Reduced Rainfall Variability Reduces Growth of Nothofagus alessandrii Espinosa (Nothofagaceae) in the Maule Region, Chile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling and Characteristics of N. alessandrii Populations
2.2. N. alessandrii Tree-Ring Chronology
2.3. Relationships between Local Climatic Variability and Radial Growth
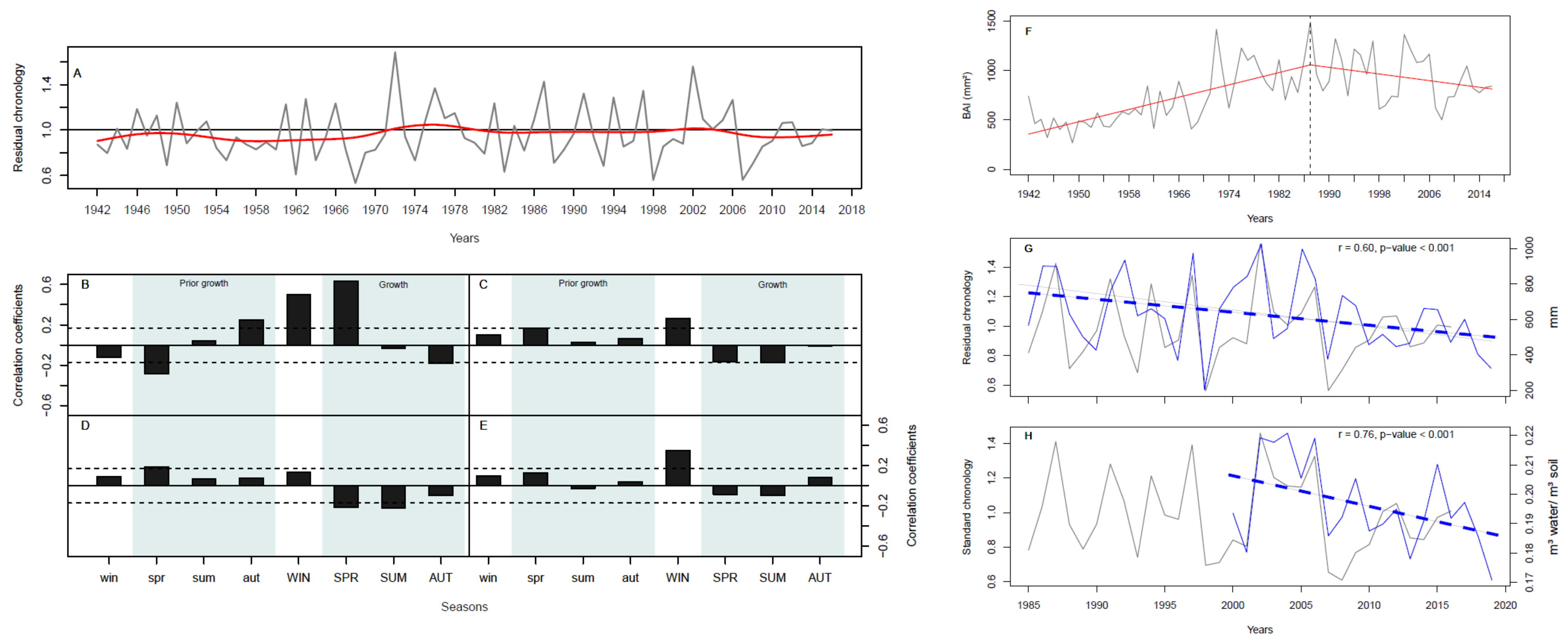
3. Results
3.1. Ring Width Chronology
3.2. Relationship of Radial Growth to the Local Climate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barstow, M.; Echeverría, C.; Baldwin, H.; Rivers, M.C.; Nothofagus alessandrii. The IUCN Red List of Threatened Species 2017: E.T32033A2808995. Available online: http://www.iucnredlist.org/details/32033/0 (accessed on 30 March 2018).
