Highlighting a New Morphospecies within the Dialium Genus Using Leaves and Wood Traits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Genus
2.2. Study Area
2.3. Herbarium Collection and Description
2.4. Wood Material
2.5. Wood Sample Preparation
2.5.1. For Chemical Analyses and Moisture Content
2.5.2. For FTIR Measurements
2.6. Wood Primary Metabolites Content and Mineral
2.6.1. Cellulose, Hemicellulose and Lignin Content
2.6.2. Ash Content
2.6.3. Nitrogen Content
2.6.4. Silica Content
2.7. Ethanol-Water Extracts Charaterisation
2.7.1. Phenolic Content
2.7.2. Condensed Tannins Content
2.8. FT-IR Acquisition
2.9. Statistical Analyses
2.9.1. Conditional Inference Tree
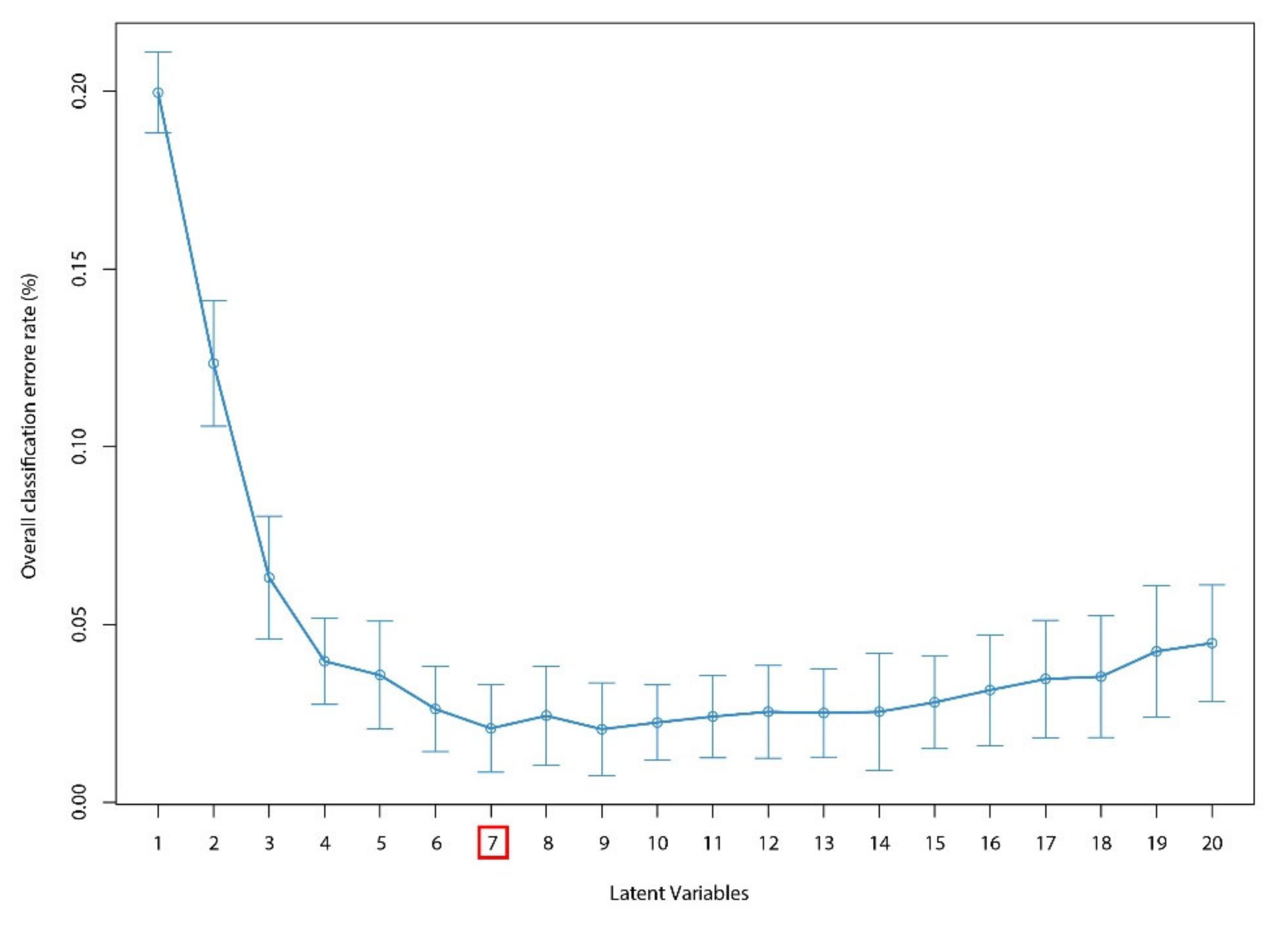
2.9.2. PLS-DA
2.9.3. Variance Analyses
3. Results
3.1. Leaf Morphological Traits
3.1.1. Conditional Inference Tree
3.1.2. Variance Analyses
3.2. Chemical Composition
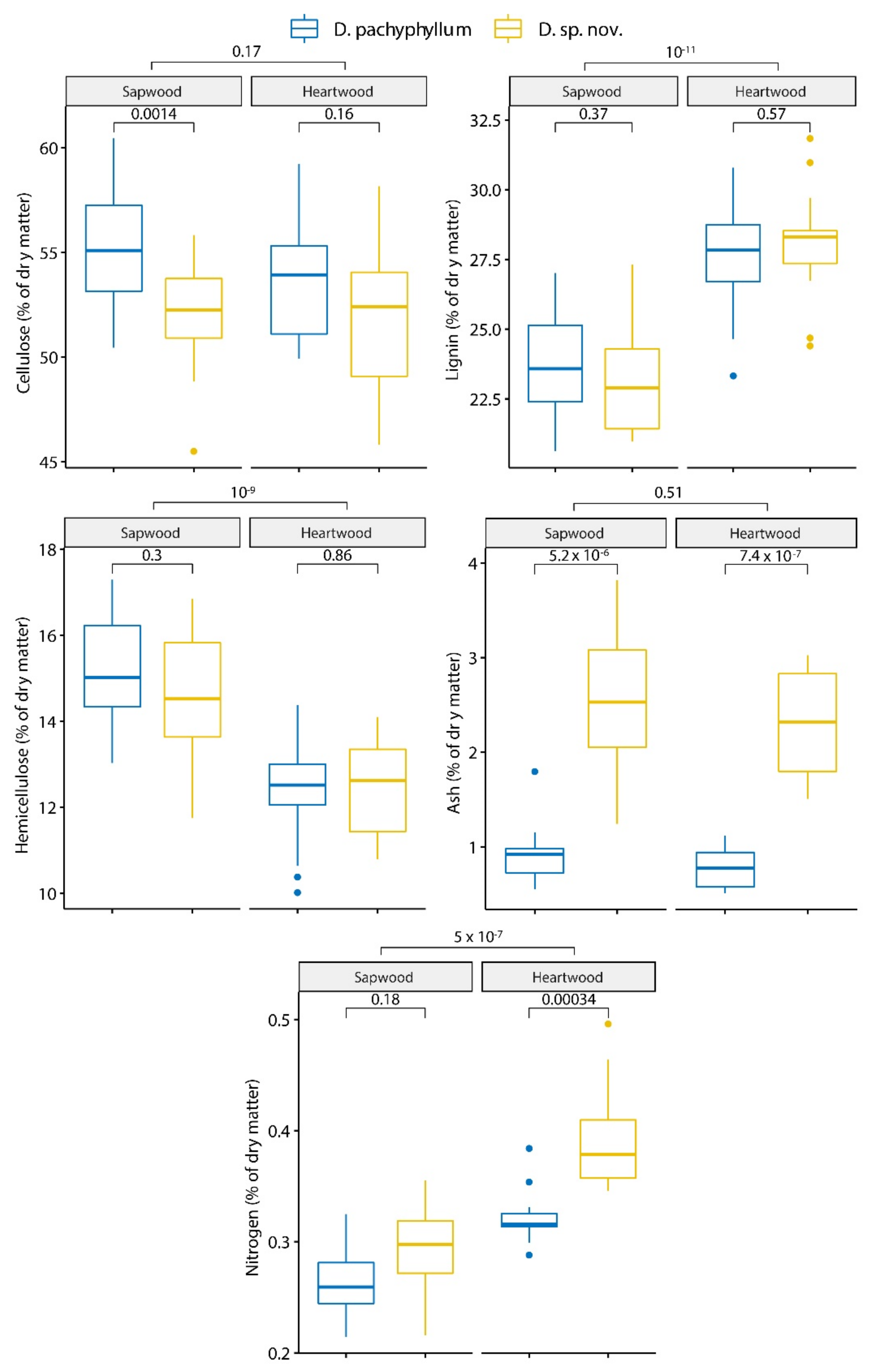
3.2.1. Primary Metabolites and Minerals
3.2.2. Ethanol Extracts
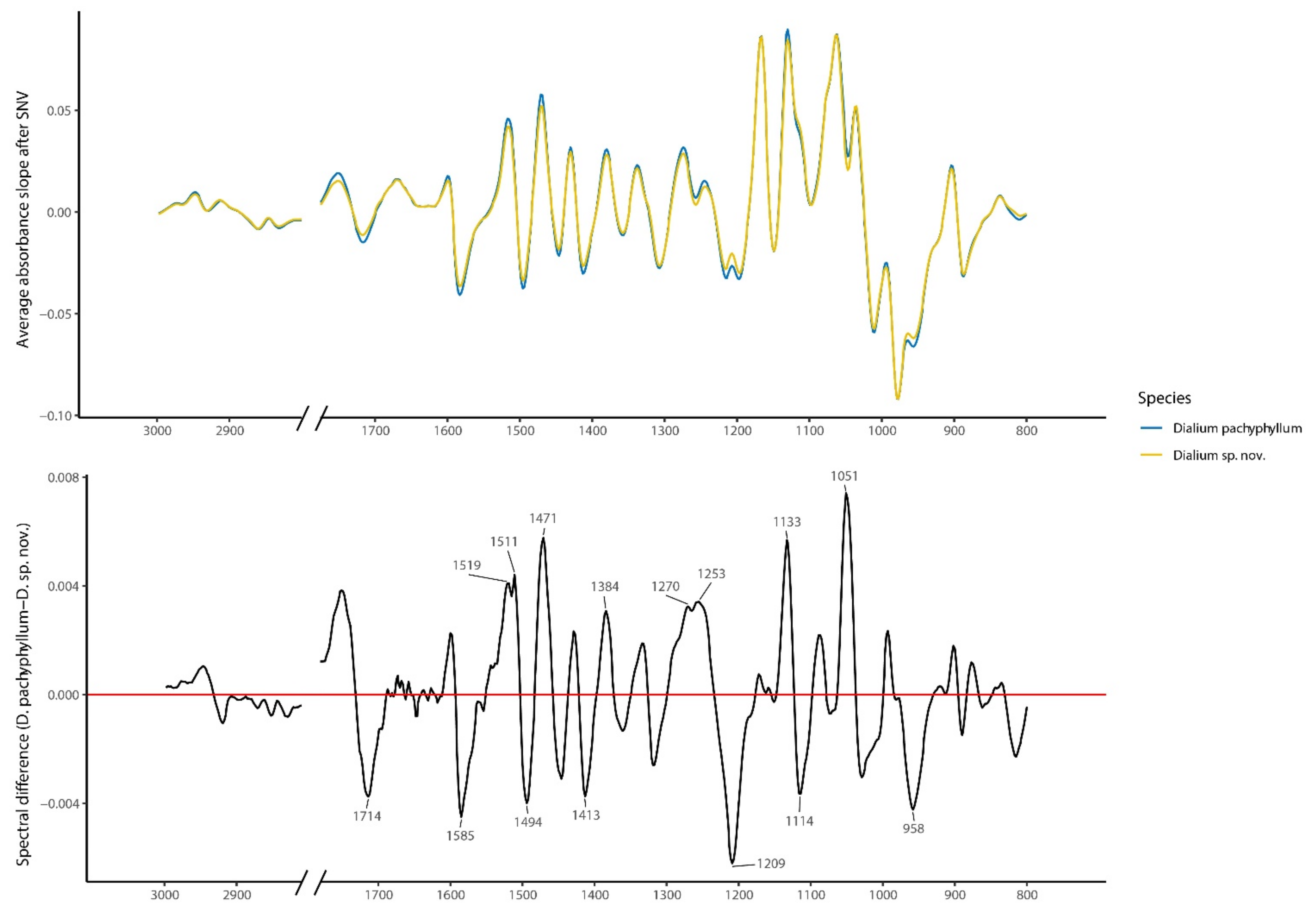
3.2.3. Wood FT-IR Distinction
4. Discussion
4.1. Species Distinction in Forest Inventories
4.2. Wood Chemical Properties and Their Variation
4.2.1. Primary Metabolites and Minerals
4.2.2. Ethanol-Water Extracts
4.3. FT-IR Wood Distinction
4.4. Impacts on Tree Ecology, Forest Management, and Wood Production
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Harvester | Herbaria Reference | Date | Identification | Country |
|---|---|---|---|---|
| de Wilde | BR0000016564503 | 19-12-96 | Dialium lopense | Gabon |
| Breteler | BR0000016564534 | 23-01-99 | Dialium lopense | Gabon |
| Wieringa | BR0000016564589 | 17-11-94 | Dialium lopense | Gabon |
| Wieringa | BR0000016564572 | 02-04-04 | Dialium lopense | Gabon |
| Breteler | BR0000016564527 | 05-02-99 | Dialium lopense | Gabon |
| Breteler | BR0000016564541 | 17-02-99 | Dialium lopense | Gabon |
| Doucet J-L | BR0000016564558 | 01-05-96 | Dialium lopense | Gabon |
| Wilk | BR0000016564565 | 17-05-87 | Dialium lopense | Gabon |
| McPherson | BR0000016564596 | 22-11-93 | Dialium lopense | Gabon |
| McPherson | BR0000016564602 | 18-05-92 | Dialium lopense | Gabon |
| McPherson | BR0000016564626 | 15-05-92 | Dialium lopense | Gabon |
| Bibang & Doucet J-L. | field | 30-03-21 | Dialium lopense | Gabon |
| Doucet R. | field | 17-06-21 | Dialium lopense | Gabon |
| Doucet R. | field | May 2019 | Dialium lopense | Gabon |
| McPherson | BR0000013636012 | 04-05-92 | Dialium pachyphyllum | DRC |
