Negative Density Restricts the Coexistence and Spatial Distribution of Dominant Species in Subtropical Evergreen Broad-Leaved Forests in China
Abstract
:1. Introduction
2. Materials and Methods
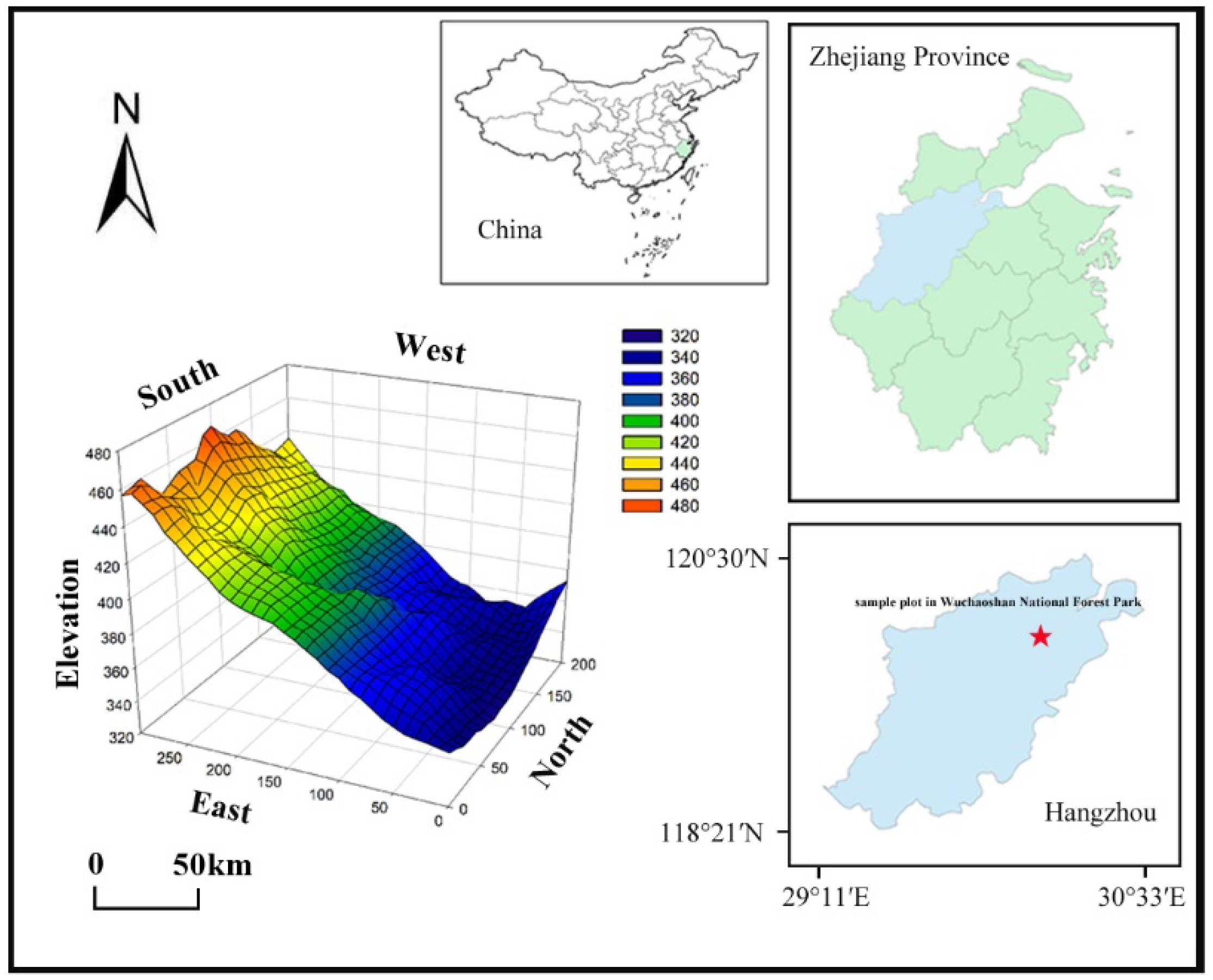
2.1. Study Site
2.2. Sample Setting and Community Survey
2.3. Research Methods
