Evidence of a Climate-Change-Induced Shift in European Beech Distribution: An Unequal Response in the Elevation, Temperature and Precipitation Gradients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data
2.2.1. Successional Life Stages
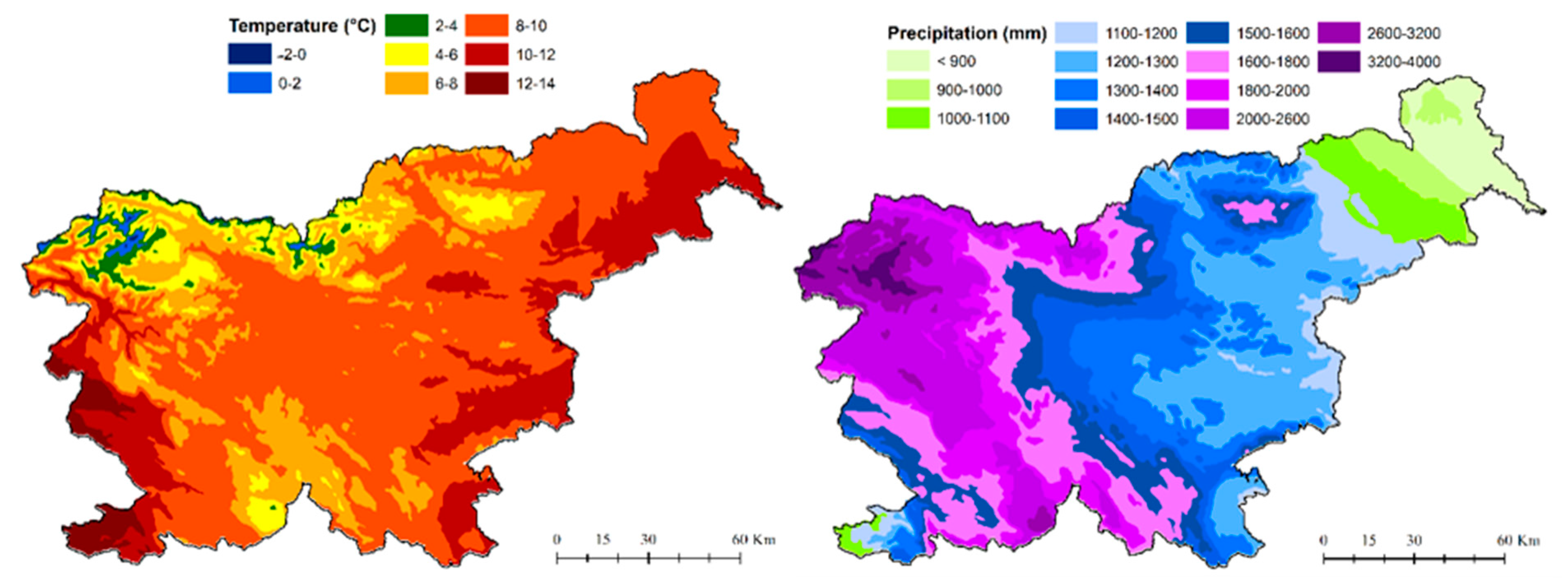
2.2.2. Environmental Data
2.3. Statistical Analyses
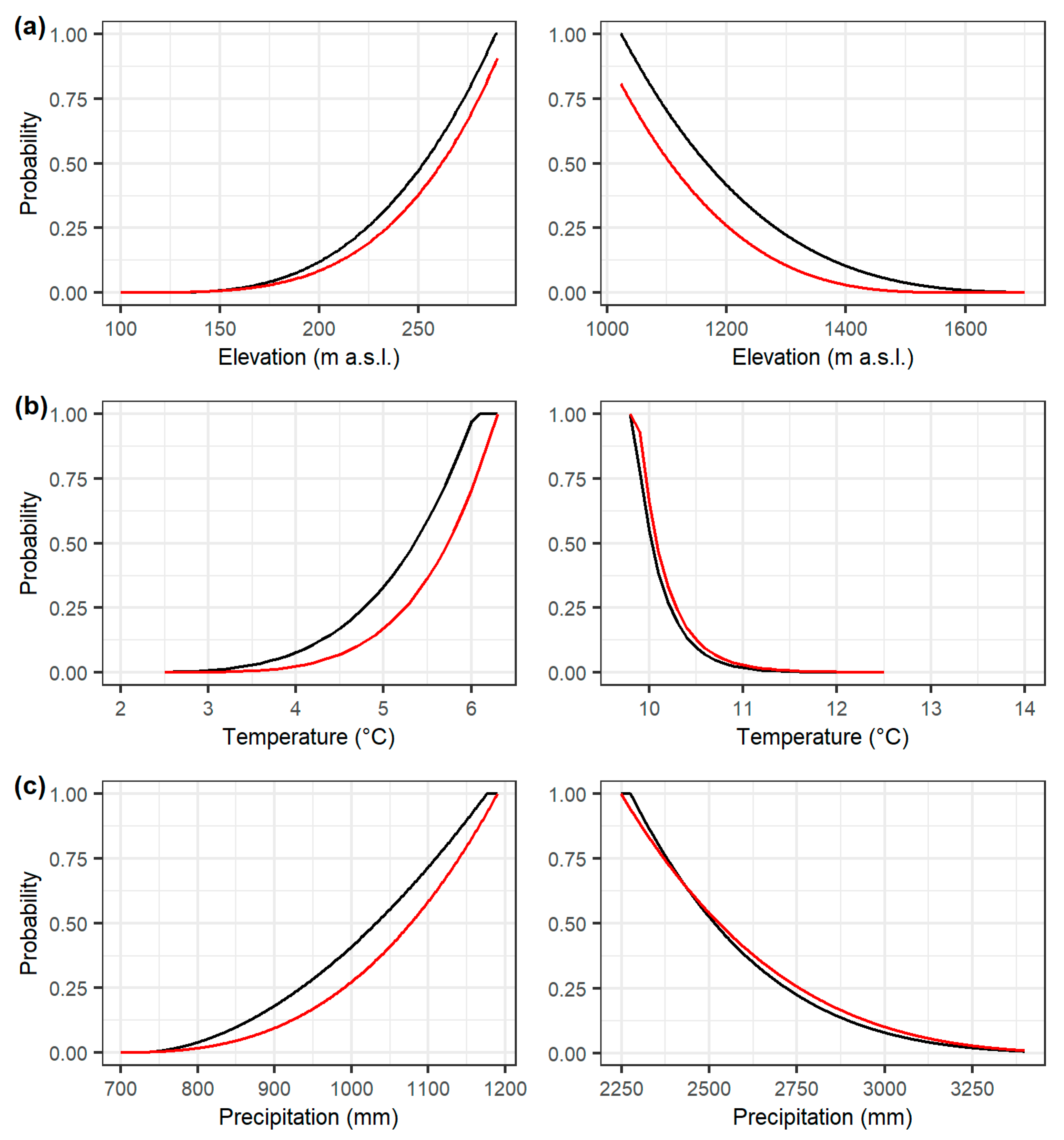
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morin, X.; Augspurger, C.; Chuine, I. Process-based modeling of species’ distributions: What limits temperate tree species’ range boundaries? Ecology 2007, 88, 2280–2291. [Google Scholar] [CrossRef]
- Woodward, F.I. Climate and Plant Distribution; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Khaine, I.; Woo, S.Y. An overview of interrelationship between climate change and forests. For. Sci. Technol. 2015, 11, 11–18. [Google Scholar] [CrossRef]
- Dolanc, C.; Safford, H.; Dobrowski, S.; Thorne, J. Twentieth century shifts in abundance and composition of vegetation types of the Sierra Nevada, CA, US. Appl. Veg. Sci. 2014, 17, 442–455. [Google Scholar] [CrossRef]
- Serra-Diaz, J.; Franklin, J.; Dillon, W.; Syphard, A.; Davis, F.; Meentemeyer, R. California forests show early indications of both range shifts and local persistence under climate change. Glob. Ecol. Biogeogr. 2016, 25, 164–175. [Google Scholar] [CrossRef]
- Boisvert-Marsh, L.; de Blois, S. Unravelling potential northward migration pathways for tree species under climate change. J. Biogeogr. 2021, 48, 1088–1100. [Google Scholar] [CrossRef]
- Lenoir, J.; Gegout, J.; Pierrat, J.; Bontemps, J.; Dhote, J. Differences between tree species seedling and adult altitudinal distribution in mountain forests during the recent warm period (1986–2006). Ecography 2009, 32, 765–777. [Google Scholar] [CrossRef]
- Rabasa, S.; Granda, E.; Benavides, R.; Kunstler, G.; Espelta, J.; Ogaya, R.; Penuelas, J.; Scherer-Lorenzen, M.; Gil, W.; Grodzki, W.; et al. Disparity in elevational shifts of European trees in response to recent climate warming. Glob. Chang. Biol. 2013, 19, 2490–2499. [Google Scholar] [CrossRef]
- Wason, J.; Dovciak, M. Tree demography suggests multiple directions and drivers for species range shifts in mountains of Northeastern United States. Glob. Chang. Biol. 2017, 23, 3335–3347. [Google Scholar] [CrossRef]
- O’Sullivan, K.; Ruiz-Benito, P.; Chen, J.; Jump, A. Onward but not always upward: Individualistic elevational shifts of tree species in subtropical montane forests. Ecography 2021, 44, 112–123. [Google Scholar] [CrossRef]