| Tshibamba | BR0000013639617 | 01-07-11 | Dialium pachyphyllum | DRC |
| van de Burgt | BR0000013639631 | 12-09-11 | Dialium pachyphyllum | Gabon |
| Wieringa | BR0000013635824 | 16-11-94 | Dialium pachyphyllum | Gabon |
| Wieringa | BR0000013635916 | 31-10-03 | Dialium pachyphyllum | Gabon |
| Doucet R. | field | May 2019 | Dialium pachyphyllum | Gabon |
| Doucet R. | field | May 2019 | Dialium pachyphyllum | Gabon |
| Doucet R. | field | 16-06-21 | Dialium pachyphyllum | Gabon |
| Doucet R. | field | 16-06-21 | Dialium pachyphyllum | Gabon |
| Doucet R. | field | 16-06-21 | Dialium pachyphyllum | Gabon |
| Doucet R. | field | 16-06-21 | Dialium pachyphyllum | Gabon |
| Doucet R. | field | 17-06-21 | Dialium pachyphyllum | Gabon |
| Doucet R. | field | 17-06-21 | Dialium pachyphyllum | Gabon |
| Doucet R. | field | 17-06-21 | Dialium pachyphyllum | Gabon |
| Doucet R. | field | 19-06-21 | Dialium pachyphyllum | Gabon |
| Doucet R. | field | 19-06-21 | Dialium pachyphyllum | Gabon |
| Bibang & Doucet J-L. | field | 30-03-21 | Dialium sp. nov. | Gabon |
| Bibang & Doucet J-L. | field | 30-03-21 | Dialium sp. nov. | Gabon |
| Bibang & Doucet J-L. | field | 31-03-21 | Dialium sp. nov. | Gabon |
| Bibang & Doucet J-L. | field | 31-03-21 | Dialium sp. nov. | Gabon |
| Bibang & Doucet J-L. | field | 31-03-21 | Dialium sp. nov. | Gabon |
| Bibang & Doucet J-L. | field | 31-03-21 | Dialium sp. nov. | Gabon |
| Bibang & Doucet J-L. | field | 31-03-21 | Dialium sp. nov. | Gabon |
| Doucet R. | field | May 2019 | Dialium sp. nov. | Gabon |
| Doucet R. | field | May 2019 | Dialium sp. nov. | Gabon |
| Doucet R. | field | May 2019 | Dialium sp. nov. | Gabon |
| Doucet R. | field | May 2019 | Dialium sp. nov. | Gabon |
| Doucet R. | field | 17-06-21 | Dialium sp. nov. | Gabon |
| Doucet R. | field | 19-06-21 | Dialium sp. nov. | Gabon |
| Doucet R. | field | 19-06-21 | Dialium sp. nov. | Gabon |
| Doucet R. | field | 19-06-21 | Dialium sp. nov. | Gabon |
References
- Biwolé, A.B.; Ouédraogo, D.-Y.; Betti, J.L.; Picard, N.; Rossi, V.; Delion, S.; Lagoute, P.; Gourlet-Fleury, S.; Lejeune, P.; Doucet, J.-L. Dynamique des populations d’azobé, Lophira alata Banks ex C. F. Gaertn., et implications pour sa gestion durable au Cameroun. Bois Forets Des Trop. 2019, 342, 55–68. [Google Scholar] [CrossRef]
- Karsenty, A.; Gourlet-Fleury, S. Assessing Sustainability of Logging Practices in the Congo Basin’s Managed Forests: The Issue of Commercial Species Recovery. Ecol. Soc. 2006, 11, art26. [Google Scholar] [CrossRef]
