Roost Selection in Relation to a Patchy, Mosaic Management Burn by a Threatened Clutter-Adapted Bat
Abstract
:1. Introduction
2. Materials and Methods
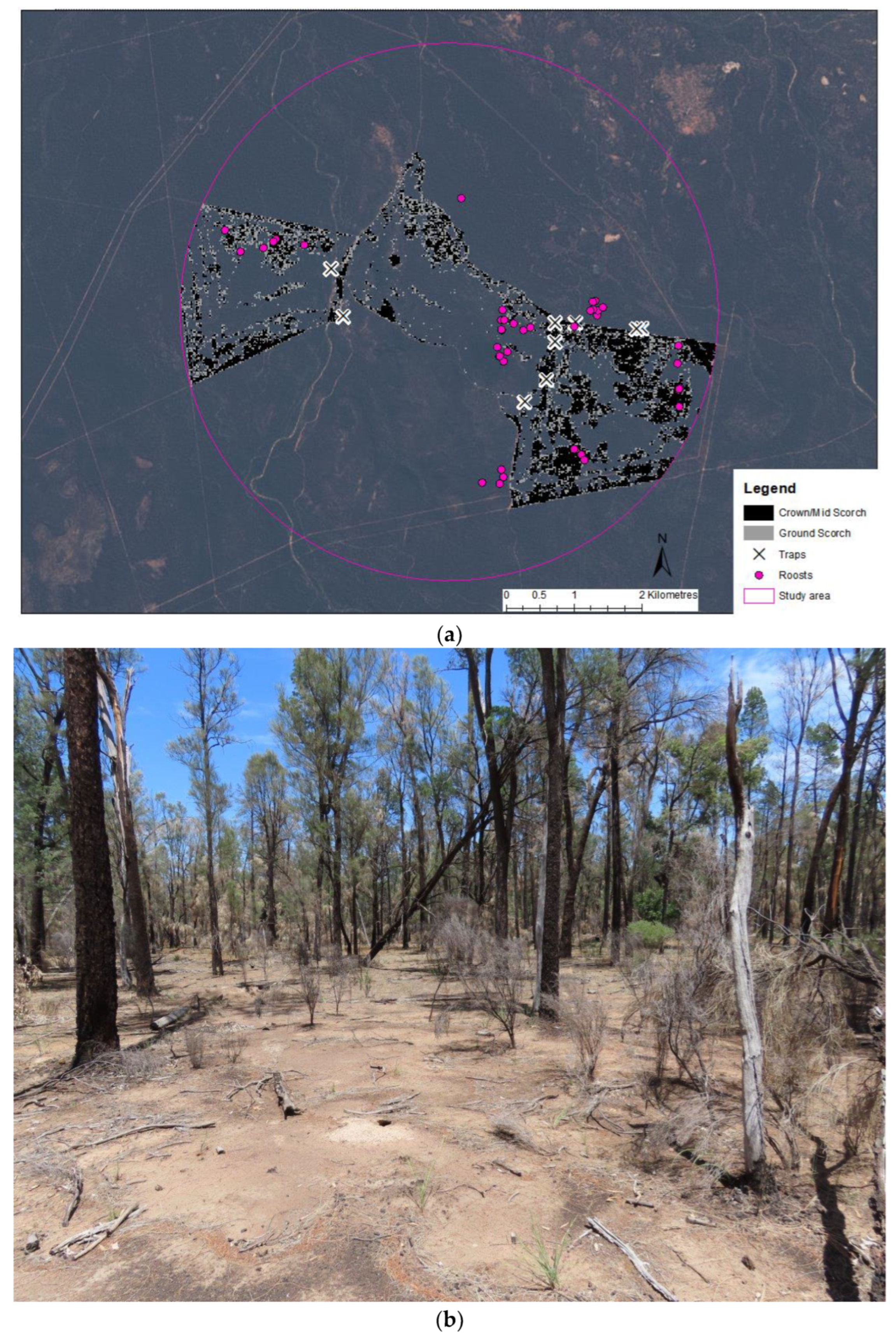
2.1. Study Area
2.2. Trapping and Radio-Tracking
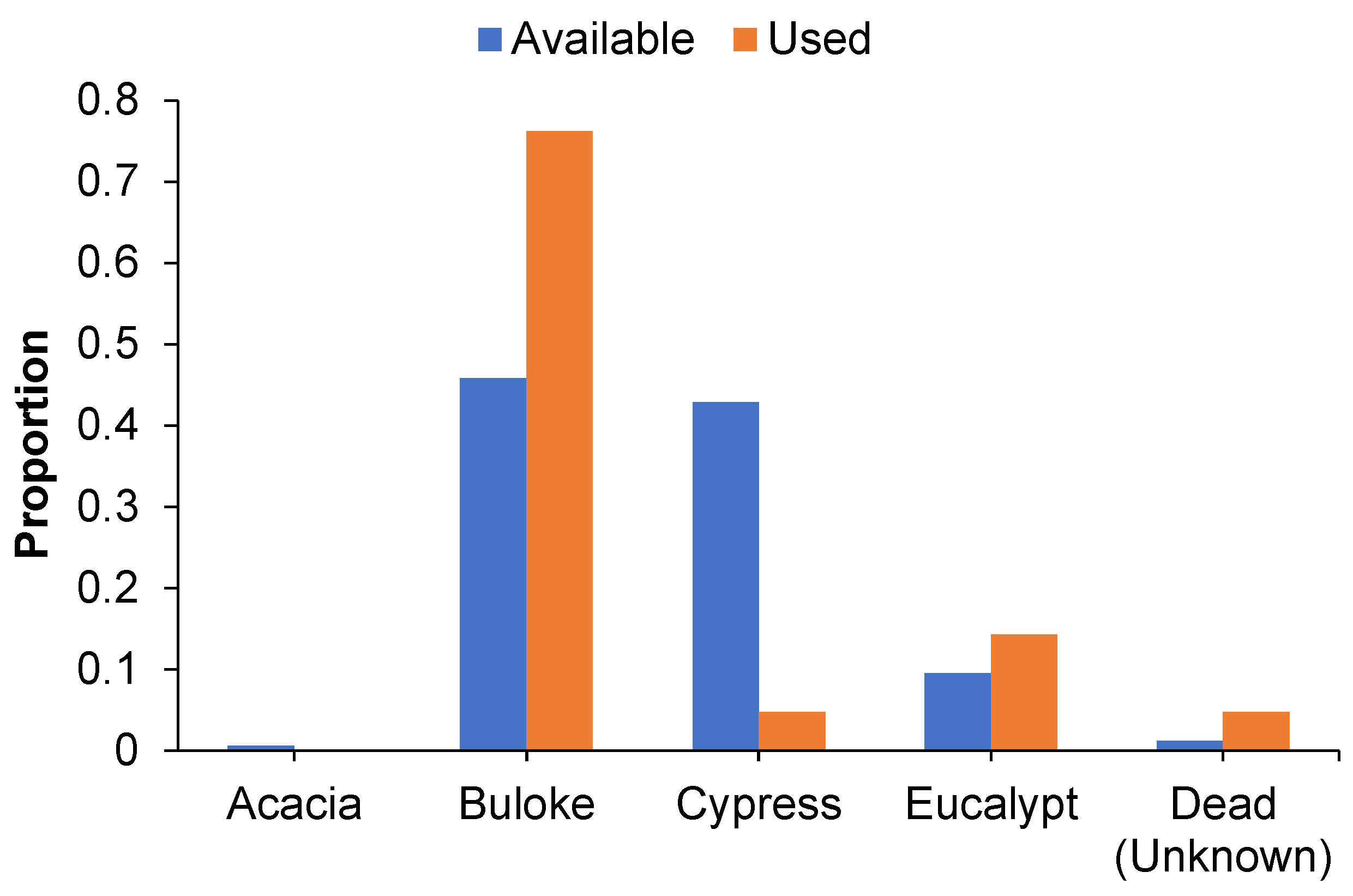
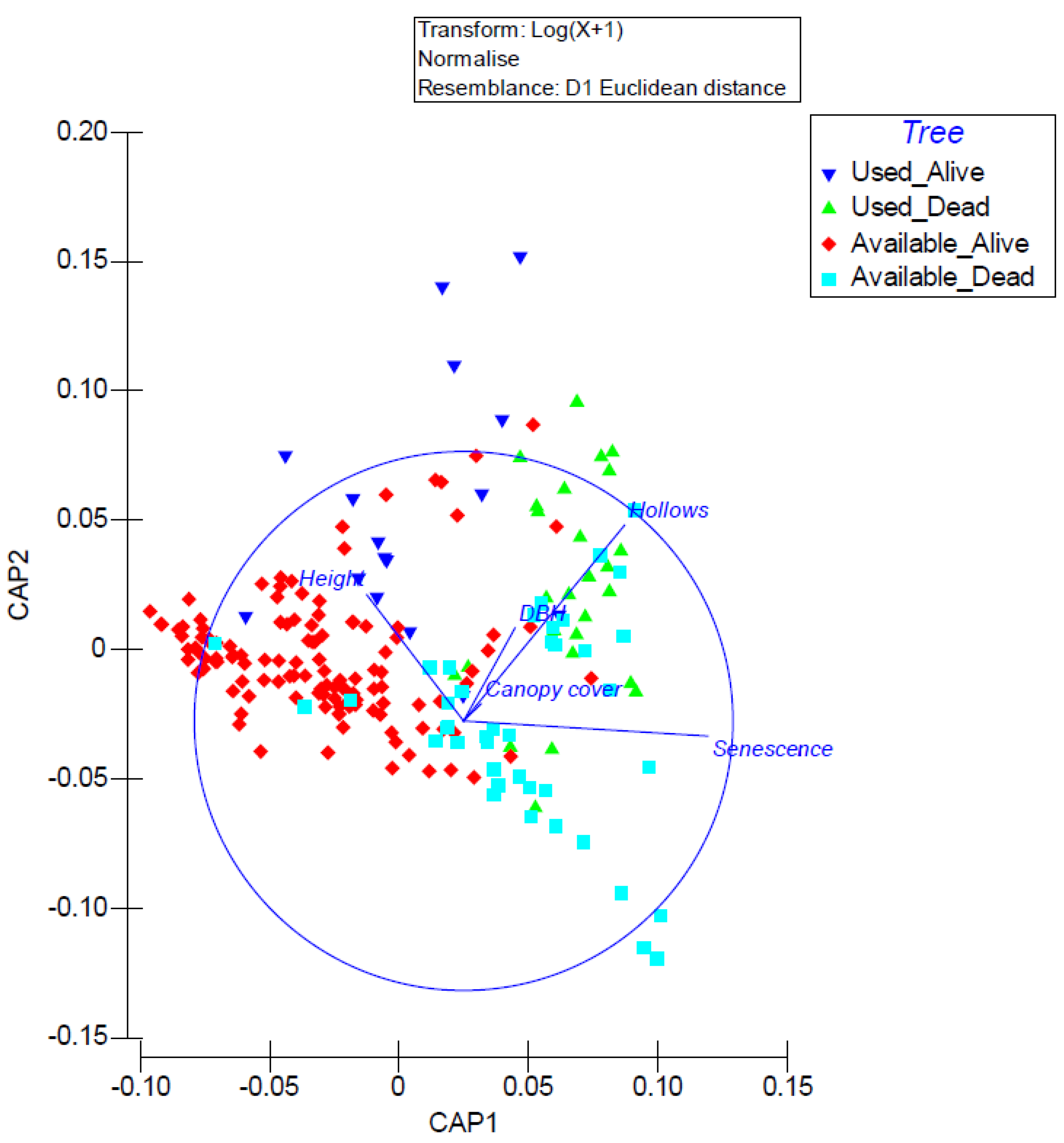
2.3. Roost Tree Assessments
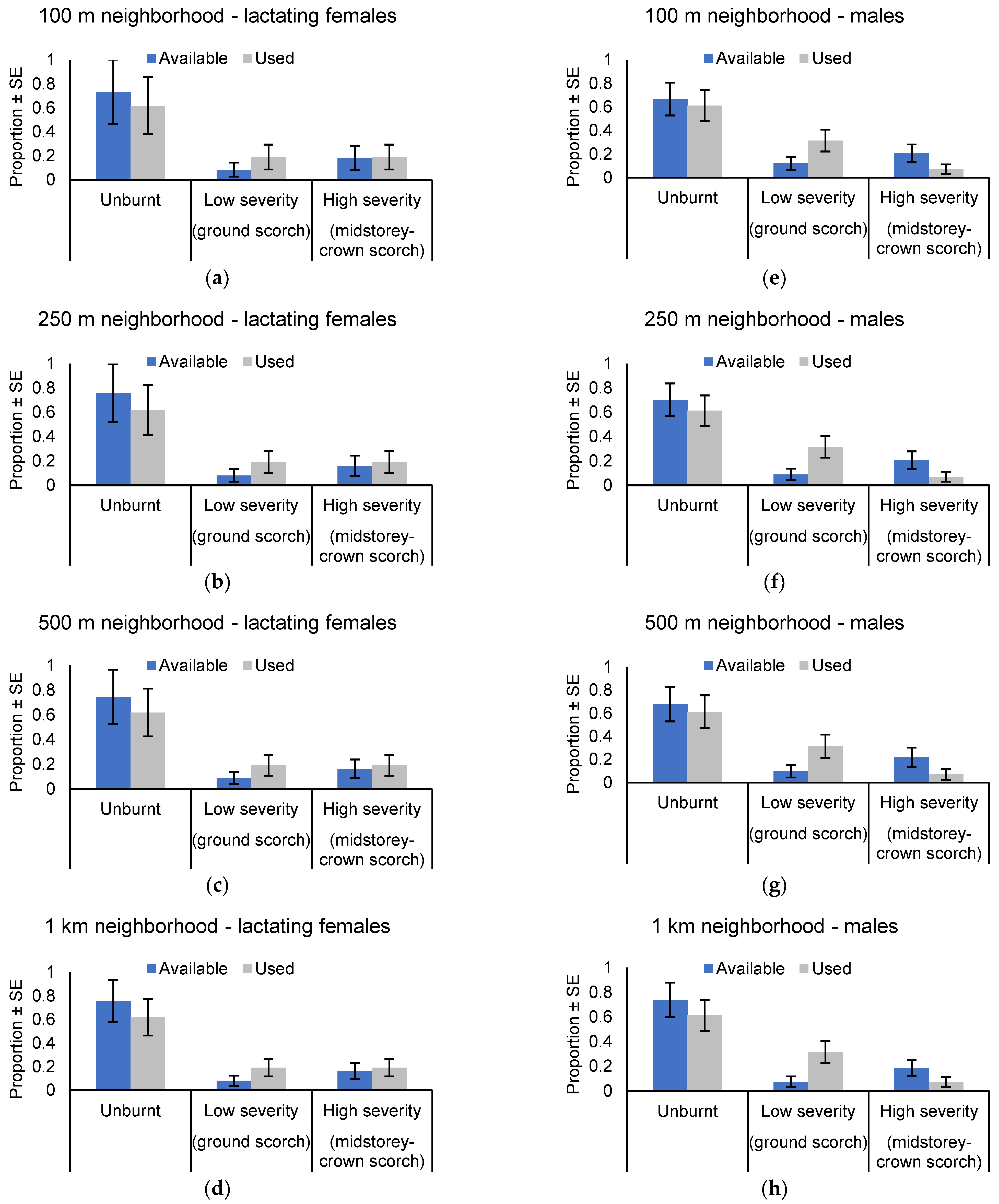
2.4. Landscape-Level Assessments of Roosts in Relation to Fire
2.5. Data Analyses
3. Results
3.1. Bat Captures
3.2. Roost Tree Use and Characteristics
3.3. Use of the Post-Burn Mosaic for Roosting
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bond, W.J.; Woodward, F.I.; Midgley, G.F. The global distribution of ecosystems in a world without fire. New Phytol. 2005, 165, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Attiwill, P.M. The disturbance of forest ecosystems: The ecological basis for conservative management. For. Ecol. Manag. 1994, 63, 247–300. [Google Scholar] [CrossRef]
- Krawchuk, M.A.; Moritz, M.A.; Parisien, M.A.; van Dorn, J.; Hayhoe, K. Global pyrogeography: The current and future distribution of wildfire. PLoS ONE 2009, 4, e5102. [Google Scholar] [CrossRef]
- Bradstock, R.A. A biogeographic model of fire regimes in Australia: Current and future implications. Glob. Ecol. Biogeogr. 2010, 19, 145–158. [Google Scholar] [CrossRef]