- Jantsch, M.; Fischer, A.; Fischer, H.; Winter, S. Shift in Plant Species Composition Reveals Environmental Changes during the Last Decades: A Long-Term Study in Beech (Fagus sylvatica) Forests in Bavaria, Germany. Folia Geobot. 2013, 48, 467–491. [Google Scholar] [CrossRef]
- Jump, A.; Hunt, J.; Penuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef]
- Dulamsuren, C.; Hauck, M.; Kopp, G.; Ruff, M.; Leuschner, C. European beech responds to climate change with growth decline at lower, and growth increase at higher elevations in the center of its distribution range (SW Germany). Trees-Struct. Funct. 2017, 31, 673–686. [Google Scholar] [CrossRef]
- Morin, X.; Lechowicz, M.; Augspurger, C.; O’ Keefe, J.; Viner, D.; Chuine, I. Leaf phenology in 22 North American tree species during the 21st century. Glob. Chang. Biol. 2009, 15, 961–975. [Google Scholar] [CrossRef]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barancok, P.; Alonso, J.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, M.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.; Zimmermann, N.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability; Summary for Policymakers; Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022.
- Saltré, F.; Duputie, A.; Gaucherel, C.; Chuine, I. How climate, migration ability and habitat fragmentation affect the projected future distribution of European beech. Glob. Chang. Biol. 2015, 21, 897–910. [Google Scholar] [CrossRef]
- Stojanovic, D.; Krzic, A.; Matovic, B.; Orlovic, S.; Duputie, A.; Djurdjevic, V.; Galic, Z.; Stojnic, S. Prediction of the European beech (Fagus sylvatica L.) xeric limit using a regional climate model: An example from southeast Europe. Agric. For. Meteorol. 2013, 176, 94–103. [Google Scholar] [CrossRef]
- Penuelas, J.; Boada, M. A global change-induced biome shift in the Montseny mountains (NE Spain). Glob. Chang. Biol. 2003, 9, 131–140. [Google Scholar] [CrossRef]
- Lenoir, J.; Gegout, J.; Marquet, P.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef]
- Jump, A.; Matyas, C.; Penuelas, J. The altitude-for-latitude disparity in the range retractions of woody species. Trends Ecol. Evol. 2009, 24, 694–701. [Google Scholar] [CrossRef]
- Boisvert-Marsh, L.; Perie, C.; de Blois, S. Shifting with climate? Evidence for recent changes in tree species distribution at high latitudes. Ecosphere 2014, 5, art83. [Google Scholar] [CrossRef]
- Monleon, V.; Lintz, H. Evidence of Tree Species’ Range Shifts in a Complex Landscape. PLoS ONE 2015, 10, e0118069. [Google Scholar] [CrossRef]
- Renwick, K.; Rocca, M. Temporal context affects the observed rate of climate-driven range shifts in tree species. Glob. Ecol. Biogeogr. 2015, 24, 44–51. [Google Scholar] [CrossRef]