- FRM. Vision Stratégique et Industrialisation de la Filière Bois dans les 6 Pays du Bassin du Congo, Horizon 2030; FRM: Montpellier, France, 2018. [Google Scholar]
- Doucet, J.-L. L’alliance Délicate de la Gestion Forestière et de la Biodiversité dans les Forêts du Centre du Gabon. Ph.D. Thesis, Université de Liège, Gembloux Agro-Bio Tech, Gembloux, Belgium, 2003. [Google Scholar]
- Kleinschroth, F.; Gourlet-Fleury, S.; Sist, P.; Mortier, F.; Healey, J.R. Legacy of logging roads in the Congo Basin: How persistent are the scars in forest cover? Ecosphere 2015, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Edwards, D.P.; Magrach, A.; Woodcock, P.; Ji, Y.; Lim, N.T.L.; Edwards, F.A.; Larsen, T.H.; Hsu, W.W.; Benedick, S.; Khen, C.V.; et al. Selective-logging and oil palm: Multitaxon impacts, biodiversity indicators, and trade-offs for conservation planning. Ecol. Appl. 2014, 24, 2029–2049. [Google Scholar] [CrossRef]
- Edwards, D.P.; Laurance, W.F. Biodiversity Despite Selective Logging. Science 2013, 339, 646–647. [Google Scholar] [CrossRef]
- Cerutti, P.O.; Lescuyer, G.; Tacconi, L.; Eba’a Atyi, R.; Essiane, E.; Nasi, R.; Tabi Eckbil, P.P.; Tsanga, R. Social impacts of the Forest Stewardship Council certification: An assessment in the Congo basin. Int. For. Rev. 2017, 19, 50–63. [Google Scholar] [CrossRef]
- Burivalova, Z.; Hua, F.; Koh, L.P.; Garcia, C.; Putz, F. A Critical Comparison of Conventional, Certified, and Community Management of Tropical Forests for Timber in Terms of Environmental, Economic, and Social Variables. Conserv. Lett. 2017, 10, 4–14. [Google Scholar] [CrossRef] [Green Version]
- FSC. FSC Forest Stewardship Council Standard for the Congo Basin; Forest Stewardship Council A.C.: Bonn, Germany, 2012. [Google Scholar]
- Putz, F.E.; Zuidema, P.A.; Synnott, T.; Peña-Claros, M.; Pinard, M.A.; Sheil, D.; Vanclay, J.K.; Sist, P.; Gourlet-Fleury, S.; Griscom, B.; et al. Sustaining conservation values in selectively logged tropical forests: The attained and the attainable. Conserv. Lett. 2012, 5, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Sist, P.; Piponiot, C.; Kanashiro, M.; Pena-Claros, M.; Putz, F.E.; Schulze, M.; Verissimo, A.; Vidal, E. Sustainability of Brazilian forest concessions. For. Ecol. Manag. 2021, 496, 119440. [Google Scholar] [CrossRef]
- Gérard, J.; Guibal, D.; Paradis, S.; Cerre, J.-C. Tropical Timber Atlas; Quae: Versailles, France, 2017. [Google Scholar]