- Bowman, D.M.; Williamson, G.J.; Abatzoglou, J.T.; Kolden, C.A.; Cochrane, M.A.; Smith, A. Human exposure and sensitivity to globally extreme wildfire events. Nat. Ecol. Evol. 2017, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pyne, S.J. Burning Bush: A Fire History of Australia; Allen & Unwin: Sydney, Australia, 1991; 520p. [Google Scholar]
- Milne, D.J.; Armstrong, M.; Fisher, A.; Flores, T.; Pavey, C.R. Structure and environmental relationships of insectivorous bat assemblages in tropical Australian savannas. Austral Ecol. 2005, 30, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Frick, W.F.; Kingston, T.; Flanders, J. A review of the major threats and challenges to global bat conservation. Ann. N. Y. Acad. Sci. 2020, 1469, 5–25. [Google Scholar] [CrossRef]
- Perry, R.W. A review of fire effects on bats and bat habitat in the eastern oak region. In Proceedings of the 4th Fire in Eastern Oak Forests Conference, Springfield, MO, USA, 17–19 May 2011. [Google Scholar]
- Loeb, S.C. Qualitative synthesis of temperate bat responses to silvicultural treatments—where do we go from here? J. Mammal. 2020, 101, 1513–1525. [Google Scholar] [CrossRef]
- Loeb, S.C.; Blakey, R.V. Bats and fire: A global review. Fire Ecol. 2021, 17, 1–8. [Google Scholar] [CrossRef]
- Inkster-Draper, T.E.; Sheaves, M.; Johnson, C.N.; Robson, S.K. Prescribed fire in eucalypt woodlands: Immediate effects on a microbat community of northern Australia. Wildl. Res. 2013, 40, 70–76. [Google Scholar] [CrossRef]