2.3.1. Testing Habitat Heterogeneity
2.3.2. Testing Negative Density Dependence
3. Results
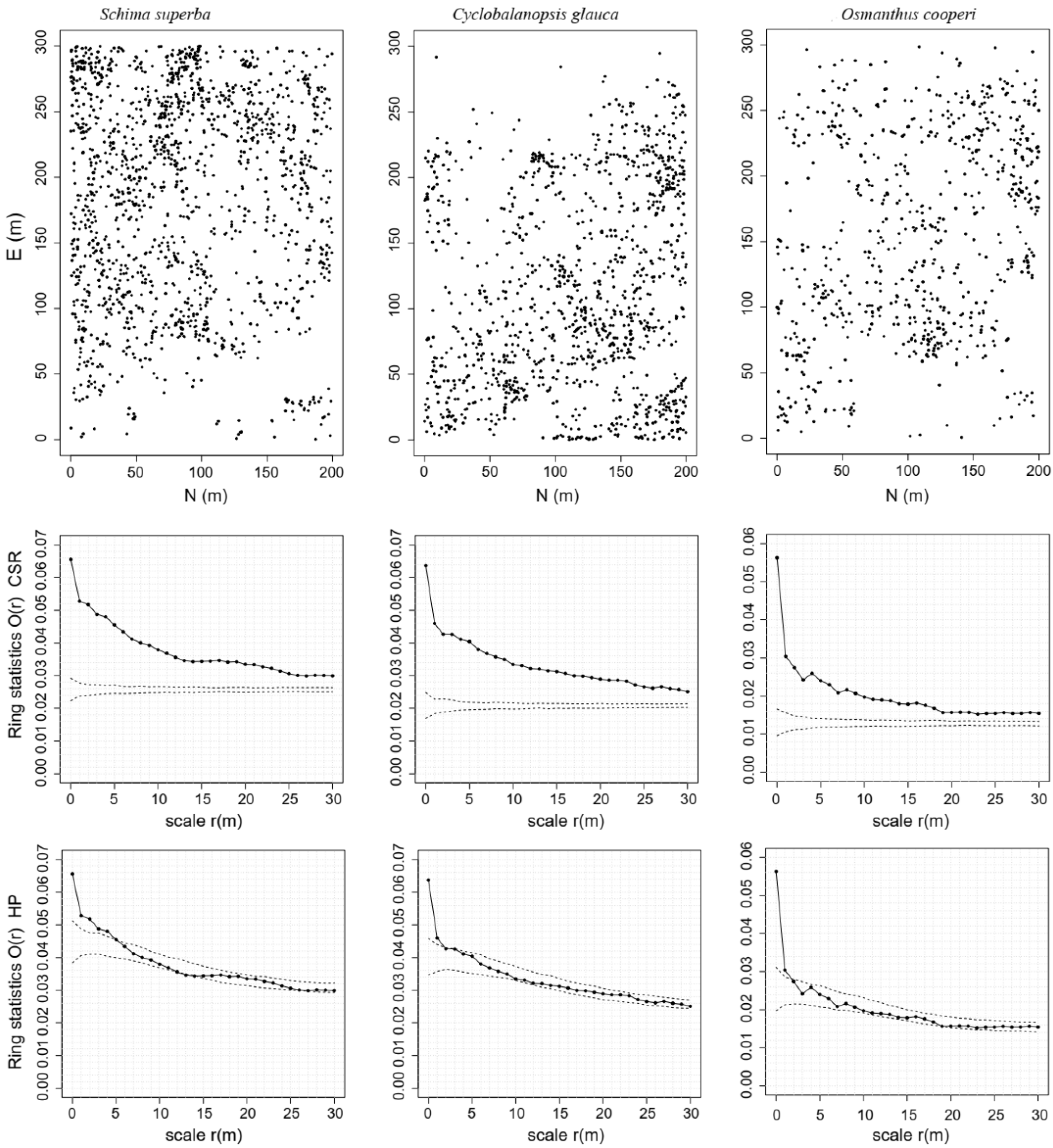
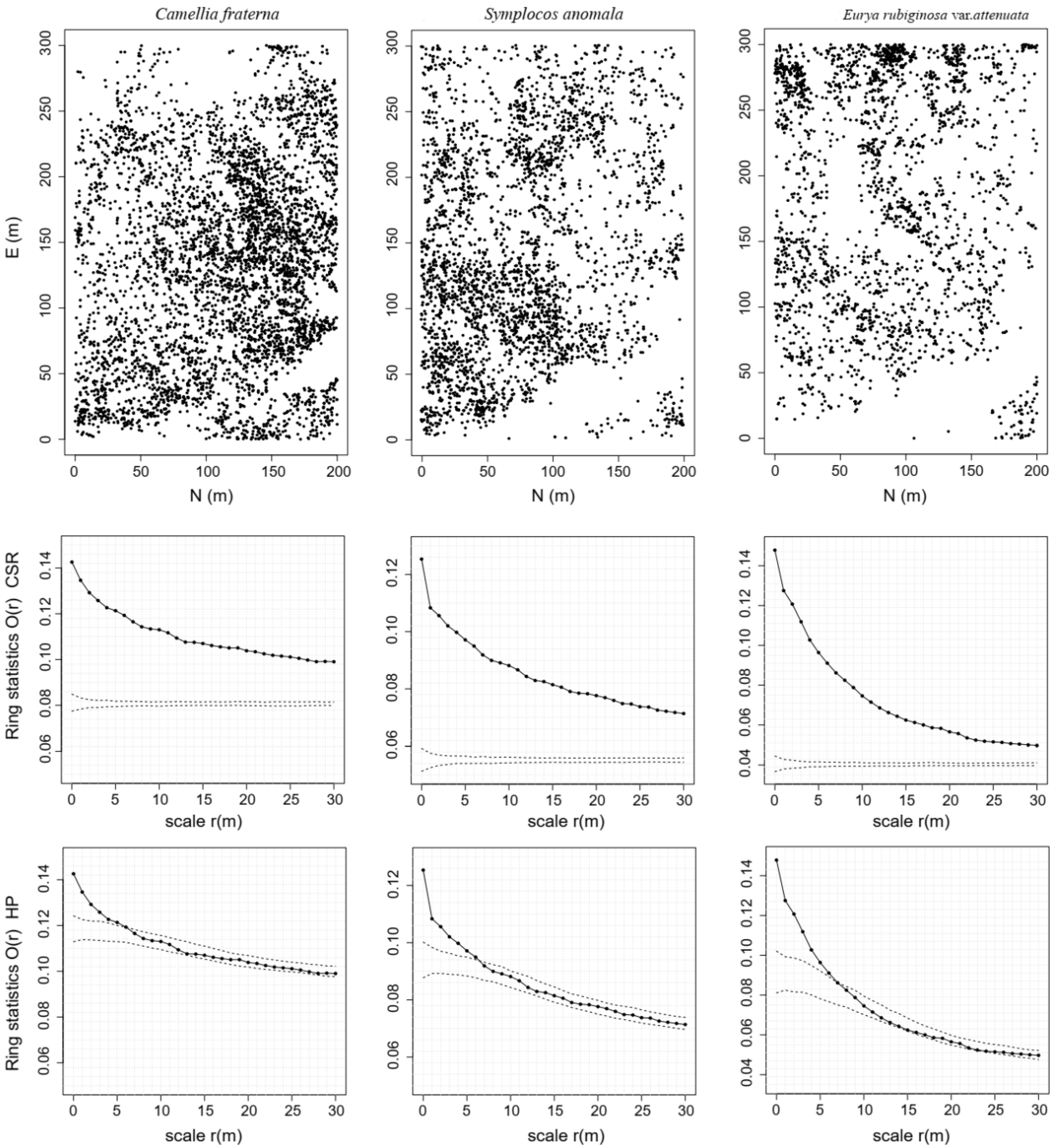
3.1. Effects of Habitat Heterogeneity
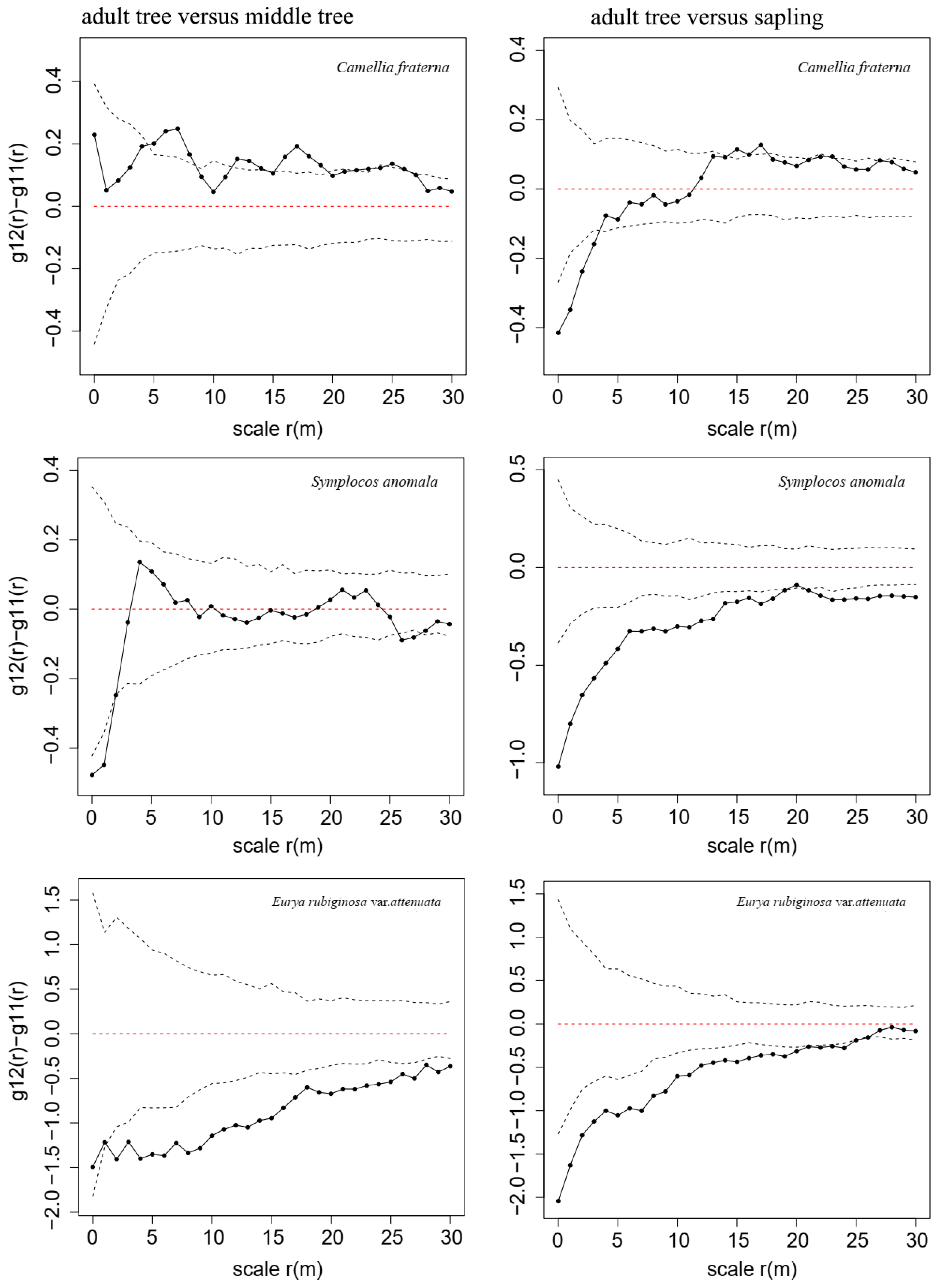
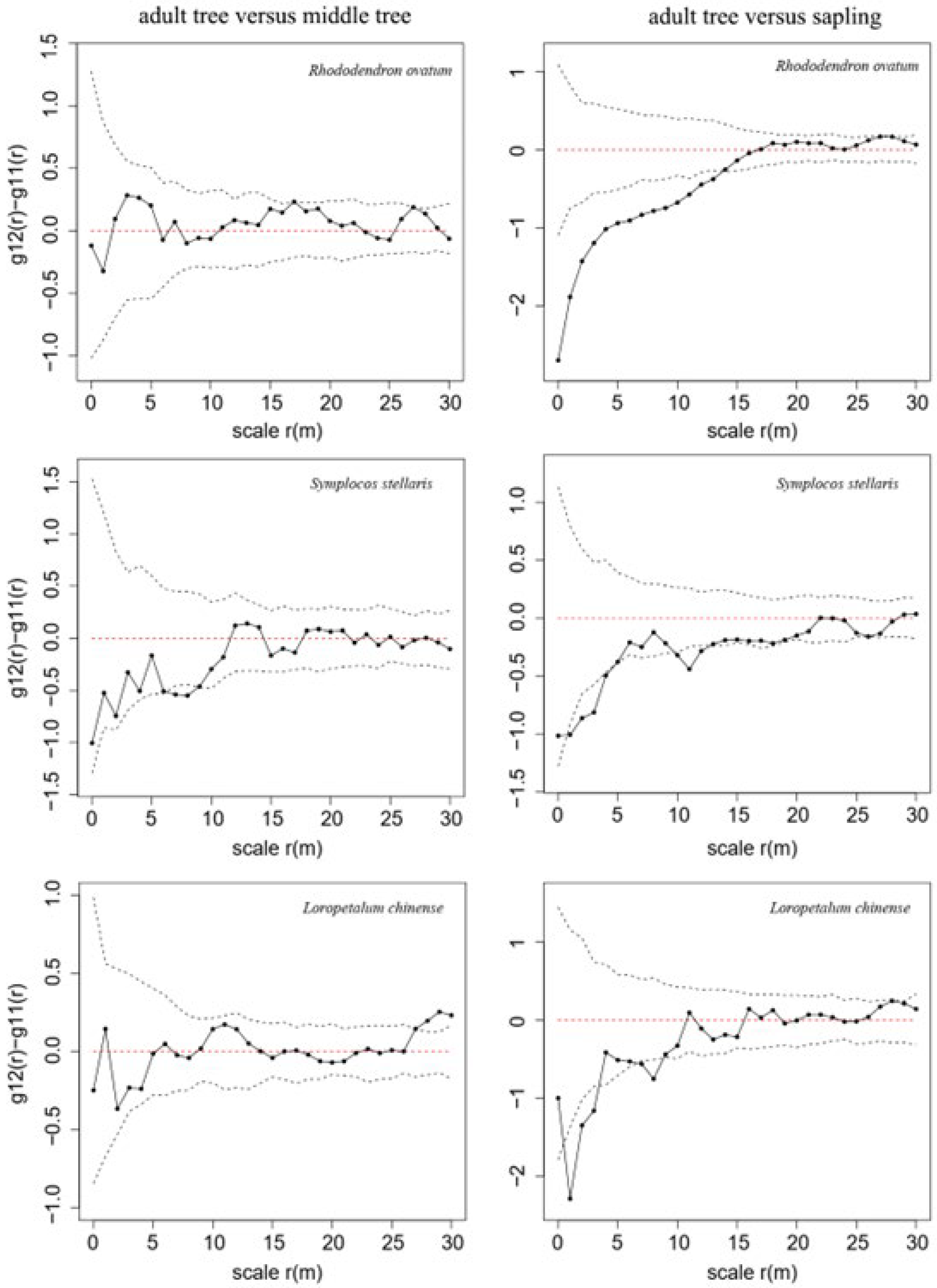
3.2. NDD Effects in a Secondary Evergreen Broad-Leaved Forest
4. Discussion
4.1. The NDD Hypothesis Explains Spatial Distributions of Woody Species in a Secondary Evergreen Broad-Leaved Forest
4.2. Habitat Heterogeneity Strongly Affects Population Spatial Distribution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sterner, R.W.; Ribic, C.A.; Schatz, G.E. Testing for Life Historical Changes in Spatial Patterns of Four Tropical Tree Species. J. Ecol. 1986, 74, 621. [Google Scholar] [CrossRef]
- Barot, S.; Gignoux, J.; Menaut, J.C. Demography of a Savanna Palm Tree: Predictions from Comprehensive Spatial Pattern Analyses. Ecology 1999, 80, 1987–2005. [Google Scholar] [CrossRef]
- Carson, W.P.; Anderson, J.T.; Leigh, J.E.G.; Schnitzer, S.A. Challenges Associated with Testing and Falsifying the Janzen-Connell Hypothesis: A Review and Critique. 2008, pp. 210–241. Available online: https://www.scienceopen.com/document?vid=2157da60-b422-4670-b88c-4c07d59f7d1a (accessed on 12 July 2022).