- Bengono, G.B.; Souza, A.; Tosso, F.; Doucet, R.; Richel, A.; Doucet, J.-L. Les Dialium de la région guinéo-congolaise (synthèse bibliographique). BASE 2021, 25, 172–191. [Google Scholar] [CrossRef]
- Ruwet, M. Caractérisations Morphologiques, Spectrales et Génétiques des Eyoum (Dialium spp.). Master’s Thesis, Université de Liège, Gembloux Agro-Bio Tech, Gembloux, Belgium, 2021. [Google Scholar]
- Allaby, M. A Dictionary of Ecology; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Wieringa, J.J.; MacKinder, B.A. Novitates Gabonensis 79: Hymenostegia elegans and H. robusta spp. nov. (Leguminosae-Caesalpinioideae) from Gabon. Nord. J. Bot. 2012, 30, 144–152. [Google Scholar] [CrossRef]
- Kenfack, D.; Sainge, M.N.; Chuyong, G.B.; Thomas, D.W. The genus cola (Malvaceae) in Cameroon’s Korup national park, with two novelties. Plant Ecol. Evol. 2018, 151, 241–251. [Google Scholar] [CrossRef]
- Bissiengou, P.; Chatrou, L.W.; Wieringa, J.J.; Sosef, M.S.M. Taxonomic novelties in the genus Campylospermum (Ochnaceae). Blumea J. Plant Taxon. Plant Geogr. 2013, 58, 1–7. [Google Scholar] [CrossRef] [Green Version]
- De Azevedo Falcão, M.J.; De Freitas Mansano, V. Dialium heterophyllum (Fabaceae: Dialioideae), a new tree species from the Amazon. Phytotaxa 2020, 477, 47–59. [Google Scholar] [CrossRef]
- Gérard, J.; Guibal, D.; Paradis, S.; Vernay, M.; Beauchêne, J.; Brancheriau, L.; Châlon, I.; Daigremont, C.; Détienne, P.; Fouquet, D.; et al. Tropix 7; CIRAD: Montpelier, France, 2011. [Google Scholar]
- TerEA. Résumé du Plan D’aménagement. Compagnie Equatoriale des Bois. Precious Wood Gabon; TerEA (Terre Environnement Aménagement): Marseille, France, 2007. [Google Scholar]
- Van Soest, P.J.; Wine, R.H. Use of Detergents in the Analysis of Fibrous Feeds. IV. Determination of Plant Cell-Wall Constituents. J. AOAC Int. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- Van Soest, P.J. Use of Detergents in the Analysis of Fibrous Feeds. II. A Rapid Method for the Determination of Fiber and Lignin. J. AOAC Int. 1990, 73, 491–497. [Google Scholar] [CrossRef]
- Chen, H.; Ferrari, C.; Angiuli, M.; Yao, J.; Raspi, C.; Bramanti, E. Qualitative and quantitative analysis of wood samples by Fourier transform infrared spectroscopy and multivariate analysis. Carbohydr. Polym. 2010, 82, 772–778. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.O.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; Renew; Energy Lab.: Golden, CO, USA; Laboratory Analytical Procedure (LAP): Golden, CO, USA, 2005. [Google Scholar]