- Benoit, I. Libro Rojo de la Flora Terrestre de Chile; Corporación Nacional Forestal: Santiago, Chile, 1989; p. 157. [Google Scholar]
- Santelices, R.; Drake, F.; Mena, C.; Ordenes, R.; Navarro-Cerrillo, R.M. Current and potential distribution areas for Nothofagus alessandrii, an endangered tree species from central Chile. Cienc. Investig. Agrar. 2012, 39, 521–531. [Google Scholar] [CrossRef] [Green Version]
- Monaghan, J. Chile, Perú, and the California Gold Rush of 1849; University of California Press: Berkeley, CA, USA, 1973; p. 312. [Google Scholar]
- Bustamante, R.O.; Castor, C. The decline of an endangered temperate ecosystem: The ruil (Nothofagus alessandrii) forest in central Chile. Biodivers. Conserv. 1998, 7, 1607–1626. [Google Scholar] [CrossRef]
- Pliscoff, P.; Folchi, M.; Aliste, E.; Cea, D.; Simonetti, J.A. Chile mega-fire 2017: An analysis of social representation of forest plantation territory. Appl. Geogr. 2020, 119, 102226. [Google Scholar] [CrossRef]
- Valencia, D.; Saavedra, J.; Brull, J.; Santelices, R. Severidad del daño causado por los incendios forestales en los bosques remanentes de Nothofagus alessandrii Espinosa en la región del Maule de Chile. Gayana Bot. 2018, 75, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Garreaud, R.D.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.H.; Veloso-Aguila, D. The Central Chile Mega Drought (2010–2018): A climate dynamics perspective. Int. J. Climatol. 2020, 40, 421–439. [Google Scholar] [CrossRef]
- Donoso, C. Estructura y Dinámica de los Bosques del Cono sur de América; Editorial Oterra: Santiago, Chile, 2015; p. 405. [Google Scholar]
- Douville, H.; Raghavan, K.; Renwick, J.; Allan, R.P.; Arias, P.A.; Barlow, M.; Cerezo-Mota, R.; Cherchi, A.; Gan, T.Y.; Gergis, J.; et al. Water Cycle Changes. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 1055–1210. [Google Scholar]
- Engelbrecht, B.M.J. Forests on the brink. Nature 2012, 491, 675–676. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.-y.; Wang, G.-x.; Huang, M.; Hu, Z.-y.; Song, C.-l. Effect of climate change on seasonal water use efficiency in subalpine Abies fabri. J. Mt. Sci. 2017, 14, 142–157. [Google Scholar] [CrossRef]
- Piao, S.; Zhang, X.; Chen, A.; Liu, Q.; Lian, X.; Wang, X.; Peng, S.; Wu, X. The impacts of climate extremes on the terrestrial carbon cycle: A review. Sci. China Earth Sci. 2019, 62, 1551–1563. [Google Scholar] [CrossRef]
- Qi, C.; Jiao, L.; Xue, R.; Wu, X.; Du, D. Timescale Effects of Radial Growth Responses of Two Dominant Coniferous Trees on Climate Change in the Eastern Qilian Mountains. Forests 2022, 13, 72. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Blackburn Press: Caldwell, ID, USA, 2001; p. 567. [Google Scholar]
- Aguilera-Betti, I.; Lucas, C.; Ferrero, M.E.; Muñoz, A.A. A Network for Advancing Dendrochronology, Dendrochemistry and Dendrohydrology in South America. Tree-Ring Res. 2020, 76, 94–101. [Google Scholar] [CrossRef]
- Gutiérrez, Á.G.; Chávez, R.O.; Domínguez-Concha, J.A.; Gibson-Carpintero, S.; Guerrero, I.P.; Rocco, R.; Urra, V.D.; Estay, S.A. Ormiscodes amphimone Outbreak Frequency Increased Since 2000 in Subantarctic Nothofagus pumilio Forests of Chilean Patagonia. In Forest Pest and Disease Management in Latin America: Modern Perspectives in Natural Forests and Exotic Plantation; Estay, S.A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 61–75. [Google Scholar]
- Morales, M.S.; Cook, E.R.; Barichivich, J.; Christie, D.A.; Villalba, R.; LeQuesne, C.; Srur, A.M.; Ferrero, M.E.; González-Reyes, Á.; Couvreux, F.; et al. Six hundred years of South American tree rings reveal an increase in severe hydroclimatic events since mid-20th century. Proc. Natl. Acad. Sci. USA 2020, 117, 16816–16823. [Google Scholar] [CrossRef] [PubMed]
- Gauli, A.; Neupane, P.R.; Mundhenk, P.; Köhl, M. Effect of Climate Change on the Growth of Tree Species: Dendroclimatological Analysis. Forests 2022, 13, 496. [Google Scholar] [CrossRef]
- Norton, D.A. Tree-growth-climate relationships in subalpine Nothofagus forests, South Island, New Zealand. N. Zeal. J. Bot. 1984, 22, 471–481. [Google Scholar] [CrossRef]
- Woollons, R.C.; Norton, D.A. Time-Series Analyses Applied To Sequences Of Nothofagus Growth-Ring Measurements. N. Zeal. J. Ecol. 1990, 13, 9–15. [Google Scholar]
- Zimmer, H.; Fox, J. Dendrochronology as a tool for understanding forest growth in Papua New Guinea’s montane forests. In Native Forest Management in Papua New Guinea: Advances in Assessment, Modelling and Decision-Making. ACIAR Proceedings No. 135; Fox, J.C., Keenan, R.J., Brack, C.L., Saulei, S., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 2011; p. 121. [Google Scholar]