- Austin, L.V.; Silvis, A.; Muthersbaugh, M.S.; Powers, K.E.; Ford, W.M. Bat activity following repeated prescribed fire in the central Appalachians, USA. Fire Ecol. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Austin, L.V.; Silvis, A.; Ford, W.M.; Muthersbaugh, M.; Powers, K.E. Bat activity following restoration prescribed burning in the central Appalachian upland and riparian habitats. Nat. Areas J. 2018, 38, 183–195. [Google Scholar] [CrossRef]
- Law, B.; Doty, A.; Chidel, M.; Brassil, T. Bat activity before and after a severe wildfire in Pilliga forests: Resilience influenced by fire extent and landscape mobility? Austral Ecol. 2018, 43, 706–718. [Google Scholar] [CrossRef]
- Law, B.; Kathuria, A.; Chidel, M.; Brassil, T. Long-term effects of repeated fuel-reduction burning and logging on bats in south-eastern Australia. Austral Ecol. 2019, 44, 1013–1024. [Google Scholar] [CrossRef]
- Ancillotto, L.; Bosso, L.; Conti, P.; Russo, D. Resilient responses by bats to a severe wildfire: Conservation implications. Anim. Conserv. 2021, 24, 470–481. [Google Scholar] [CrossRef]
- Broken-Brow, J.; Hitch, A.T.; Armstrong, K.N.; Leung, L.K. Effect of fire on insectivorous bat activity in northern Australia: Does fire intensity matter on a local scale? Aust. J. Zool. 2020, 67, 260–268. [Google Scholar] [CrossRef]
- Boyles, J.G.; Aubrey, D.P. Managing forests with prescribed fire: Implications for a cavity-dwelling bat species. For. Ecol. Manag. 2006, 222, 108–115. [Google Scholar] [CrossRef]