- Suzuki, S.; Ishihara, M.; Hidaka, A. Regional-scale directional changes in abundance of tree species along a temperature gradient in Japan. Glob. Chang. Biol. 2015, 21, 3436–3444. [Google Scholar] [CrossRef]
- Silva, D.; Mazzella, P.; Legay, M.; Corcket, E.; Dupouey, J. Does natural regeneration determine the limit of European beech distribution under climatic stress? For. Ecol. Manag. 2012, 266, 263–272. [Google Scholar] [CrossRef]
- Lenoir, J.; Gegout, J.; Guisan, A.; Vittoz, P.; Wohlgemuth, T.; Zimmermann, N.; Dullinger, S.; Pauli, H.; Willner, W.; Svenning, J. Going against the flow: Potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography 2010, 33, 295–303. [Google Scholar] [CrossRef]
- Von Wühlisch, G. EUFORGEN Technical Guidelines for Genetic Conservation and Use for European Beech (Fagus sylvatica); Bioversity International: Rome, Italy, 2008. [Google Scholar]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas mit den Alpen in ökologischer, Dynamischer und Historischer Sicht, 6th ed.; Verlag Eugen Ulmer: Stuttgart, Germany, 2010; p. 1334. [Google Scholar]
- Bončina, A.; Rozman, A.; Dakskobler, I.; Klopčič, M.; Babij, V.; Poljanec, A. Forest Site Types in Slovenia. Vegetation, Stand and Management Characteristics; University of Ljubljana, Biotechnical Faculty, Department of Forestry and Renewable Forest Resources; Slovenia Forest Service: Ljubljana, Slovenia, 2021. [Google Scholar]
- Pezzi, G.; Ferrari, C.; Corazza, M. The Altitudinal Limit of Beech Woods in the Northern Apennines (Italy). Its Spatial Pattern and Some Thermal Inferences. Folia Geobot. 2008, 43, 447–459. [Google Scholar] [CrossRef]
- Boncina, A. History, current status and future prospects of uneven-aged forest management in the Dinaric region: An overview. Forestry 2011, 84, 467–478. [Google Scholar] [CrossRef]
- Johann, E. Traditional forest management under the influence of science and industry: The story of the alpine cultural landscapes. For. Ecol. Manag. 2007, 249, 54–62. [Google Scholar] [CrossRef]
- Kocher, P.; Gebauer, T.; Horna, V.; Leuschner, C. Leaf water status and stem xylem flux in relation to soil drought in five temperate broad-leaved tree species with contrasting water use strategies. Ann. For. Sci. 2009, 66, 101. [Google Scholar] [CrossRef]
- Mátyás, C.; Berki, I.; Czúcz, B.; Gálos, B.; Móricz, N.; Rasztovits, E. Future of Beech in Southeast Europe from the Perspective of Evolutionary Ecology. Acta Silv. Lignaria Hung. 2010, 6, 91–110. [Google Scholar]