- Luo, Z.; Mi, X.; Chen, X.; Ye, Z.; Ding, B. Density Dependence Is Not Very Prevalent in a Heterogeneous Subtropical Forest. Oikos 2012, 121, 1239–1250. [Google Scholar] [CrossRef]
- Zang, L.; Xu, H.; Li, Y.; Zang, R. Conspecific Negative Density Dependence of Trees Varies with Plant Functional Traits and Environmental Conditions across Scales in a 60-ha Tropical Rainforest Dynamics Plot. Biotropica 2021, 53, 693–702. [Google Scholar] [CrossRef]
- Wills, C.; Condit, R.; Foster, R.B.; Hubbell, S.P. Strong Density- and Diversity-Related Effects Help to Maintain Tree Species Diversity in a Neotropical Forest. Proc. Natl. Acad. Sci. USA 1997, 94, 1252–1257. [Google Scholar] [CrossRef] [Green Version]
- Connell, J.H.; Tracey, J.G.; Webb, L.J. Compensatory Recruitment, Growth, and Mortality as Factors Maintaining Rain Forest Tree Diversity. Ecol. Monogr. 1984, 54, 141–164. [Google Scholar] [CrossRef]
- Peters, H.A. Neighbour-Regulated Mortality: The Influence of Positive and Negative Density Dependence on Tree Populations in Species-Rich Tropical Forests. Ecol. Lett. 2003, 6, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Xu, Y.; Jiang, B.; Wu, C.; Yuan, W.; Zhu, J.; Li, T.; Wang, Z. Competition Restricts the Growth, Development, and Propagation of Carpinus Tientaiensis: A Rare and Endangered Species in China. Forests 2021, 12, 503. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Gunatilleke, N.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; LaFrankie, J.V.; et al. Spatial Patterns in the Distribution of Tropical Tree Species. Science 2020, 288, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Getzin, S.; Wiegand, T.; Wiegand, K.; He, F. Heterogeneity Influences Spatial Patterns and Demographics in Forest Stands. J. Ecol. 2008, 96, 807–820. [Google Scholar] [CrossRef]
- Zhu, Y.; Comita, L.S.; Hubbell, S.P.; Ma, K. Conspecific and Phylogenetic Density-Dependent Survival Differs across Life Stages in a Tropical Forest. J. Ecol. 2015, 103, 957–966. [Google Scholar] [CrossRef]
- Janzen, D.H. Herbivores and the Number of Tree Species in Tropical Forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Connell, J.H. On the Role of Natural Enemies in Preventing Competitive Exclusion in Some Marine Animals and in Rain Forest Trees. Dyn. Popul. 1971, 298, 312. [Google Scholar]
- Wiegand, T.; Moloney, K.A. Rings, Circles, and Null-Models for Point Pattern Analysis in Ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Liu, H.; Johnson, D.J.; Yang, Q.; Xu, M.; Ma, Z.; Fang, X.; Shang, Y.; Wang, X. The Dynamics of Conspecific Tree and Seedling Neighbors on Seedling Survival in a Subtropical Forest. For. Ecol. Manag. 2021, 483, 118924. [Google Scholar] [CrossRef]
- Yao, L.; Wang, Z.; Zhan, X.; Wu, W.; Jiang, B.; Jiao, J.; Yuan, W.; Zhu, J.; Ding, Y.; Li, T.; et al. Assessment of Species Composition and Community Structure of the Suburban Forest in Hangzhou, Eastern China. Sustainability 2022, 14, 4304. [Google Scholar] [CrossRef]
- Ripley, B.D. Modelling Spatial Patterns. J. R. Stat. Soc. Ser. B 1977, 39, 172–192. [Google Scholar] [CrossRef]
- MacKay, D.B.; Ripley, B.D. Spatial Statistics. J. Mark. Res. 1982, 19, 279. [Google Scholar] [CrossRef]