- Sheppard, P.R.; Thompson, T.L. Effect of Extraction Pretreatment on Radial Variation of Nitrogen Concentration in Tree Rings. J. Environ. Qual. 2000, 29, 2037–2042. [Google Scholar] [CrossRef]
- Gérard, J.; Paradis, S.; Thibaut, B. Survey on the chemical composition of several tropical wood species. Bois Forets Des Trop. 2019, 342, 79–91. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols from Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–259. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Engozogho Anris, S.P.; Bi Athomo, A.B.; Safou Tchiama, R.; Santiago-Medina, F.J.; Cabaret, T.; Pizzi, A.; Charrier, B. The condensed tannins of Okoume (Aucoumea klaineana Pierre): A molecular structure and thermal stability study. Sci. Rep. 2020, 10, 1773. [Google Scholar] [CrossRef]
- Kucheryavskiy, S. mdatools—R package for chemometrics. Chemom. Intell. Lab. Syst. 2020, 198, 103937. [Google Scholar] [CrossRef]
- Hobro, A.J.; Kuligowski, J.; Döll, M.; Lendl, B. Differentiation of walnut wood species and steam treatment using ATR-FTIR and partial least squares discriminant analysis (PLS-DA). Anal. Bioanal. Chem. 2010, 398, 2713–2722. [Google Scholar] [CrossRef]
- Wang, S.N.; Da Zhang, F.; Huang, A.M.; Zhou, Q. Distinction of four Dalbergia species by FTIR, 2nd derivative IR, and 2D-IR spectroscopy of their ethanol-benzene extractives. Holzforschung 2016, 70, 503–510. [Google Scholar] [CrossRef]
- R Studio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Hothorn, T.; Zeileis, A. Partykit: A modular toolkit for recursive partytioning in R. J. Mach. Learn. Res. 2015, 16, 3905–3909. [Google Scholar]
- Pomerantsev, A.L. Acceptance areas for multivariate classification derived by projection methods. J. Chemom. 2008, 22, 601–609. [Google Scholar] [CrossRef]
- Rohart, F.; Gauthier, B.; Signh, A.; Lê Cao, K.A. mixOmics: An R package for omics feature selection and multiple data integration. PLoS Comput Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [Green Version]
- Mahalanobis, P.C. On the Generalised Distance in Statistics. Proc. Natl. Inst. Sci. India 1936, 2, 49–55. [Google Scholar]
- Chong, I.G.; Jun, C.H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests; (v 0.7.0); R Project for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Grasel, F.D.S.; Ferrão, M.F.; Wolf, C.R. Development of methodology for identification the nature of the polyphenolic extracts by FTIR associated with multivariate analysis. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2016, 153, 94–101. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.M.; Hale, M.D.C. Rapid assessment of physical properties and chemical composition of thermally modified wood by mid-infrared spectroscopy. Wood Sci. Technol. 2011, 45, 83–102. [Google Scholar] [CrossRef]
