McClaugherty, C.; Rayburn, L.; Repert, D.; Weiland, T. Wood decomposition: Nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 1993, 74, 1586–1593. [Google Scholar] [CrossRef]
- Sinsabaugh, R.; Antibus, R.; Linkins, A.; McClaugherty, C.; Rayburn, L.; Repert, D.; Weiland, T. Wood decomposition over a first-order watershed: Mass loss as a function of lignocellulase activity. Soil Biol. Biochem. 1992, 24, 743–749. [Google Scholar] [CrossRef]
- Johnson, M.G.; Kern, J.S. Quantifying the organic carbon held in forested soils of the United States and Puerto Rico, chapter 4. In The Potential of U.S. Forest Soils to Sequester Carbon and Mitigate the Greenhouse Effect; Kimble, J.S., Ed.; CRC Press LLC.: Boca Raton, FL, USA, 2003. [Google Scholar]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, E.M.; Berrier, D.J.; Neubauer, S.C.; Franklin, R.B. Using microbial communities and extracellular enzymes to link soil organic matter characteristics to greenhouse gas production in a tidal freshwater wetland. Biogeochemistry 2014, 117, 473–490. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Jicha, T.M.; Kolka, R.K.; Lehto, L.L.; Sebestyen, S.D.; Seifert-Monson, L.R. Ecoenzymatic stoichiometry and microbial processing of organic matter in northern bogs and fens reveals a common P-limitation between peatland types. Biogeochemistry 2014, 120, 203–224. [Google Scholar] [CrossRef]
- Steinweg, J.M.; Kostka, J.E.; Hanson, P.J.; Schadt, C.W. Temperature sensitivity of extracellular enzymes differs with peat depth but not with season in an ombrotrophic bog. Soil Biol. Biochem. 2018, 125, 244–250. [Google Scholar] [CrossRef]
- Kramer, S.; Marhan, S.; Haslwimmer, H.; Ruess, L.; Kandeler, E. Temporal variation in surface and subsoil abundance and function of the soil microbial community in an arable soil. Soil Biol. Biochem. 2013, 61, 76–85. [Google Scholar] [CrossRef]
- Šnajdr, J.; Valášková, V.; Merhautová, V.; Herinková, J.; Cajthaml, T.; Baldrian, P. Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol. Biochem. 2008, 40, 2068–2075. [Google Scholar] [CrossRef]
- Gelsomino, A.; Azzellino, A. Multivariate analysis of soils: Microbial biomass, metabolic activity, and bacterial-community structure and their relationships with soil depth and type. J. Plant Nutr. Soil Sci. 2011, 174, 381–394. [Google Scholar] [CrossRef]
- Taylor, J.P.; Wilson, B.; Mills, M.S.; Burns, R.G. Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biol. Biochem. 2002, 34, 387–401. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Inglett, K.; Inglett, P.; Reddy, K.; Osborne, T. Temperature sensitivity of greenhouse gas production in wetland soils of different vegetation. Biogeochemistry 2012, 108, 77–90. [Google Scholar] [CrossRef]