- Lacki, M.J.; Cox, D.R.; Dodd, L.E.; Dickinson, M.B. Response of northern bats (Myotis septentrionalis) to prescribed fires in eastern Kentucky forests. J. Mammal. 2009, 90, 1165–1175. [Google Scholar] [CrossRef]
- Johnson, J.B.; Edwards, J.W.; Ford, W.M.; Gates, J.E. Roost tree selection by northern myotis (Myotis septentrionalis) maternity colonies following prescribed fire in a Central Appalachian Mountains hardwood forest. For. Ecol. Manag. 2009, 258, 233–242. [Google Scholar] [CrossRef]
- Law, B.; Gonsalves, L.; Chidel, M.; Brassil, T. Subtle use of a disturbance mosaic by the south-eastern long-eared bat (Nyctophilus corbeni): An extinction-prone, narrow-space bat. Wildl. Res. 2016, 43, 153–168. [Google Scholar] [CrossRef]
- Law, B.; Gonsalves, L.; Brassil, T.; Hill, D. Does thinning homogenous and dense regrowth benefit bats? Radio-tracking, ultrasonic detection and trapping. Diversity 2018, 10, 45. [Google Scholar] [CrossRef]
- Lumsden, L.; Nelson, J.; Lindeman, M. Ecological Research on the Eastern Long-Eared Bat Nyctophilus timoriensis (South-Eastern Form). A Report to the Mallee Catchment Management Authority; Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment: Melbourne, Australian, 2018. [Google Scholar]
- Hill, D.A.; Armstrong, K.N.; Barden, P.A. Preliminary assessment suggests that acoustic lures can increase capture rates of Australian echolocating bats. Aust. Mammal. 2014, 37, 104–106. [Google Scholar] [CrossRef]