- Yap, M.G.S.; Que, Y.T.; Chia, L.H.L. FTIR characterization of tropical wood–polymer composites. J. Appl. Polym. Sci. 1991, 43, 2083–2090. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Jones, D.; Kržišnik, D.; Humar, M. Determination of the effectiveness of a combined thermal/chemical wood modification by the use of FT–IR spectroscopy and chemometric methods. J. Mol. Struct. 2020, 1200, 127133. [Google Scholar] [CrossRef]
- Backa, S.; Brolin, A.; Nilsson, T. Characterisation of fungal degraded birch wood by FTIR and Py-GC. Holzforschung 2001, 55, 225–232. [Google Scholar] [CrossRef]
- Shen, D.; Liu, G.; Zhao, J.; Xue, J.; Guan, S.; Xiao, R. Thermo-chemical conversion of lignin to aromatic compounds: Effect of lignin source and reaction temperature. J. Anal. Appl. Pyrolysis 2015, 112, 56–65. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef]
- Abdul Latif, M.H.; Attiya, H.G.; Al–Abayaji, M.A. Lignin FT-IR study of Iraqi date palm Phoenix dactylifera frond bases wood. Plant Arch. 2019, 19, 327–332. [Google Scholar]
- Nuopponen, M.H.; Wikberg, H.I.; Birch, G.M.; Jääskeläinen, A.S.; Maunu, S.L.; Vuorinen, T.; Stewart, D. Characterization of 25 tropical hardwoods with fourier transform infrared, ultraviolet resonance raman, and13C-NMR cross-polarization/magic- angle spinning spectroscopy. J. Appl. Polym. Sci. 2006, 102, 810–819. [Google Scholar] [CrossRef]
- Huang, A.; Zhou, Q.; Liu, J.; Fei, B. Distinction of three wood species by Fourier transform infrared spectroscopy and two-dimensional correlation IR spectroscopy. J. Mol. Struct. 2008, 883–884, 160–166. [Google Scholar] [CrossRef]
- Özgenç, Ö.; Durmaz, S.; Boyaci, I.H.; Eksi-Kocak, H. Determination of chemical changes in heat-treated wood using ATR-FTIR and FT Raman spectrometry. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2017, 171, 395–400. [Google Scholar] [CrossRef]
- Pasquet, R.S. Classification infraspecifique des formes spontanees de Vigna unguiculata (L.) Walp. (Fabaceae) a partir de donnees morphologiques. Bull. Du Jard. Bot. Natl. Belg. 1993, 62, 127. [Google Scholar] [CrossRef]
- Vandrot, H. Nomenclature of the new caledonian genera arthroclianthus Baill. and nephrodesmus schindl. (Fabaceae-Desmodieae). Adansonia 2018, 40, 103–129. [Google Scholar] [CrossRef]