- Mellert, K.; Ewald, J.; Hornstein, D.; Dorado-Linan, I.; Jantsch, M.; Taeger, S.; Zang, C.; Menzel, A.; Kolling, C. Climatic marginality: A new metric for the susceptibility of tree species to warming exemplified by Fagus sylvatica (L.) and Ellenberg’s quotient. Eur. J. For. Res. 2016, 135, 137–152. [Google Scholar] [CrossRef]
- Bertalanič, R.; Dolinar, M.; Draksler, A.; Honzak, L.; Kobold, M.; Kozjek, K.; Lokošek, N.; Medved, A.; Vertačnik, G.; Vlahović, Ž.; et al. Assessment of Climate Change in Slovenia until the End of the 21st Century; Ministry of the Environment and Spatial Planning, Slovenian Environmental Agency: Ljubljana, Slovenia, 2018. [Google Scholar]
- Cheddadi, R.; Araujo, M.; Maiorano, L.; Edwards, M.; Guisan, A.; Carre, M.; Chevalier, M.; Pearman, P. Temperature range shifts for three European tree species over the last 10,000 years. Front. Plant Sci. 2016, 7, 1581. [Google Scholar] [CrossRef]
- Vitasse, Y.; Hoch, G.; Randin, C.; Lenz, A.; Kollas, C.; Korner, C. Tree recruitment of European tree species at their current upper elevational limits in the Swiss Alps. J. Biogeogr. 2012, 39, 1439–1449. [Google Scholar] [CrossRef]
- Ficko, A.; Klopčič, M.; Matijašič, D.; Poljanec, A.; Bončina, A. The distribution of beech and structural characteristics of beech stands in Slovenia. Res. Rep. For. Wood Sci. Technol. 2008, 87, 45–60. (In Slovene) [Google Scholar]
- SFS. Forest Inventory Databases; Slovenia Forest Service: Ljubljana, Slovenia, 2018. [Google Scholar]
- Klopcic, M.; Simoncic, T.; Boncina, A. Comparison of regeneration and recruitment of shade-tolerant and light-demanding tree species in mixed uneven-aged forests: Experiences from the Dinaric region. Forestry 2015, 88, 552–563. [Google Scholar] [CrossRef]
- GURS. Digital Relief Model; Surveying and Mapping Authority of the Republic of Slovenia: Ljubljana, Slovenia, 2021. [Google Scholar]
- ARSO. Databases and Maps of the Slovenian Environment Agency (ARSO); Slovenian Environment Agency: Ljubljana, Slovenia, 2022. [Google Scholar]
- Mood, A.M. On the asymptotic efficiency of certain nonparametric 2-sample tests. Ann. Math. Stat. 1954, 25, 514–522. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analyzing Ecological Data; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Hastie, T.; Tibshirani, R. Generalized additive models. Stat. Sci. 1987, 1, 297–318. [Google Scholar]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman & Hall/CRC: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2006. [Google Scholar]
- Coles, S. An Introduction to Statistical Modeling of Extreme Values; Springer: Berlin/Heidelberg, Germany; London, UK, 2004. [Google Scholar]