- Howroyd, J.D.; Stoyan, D.; Stoyan, H. Fractals, Random Shapes and Point Fields: Methods of Geometrical Statistics. Biometrics 1996, 52, 377. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Liu, P.; Wang, W.; Bai, Z.; Guo, Z.; Ren, W.; Huang, J.; Xu, Y.; Yao, J.; Ding, Y.; Zang, R. Competition and Facilitation Co-Regulate the Spatial Patterns of Boreal Tree Species in Kanas of Xinjiang, Northwest China. For. Ecol. Manag. 2020, 467, 118167. [Google Scholar] [CrossRef]
- Hille Ris Lambers, J.; Clark, J.S.; Beckage, B. Density-Dependent Mortality and the Latitudinal Gradient in Species Diversity. Nature 2002, 417, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Piao, T.; Comita, L.S.; Jin, G.; Kim, J.H. Density Dependence across Multiple Life Stages in a Temperate Old-Growth Forest of Northeast China. Oecologia 2013, 172, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Queenborough, S.A.; Chen, L.; Wang, Y.; Mi, X.; Ma, K.; Comita, L.S. Intraspecific and Phylogenetic Density-Dependent Seedling Recruitment in a Subtropical Evergreen Forest. Oecologia 2017, 184, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, Y.; Yu, S. Conspecific Negative Density Dependence Decreases with Increasing Species Abundance. Ecosphere 2015, 6, art257. [Google Scholar] [CrossRef]
- LaManna, J.A.; Walton, M.L.; Turner, B.L.; Myers, J.A. Negative Density Dependence Is Stronger in Resource-rich Environments and Diversifies Communities When Stronger for Common but Not Rare Species. Ecol. Lett. 2016, 19, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Chang, L.-W.; Yang, K.-C.; Wang, H.-H.; Sun, I.-F. Point Patterns of Tree Distribution Determined by Habitat Heterogeneity and Dispersal Limitation. Oecologia 2011, 165, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.R.; Finley, A.O.; D’Amato, A.W.; Bradford, J.B.; Banerjee, S. Predicting Tree Biomass Growth in the Temperate–Boreal Ecotone: Is Tree Size, Age, Competition, or Climate Response Most Important? Glob. Change Biol. 2016, 22, 2138–2151. [Google Scholar] [CrossRef]
- Magee, L.; Wolf, A.; Howe, R.; Schubbe, J.; Hagenow, K.; Turner, B. Density Dependence and Habitat Heterogeneity Regulate Seedling Survival in a North American Temperate Forest. For. Ecol. Manag. 2021, 480, 118722. [Google Scholar] [CrossRef]
- Wright, J.S. Plant Diversity in Tropical Forests: A Review of Mechanisms of Species Coexistence. Oecologia 2002, 130, 1–14. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, J.; Wu, C.; Jiang, B.; Wang, Z.; Yuan, W.; Zhu, J.; Li, T.; Yang, S.; Yao, L. Negative Density Restricts the Coexistence and Spatial Distribution of Dominant Species in Subtropical Evergreen Broad-Leaved Forests in China. Forests 2022, 13, 1227. https://doi.org/10.3390/f13081227
Jiao J, Wu C, Jiang B, Wang Z, Yuan W, Zhu J, Li T, Yang S, Yao L. Negative Density Restricts the Coexistence and Spatial Distribution of Dominant Species in Subtropical Evergreen Broad-Leaved Forests in China. Forests. 2022; 13(8):1227. https://doi.org/10.3390/f13081227
Chicago/Turabian StyleJiao, Jiejie, Chuping Wu, Bo Jiang, Zhigao Wang, Weigao Yuan, Jinru Zhu, Tingting Li, Shaozong Yang, and Liangjin Yao. 2022. "Negative Density Restricts the Coexistence and Spatial Distribution of Dominant Species in Subtropical Evergreen Broad-Leaved Forests in China" Forests 13, no. 8: 1227. https://doi.org/10.3390/f13081227