- Rojo, J.P. Studies in the Genus Dialium (Cassieae-Caesalpinioideae). Ph.D. Thesis, Oxford University, Oxford, UK, 1982. [Google Scholar]
- Zimmerman, E.; Prenner, G.; Bruneau, A. Floral ontogeny in Dialiinae (Caesalpinioideae: Cassieae), a study in organ loss and instability. S. Afr. J. Bot. 2013, 89, 188–209. [Google Scholar] [CrossRef] [Green Version]
- Bosco Mbagou, J. Variabilité Intra-Arbre des Propriétés Physico-Mécaniques Et Chimiques du Tessmania Africana en Provenance du Gabon. Ph.D. Thesis, Université de Laval, Québec, QC, Canada, 2017. [Google Scholar]
- Lourenço, A.; Neiva, D.M.; Gominho, J.; Marques, A.V.; Pereira, H. Characterization of lignin in heartwood, sapwood and bark from Tectona grandis using Py–GC–MS/FID. Wood Sci. Technol. 2015, 49, 159–175. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Voulgaridis, E.; Passialis, C. Variation of certain chemical properties within the stemwood of black locust (Robinia pseudoacacia L.). Eur. J. Wood Wood Prod. 2005, 63, 327–333. [Google Scholar] [CrossRef]
- Lhate, I.; Cuvilas, C.; Terziev, N.; Jirjis, R. Chemical composition of traditionally and lesser used wood species from Mozambique. Wood Mater. Sci. Eng. 2010, 5, 143–150. [Google Scholar] [CrossRef]
- Doat, J. Etude papetière de l’Okoumé. Essais de laboratoire, semi-industriels et industriels (1re partie). Bois Forets Des Trop. 1972, 146, 31–52. [Google Scholar]
- Nuopponen, M.H.; Birch, G.M.; Sykes, R.J.; Lee, S.J.; Stewart, D. Estimation of wood density and chemical composition by means of diffuse reflectance mid-infrared fourier transform (DRIFT-MIR) spectroscopy. J. Agric. Food Chem. 2006, 54, 34–40. [Google Scholar] [CrossRef]
- Martin, A.R.; Erickson, D.L.; Kress, W.J.; Thomas, S.C. Wood nitrogen concentrations in tropical trees: Phylogenetic patterns and ecological correlates. N. Phytol. 2014, 204, 484–495. [Google Scholar] [CrossRef]
- Gérardin, P.; Fritsch, C.; Cosgun, S.; Brennan, M.; Dumarçay, S.; Colin, F.; Gérardin, P. Effet de la hauteur de prélèvement sur la composition quantitative et qualitative des polyphénols de l’écorce d’Abies alba Mill. Rev. For. Française 2020, 72, 411–423. [Google Scholar] [CrossRef]