- Gilleland, E.; Katz, R. extRemes 2.0: An Extreme Value Analysis Package in R. J. Stat. Softw. 2016, 72, 1–39. [Google Scholar] [CrossRef]
- Gomes, M.; Guillou, A. Extreme Value Theory and Statistics of Univariate Extremes: A Review. Int. Stat. Rev. 2015, 83, 263–292. [Google Scholar] [CrossRef]
- DuMouchel, W.H. Estimating the stable index-alpha in order to measure tail thickness—A critique. Ann. Stat. 1983, 11, 1019–1031. [Google Scholar] [CrossRef]
- Scarrott, C.; MacDonald, A. A review of extreme value threshold estimation and uncertainty quantification. Revstat-Stat. J. 2012, 10, 33–60. [Google Scholar]
- Pfaff, B.; Zivot, E.; McNeil, A.; Stephenson, A. Package ‘evir’. 2018. Available online: https://cran.r-project.org/web/packages/evir/evir.pdf (accessed on 1 June 2022).
- Gazda, A.; Koscielniak, P.; Hardy, M.; Muter, E.; Kedra, K.; Bodziarczyk, J.; Fraczek, M.; Chwistek, K.; Rozanski, W.; Szwagrzyk, J. Upward expansion of distribution ranges of tree species: Contrasting results from two national parks in Western Carpathians. Sci. Total Environ. 2019, 653, 920–929. [Google Scholar] [CrossRef]
- Walthert, L.; Meier, E.S. Tree species distribution in temperate forests is more influenced by soil than by climate. Ecol. Evol. 2017, 7, 9473–9484. [Google Scholar] [CrossRef]
- Vertačnik, G.; Dolinar, M.; Bertalanič, R.; Klančar, M.; Dvoršek, D.; Nadbath, M. Climatic Variability of Slovenia. Main Characteristics of Air Temperature Trends in the Period 1961–2011; Ministry of the Environment and Spatial Planning, Slovenian Environment Agency: Ljubljana, Slovenia, 2013. (In Slovene) [Google Scholar]
- Klopcic, M.; Boncina, A. Stand dynamics of silver fir (Abies alba Mill.)-European beech (Fagus sylvatica L.) forests during the past century: A decline of silver fir? Forestry 2011, 84, 259–271. [Google Scholar] [CrossRef]
- Axer, M.; Martens, S.; Schlicht, R.; Wagner, S. Modelling natural regeneration of European beech in Saxony, Germany: Identifying factors influencing the occurrence and density of regeneration. Eur. J. For. Res. 2021, 140, 947–968. [Google Scholar] [CrossRef]
- Henne, P.; Elkin, C.; Reineking, B.; Bugmann, H.; Tinner, W. Did soil development limit spruce (Picea abies) expansion in the Central Alps during the Holocene? Testing a palaeobotanical hypothesis with a dynamic landscape model. J. Biogeogr. 2011, 38, 933–949. [Google Scholar] [CrossRef]
- Dobrowski, S. A climatic basis for microrefugia: The influence of terrain on climate. Glob. Change Biol. 2011, 17, 1022–1035. [Google Scholar] [CrossRef]
- Chen, J.Q.; Franklin, J.F.; Spies, T.A. Contrasting microclimates among clear-cut, edge, and interior of old-growth Douglas-fir forest. Agric. For. Meteorol. 1993, 63, 219–237. [Google Scholar] [CrossRef]