- Gibbons, P.; Lindenmayer, D.B.; Barry, S.C.; Tanton, M.T. Hollow formation in eucalypts from temperate forests in southeastern Australia. Pac. Conserv. Biol. 2000, 6, 218–228. [Google Scholar] [CrossRef]
- Pollard, J.H. On distance estimators of density in randomly distributed forests. Biometrics 1971, 4, 991–1002. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; Van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 97, 378–400. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Duncan, A.M.; Baker, G.B.; Montgomery, N. The Action Plan for Australian Bats; Biodiversity Group Environment Australia: Canberra, Australia, 1999. [Google Scholar]
- Parnaby, H.; Lunney, D.; Shannon, I.; Fleming, M. Collapse rates of hollow-bearing trees following low intensity prescription burns in the Pilliga forests, New South Wales. Pac. Conserv. Biol. 2010, 16, 209–220. [Google Scholar] [CrossRef]
- Salmona, J.; Dixon, K.M.; Banks, S.C. The effects of fire history on hollow-bearing tree abundance in montane and subalpine eucalypt forests in southeastern Australia. For. Ecol. Manag. 2018, 428, 93–103. [Google Scholar] [CrossRef]
- Radford, I.J.; Oliveira, S.L.; Byrne, B.; Woolley, L.A. Tree hollow densities reduced by frequent late dry-season wildfires in threatened Gouldian finch (Erythrura gouldiae) breeding habitat. Wildl. Res. 2021, 48, 511–520. [Google Scholar] [CrossRef]