- Silvola, J.; Alm, J.; Ahlholm, U.; Nykanen, H.; Martikainen, P.J. CO2 fluxes from peat in boreal mires under varying temperature and moisture conditions. J. Ecol. 1996, 84, 219–228. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Melillo, J.M.; Aber, J.D.; Linkins, A.E.; Ricca, A.; Fry, B.; Nadelhoffer, K.J. Carbon and nitrogen dynamics along the decay continuum: Plant litter to soil organic matter. Plant Soil 1989, 115, 189–198. [Google Scholar] [CrossRef]
- MacLean, D.A.; Wein, R.W. Litter production and forest floor nutrient dynamics in pine and hardwood stands of New Brunswick, Canada. Ecography 1978, 1, 1–15. [Google Scholar] [CrossRef]
- Johansson, M.B. The chemical composition of needle and leaf litter from Scots pine, Norway spruce and white birch in Scandinavian forests. For. Int. J. For. Res. 1995, 68, 49–62. [Google Scholar] [CrossRef]
- Osono, T.; Azuma, J.I.; Hirose, D. Plant species effect on the decomposition and chemical changes of leaf litter in grassland and pine and oak forest soils. Plant Soil 2014, 376, 411–421. [Google Scholar] [CrossRef]
- Mitra, B.; Miao, G.; Minick, K.J.; McNulty, S.; Sun, G.; Gavazzi, M.; King, J.S.; Noormets, A. Disentangling the effects of temperature, moisture and substrate availability on soil CO2 efflux. Biogeosciences 2019, 124, 2060–2075. [Google Scholar] [CrossRef]
- Mitra, B.; Minick, K.J.; Miao, G.; Domec, J.C.; Prajapati, P.; McNulty, S.; Sun, G.; King, J.S.; Noormets, A. Spectral evidence for substrate availability rather than environmental control of methane emissions from a coastal forested wetland. Agric. For. Meteorol. 2020, 291, 108062. [Google Scholar] [CrossRef]
- Karegar, M.A.; Dixon, T.H.; Malservisi, R.; Kusche, J.; Engelhart, S.E. Nuisance flooding and relative sea-level rise: The importance of present-day land motion. Sci. Rep. 2017, 7, 1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallenger, A.H.; Doran, K.S.; Howd, P.A. Hotspot of accelerated sea-level rise on the Atlantic coast of North America. Nat. Clim. Change 2012, 2, 884. [Google Scholar] [CrossRef]


| pH | Soil Water Content (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Horizon | Natural Wetland | Drained Wetland | Natural Wetland | Drained Wetland | ||||
| Hummock | Hollow | Bed | Interbed | Hummock | Hollow | Bed | Interbed | |
| Oi | 4.8 ± 0.1 Ab | 4.3 ± 0.1 Bb | 5.6 ± 0.3 Aa | 5.6 ± 0.2 Aa | 67 ± 3 Bb | 90 ± 1 Aa | 48 ± 5 BCc | 55 ± 5 BCc |
| Oe | 4.2 ± 0.1 Ba | 4.3 ± 0.1 Ba | 4.2 ± 0.2 DCEa | 4.5 ± 0.2 CDEa | 85 ± 1 Ab | 90 ± 1 Aa | 66 ± 2 Ad | 78 ± 2 Ac |
| Oa1 | 4.4 ± 0.1 ABa | 4.4 ± 0.1 Ba | 4.0 ± 0.1 Eb | 4.3 ± 0.1 Ea | 84 ± 4 Aa | 86 ± 2 Aa | 61 ± 1 Ab | 64 ± 3 Bb |
| Oa2 | 4.3 ± 0.1 ABa | 4.4 ± 0.2 ABa | 4.2 ± 0.1 DEa | 4.4 ± 0.1 DEa | 78 ± 6 Aa | 74 ± 5 Ba | 60 ± 3 Ab | 61 ± 1 BCb |
| Oa3 | 4.4 ± 0.1 ABb | 4.4 ± 0.2 Bb | 4.6 ± 0.2 Cab | 4.7 ± 0.0 BCa | 74 ± 5 ABa | 71 ± 6 BCa | 45 ± 5 Cb | 50 ± 5 Cb |
| A | 4.4 ± 0.2 ABa | 4.4 ± 0.2 ABa | 4.5 ± 0.2 DCa | 4.7 ± 0.1 BCDa | 60 ± 4 BCa | 59 ± 5 Ca | 31 ± 3 Db | 29 ± 3 Db |