- Del Castillo, E.; Zang, C.; Buras, A.; Hacket-Pain, A.; Esper, J.; Serrano-Notivoli, R.; Hartl, C.; Weigel, R.; Klesse, S.; de Dios, V.; et al. Climate-change-driven growth decline of European beech forests. Commun. Biol. 2022, 5, 163. [Google Scholar] [CrossRef]
- Ogris, N.; Skudnik, M. Beech defoliation in Slovenia is increasing. Slov. J. For. 2021, 79, 226–237. (In Slovene) [Google Scholar]
- Ognjenovic, M.; Seletkovic, I.; Potocic, N.; Marusic, M.; Tadic, M.; Jonard, M.; Rautio, P.; Timmermann, V.; Lovreskov, L.; Ugarkovic, D. Defoliation Change of European Beech (Fagus sylvatica L.) Depends on Previous Year Drought. Plants 2022, 11, 730. [Google Scholar] [CrossRef]



| European Beech Life Stage | p-Value | |||||
|---|---|---|---|---|---|---|
| Regeneration | Recruitment | Thin Trees | Mature Trees | |||
| ELV (m) | median | 609 | 628 | 624 | 596 | 0.151 |
| mean | 640 | 635 | 648 | 618 | 0.090 | |
| min | 81 | 134 | 182 | 134 | ||
| max | 1755 | 1424 | 1472 | 1472 | ||
| 10th percentile | 289 | 292 | 308 | 295 | ||
| 90th percentile | 1024 | 989 | 1008 | 982 | ||
| TEMP (°C) | median | 8.28 | 8.28 | 8.31 | 8.46 | 0.110 |
| mean | 8.09 a | 8.15 a,b | 8.11 a,b | 8.28 b | 0.001 | |
| min | 3.11 | 3.85 | 3.66 | 3.66 | ||
| max | 12.28 | 12.15 | 11.44 | 12.15 | ||
| 10th percentile | 6.03 | 6.27 | 6.15 | 6.29 | ||
| 90th percentile | 9.83 | 9.87 | 9.73 | 9.88 | ||
| PREC (mm) | median | 1573 a | 1581 a | 1606 a,b | 1495 b | 0.009 |
| mean | 1632 | 1645 | 1673 | 1605 | 0.050 | |
| min | 807 | 840 | 875 | 825 | ||
| max | 3607 | 3160 | 3247 | 3607 | ||
| 10th percentile | 1177 | 1174 | 1207 | 1190 | ||
| 90th percentile | 2276 | 2309 | 2353 | 2249 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klopčič, M.; Rozman, A.; Bončina, A. Evidence of a Climate-Change-Induced Shift in European Beech Distribution: An Unequal Response in the Elevation, Temperature and Precipitation Gradients. Forests 2022, 13, 1311. https://doi.org/10.3390/f13081311
Klopčič M, Rozman A, Bončina A. Evidence of a Climate-Change-Induced Shift in European Beech Distribution: An Unequal Response in the Elevation, Temperature and Precipitation Gradients. Forests. 2022; 13(8):1311. https://doi.org/10.3390/f13081311
Chicago/Turabian StyleKlopčič, Matija, Andrej Rozman, and Andrej Bončina. 2022. "Evidence of a Climate-Change-Induced Shift in European Beech Distribution: An Unequal Response in the Elevation, Temperature and Precipitation Gradients" Forests 13, no. 8: 1311. https://doi.org/10.3390/f13081311