- McLean, C.M.; Bradstock, R.; Price, O.; Kavanagh, R.P. Tree hollows and forest stand structure in Australian warm temperate Eucalyptus forests are adversely affected by logging more than wildfire. For. Ecol. Manag. 2015, 341, 37–44. [Google Scholar] [CrossRef]




| Species | No. of Captures |
|---|---|
| Chalinolobus gouldii | 58 |
| Chalinolobus picatus | 3 |
| Nyctophilus corbeni | 17 |
| Nyctophilus geoffroyi | 23 |
| Nyctophilus gouldi | 56 |
| Ozimops planiceps | 1 |
| Ozimops ridei | 2 |
| Scotorepens balstoni | 27 |
| Scotorepens greyii | 31 |
| Scotorepens sp. | 59 |
| Vespadelus vulturnus | 115 |
| Unidentified * | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonsalves, L.; Law, B.; Brassil, T.; Kerr, I.; O’Loughlin, C. Roost Selection in Relation to a Patchy, Mosaic Management Burn by a Threatened Clutter-Adapted Bat. Forests 2022, 13, 1327. https://doi.org/10.3390/f13081327
Gonsalves L, Law B, Brassil T, Kerr I, O’Loughlin C. Roost Selection in Relation to a Patchy, Mosaic Management Burn by a Threatened Clutter-Adapted Bat. Forests. 2022; 13(8):1327. https://doi.org/10.3390/f13081327
Chicago/Turabian StyleGonsalves, Leroy, Brad Law, Traecey Brassil, Isobel Kerr, and Christopher O’Loughlin. 2022. "Roost Selection in Relation to a Patchy, Mosaic Management Burn by a Threatened Clutter-Adapted Bat" Forests 13, no. 8: 1327. https://doi.org/10.3390/f13081327