| AB | 4.5 ± 0.3 ABa | 4.5 ± 0.3 ABa | 5.0 ± 0.1 Ba | 5.0 ± 0.1 ABa | 52 ± 6 Ca | 45 ± 4 Da | 20 ± 2 DEb | 24 ± 3 DEb |
| B | 4.7 ± 0.2 ABb | 4.9 ± 0.3 Aab | 5.4 ± 0.2 ABa | 5.4 ± 0.2 Aa | 36 ± 1 Da | 32 ± 1 Ea | 21 ± 1 Eb | 20 ± 1 Eb |
| Source | AG | BG | CBH | XYL | POX | PER | NAG |
|---|---|---|---|---|---|---|---|
| Site | 16.1 *** | 1.2 | 2.42 | 0.7 | 1.5 | 45.5 *** | 14.6 *** |
| Microsite | 1.8 | 0.3 | 0.0 | 0.4 | 1.0 | 0.0 | 1.8 |
| Horizon | 194.3 *** | 394.2 *** | 276.6 *** | 235.3 *** | 102.2 *** | 49.6 *** | 198.2 *** |
| Site * Microsite | 0.1 | 1.2 | 5.6 * | 1.1 | 0.8 | 0.0 | 3.6 |
| Site * Horizon | 15.3 *** | 0.3 | 1.4 | 2.8 * | 1.0 | 10.8 *** | 12.4 *** |
| Microsite * Horizon | 1.4 | 0.8 | 0.2 | 0.4 | 0.7 | 2.5 * | 0.5 |
| Site * Microsite * Horizon | 0.3 | 0.4 | 2.8 * | 1.5 | 0.5 | 3.5 ** | 1.4 |
| Source | AG | BG | CBH | XYL | POX | PER | NAG |
|---|---|---|---|---|---|---|---|
| Site | 0.8 | 0.1 | 10.2 ** | 5.8 ** | 0.4 | 0.6 | 23.3 *** |
| Microsite | 0.8 | 0.1 | 0.1 | 0.4 | 0.8 | 0.0 | 1.0 |
| Horizon | 106.7 *** | 216.1 *** | 249.8 *** | 41.1 *** | 28.8 *** | 16.1 *** | 107.7 *** |
| Site * Microsite | 0.2 | 0.5 | 4.6 * | 0.1 | 0.2 | 0.1 | 6.5 * |
| Site * Horizon | 13.3 *** | 0.3 | 2.3 * | 2.3 * | 1.1 | 12.4 *** | 5.4 ** |
| Microsite * Horizon | 0.8 | 0.7 | 0.2 | 0.3 | 0.3 | 1.1 | 0.8 |
| Site * Microsite * Horizon | 0.3 | 0.5 | 2.1 | 0.8 | 0.5 | 1.8 | 0.4 |
| Source | AG | BG | CBH | XYL | POX | PER | NAG |
|---|---|---|---|---|---|---|---|
| Site | 2.8 | 0.0 | 2.8 | 0.7 | 0.5 | 6.2 ** | 15.2 *** |
| Microsite | 1.0 | 0.0 | 0.9 | 0.2 | 0.0 | 0.5 | 0.1 |
| Horizon | 11.2 *** | 54.8 *** | 71.4 *** | 3.0 * | 4.3 ** | 32.0 *** | 23.3 *** |
| Site * Microsite | 1.2 | 0.1 | 0.5 | 0.9 | 0.0 | 0.8 | 1.7 |
| Site * Horizon | 10.3 *** | 5.0 ** | 4.2 ** | 4.4 ** | 3.5 ** | 11.5 *** | 4.5 ** |
| Microsite * Horizon | 1.7 | 1.2 | 0.9 | 1.6 | 0.9 | 2.9 * | 0.9 |
| Site * Microsite * Horizon | 0.7 | 0.4 | 1.3 | 0.2 | 0.4 | 1.5 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minick, K.J.; Aguilos, M.; Li, X.; Mitra, B.; Prajapati, P.; King, J.S. Effects of Spatial Variability and Drainage on Extracellular Enzyme Activity in Coastal Freshwater Forested Wetlands of Eastern North Carolina, USA. Forests 2022, 13, 861. https://doi.org/10.3390/f13060861
Minick KJ, Aguilos M, Li X, Mitra B, Prajapati P, King JS. Effects of Spatial Variability and Drainage on Extracellular Enzyme Activity in Coastal Freshwater Forested Wetlands of Eastern North Carolina, USA. Forests. 2022; 13(6):861. https://doi.org/10.3390/f13060861
Chicago/Turabian StyleMinick, Kevan J., Maricar Aguilos, Xuefeng Li, Bhaskar Mitra, Prajaya Prajapati, and John S. King. 2022. "Effects of Spatial Variability and Drainage on Extracellular Enzyme Activity in Coastal Freshwater Forested Wetlands of Eastern North Carolina, USA" Forests 13, no. 6: 861. https://doi.org/10.3390/f13060861