- Fajardo, A.; Gazol, A.; Mayr, C.; Camarero, J.J. Recent decadal drought reverts warming-triggered growth enhancement in contrasting climates in the southern Andes tree line. J. Biogeogr. 2019, 46, 1367–1379. [Google Scholar] [CrossRef]
- Lara, A.; Villalba, R.; Wolodarsky-Franke, A.; Aravena, J.C.; Luckman, B.H.; Cuq, E. Spatial and temporal variation in Nothofagus pumilio growth at tree line along its latitudinal range (35°40′–55°S) in the Chilean Andes. J. Biogeogr. 2005, 32, 879–893. [Google Scholar] [CrossRef]
- Suarez, M.L. Tree-ring records from Nothofagus dombeyi: A preliminary chronology network in Northern Patagonia, Argentina. Dendrochronologia 2010, 28, 65–72. [Google Scholar] [CrossRef]
- Tognetti, R.; Lombardi, F.; Lasserre, B.; Cherubini, P.; Marchetti, M. Tree-Ring Stable Isotopes Reveal Twentieth-Century Increases in Water-Use Efficiency of Fagus sylvatica and Nothofagus spp. in Italian and Chilean Mountains. PLoS ONE 2014, 9, e113136. [Google Scholar] [CrossRef] [Green Version]
- Venegas-González, A.; Juñent, F.R.; Gutiérrez, A.G.; Filho, M.T. Recent radial growth decline in response to increased drought conditions in the northernmost Nothofagus populations from South America. For. Ecol. Manag. 2018, 409, 94–104. [Google Scholar] [CrossRef]
- Venegas-González, A.; Roig, F.A.; Peña-Rojas, K.; Hadad, M.A.; Aguilera-Betti, I.; Muñoz, A.A. Recent Consequences of Climate Change Have Affected Tree Growth in Distinct Nothofagus macrocarpa (DC.) FM Vaz Rodr Age Classes in Central Chile. Forests 2019, 10, 653. [Google Scholar] [CrossRef] [Green Version]
- San Martín, J.; Santelices, R.; Henríquez, R. Nothofagus alessandrii Espinosa, Ruil. Familia: Nothofagaceae. In Las Especies Arbóreas de los Bosques Templados de Chile y Argentina: Autoecología, Segunda Edición ed.; Donoso, C., Ed.; Marisa Cuneo Ediciones: Valdivia, Chile, 2013; pp. 391–401. [Google Scholar]
- CIREN. Estudio Agrológico de la VII Región. Descripciones de Suelos. Materiales y Símbolos. Publicación CIREN N° 117; Centro de Información de Recursos Naturales (CIREN): Santiago, Chile, 1997; p. 624. [Google Scholar]
- Sarricolea, P.; Herrera, M.; Meseguer-Ruiz, O. Climatic regionalization of continental Chile. J. Maps 2017, 13, 66–73. [Google Scholar] [CrossRef]
- Schulman, E. Dendroclimatic Changes in Semiarid America; University of Arizona Press: Tucson, AZ, USA, 1957; p. 142. [Google Scholar]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Cook, E.R. A Time Series Analysis Approach to Tree-Ring Standardization; University of Arizona: Tucson, AZ, USA, 1985. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. RStudio. Available online: http://www.rstudio.com/ (accessed on 20 April 2022).
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Muggeo, V. Segmented: An R package to fit regression models with broken-line relationships. R News 2008, 8, 20–25. [Google Scholar]
- Zang, C.; Biondi, F. treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Corvalán, P.; Hernández, J. Impacto de la megasequía en el crecimiento radial de Nothofagus obliqua (Mirb.) Oerst. (Roble), Chile. Rev. Cuba. De Cienc. For. 2019, 7, 184–196. [Google Scholar]
- Urrutia, R.B.; Lara, A.; Villalba, R.; Christie, D.A.; Le Quesne, C.; Cuq, A. Multicentury tree ring reconstruction of annual streamflow for the Maule River watershed in south central Chile. Water Resour. Res. 2011, 47. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of GLOBAL warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., JSkea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; p. 616. [Google Scholar]

| Chronology | Ruiles National Reserve |
|---|---|
| Number of samples | 18 |
| Number of trees | 10 |
| Correlation among series | 0.48 |
| Total length | 1888–2016 |
| Age Min–Average–Max. (years) | 75–95–129 |
| EPS > 0.85 | 1942–2016 (with 10 trees during whole period) |
| Mean Rbar | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santelices-Moya, R.; Gibson-Carpintero, S.; Cabrera-Ariza, A.; Santini-Junior, L.; Venegas-González, A. Reduced Rainfall Variability Reduces Growth of Nothofagus alessandrii Espinosa (Nothofagaceae) in the Maule Region, Chile. Forests 2022, 13, 1184. https://doi.org/10.3390/f13081184
Santelices-Moya R, Gibson-Carpintero S, Cabrera-Ariza A, Santini-Junior L, Venegas-González A. Reduced Rainfall Variability Reduces Growth of Nothofagus alessandrii Espinosa (Nothofagaceae) in the Maule Region, Chile. Forests. 2022; 13(8):1184. https://doi.org/10.3390/f13081184
Chicago/Turabian StyleSantelices-Moya, Rómulo, Stephanie Gibson-Carpintero, Antonio Cabrera-Ariza, Luiz Santini-Junior, and Alejandro Venegas-González. 2022. "Reduced Rainfall Variability Reduces Growth of Nothofagus alessandrii Espinosa (Nothofagaceae) in the Maule Region, Chile" Forests 13, no. 8: 1184. https://doi.org/10.3390/f13081184