- Bikoro Bi Athomo, A.; Engozogho Anris, S.P.; Safou-Tchiama, R.; Santiago-Medina, F.J.; Cabaret, T.; Pizzi, A.; Charrier, B. Chemical composition of African mahogany (K. ivorensis A. Chev) extractive and tannin structures of the bark by MALDI-TOF. Ind. Crops Prod. 2018, 113, 167–178. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative Study Regarding the Chemical Composition and Biological Activity of Pine (Pinus nigra and P. sylvestris) Bark Extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef]
- Tchinda Saha, J.-B.; Abia, D.; Dumarç Ay, S.; Kor Ndikontar, M.; Gérardin, P.; Noah, J.N.; Perrin, D. Antioxidant activities, total phenolic contents and chemical compositions of extracts from four Cameroonian woods: Padouk (Pterocarpus soyauxii Taubb), tali (Erythrophleum suaveolens), moabi (Baillonella toxisperma), and movingui (Distemonanthus benthamianus). Ind. Crops Prod. 2012, 41, 71–77. [Google Scholar]
- Poorter, L. Leaf traits show different relationships with shade tolerance in moist versus dry tropical forests. N. Phytol. 2009, 181, 890–900. [Google Scholar] [CrossRef]
- Sack, L.; Cowan, P.D.; Jaikumar, N.; Holbrook, N.M. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant. Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef] [Green Version]
- Barnett, J.; Jeronimidis, G. Wood Quality and Its Biological Basis; Blackwell Publishing Ltd.: Carlton, VIC, Australia, 2003. [Google Scholar]
- Cruz, N.; Bustos, C.; Aguayo, M.G.; Cloutier, A.; Castillo, R. THM densification of wood. BioResources 2018, 13, 2268–2282. [Google Scholar]
- Dirol, D.; Deglise, X. Durabilité des Bois et Problèmes Associés; Hermès Science: Paris, France, 2001. [Google Scholar]
- Anouhe, B.J.S.; Niamké, F.B.; Faustin, M.; Virieux, D.; Pirat, J.L.; Adima, A.A.; Kati-Coulibaly, S.; Amusant, N. The role of extractives in the natural durability of the heartwood of Dicorynia guianensis Amsh: New insights in antioxydant and antifungal properties. Ann. For. Sci. 2018, 75, 15. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A. Tannins in Woods and Their Contribution to Microbial Decay Prevention. In Plant Polyphenols; Springer: Boston, MA, USA, 1992; pp. 935–952. [Google Scholar]
- Akai, S.; Fukutomi, M. Preformed Internal Physical Defenses. In How Plants Defend Themselves; Horsfall, J.G., Cowling, E.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1980; pp. 139–159. [Google Scholar]




| Trait | Units/Modalities |
|---|---|
| Petiole type | “cylindrical”; “bulged”; “canaliculated” |
| Petiole length | mm |
| Rachis length | mm |
| Foliole number | - |
| Pilosity on the upper face | “pubescent”; “glabrous” |
| Pilosity on the above face | “pubescent”; “glabrous” |
| Curved margin | “yes”; “no” |
| Acumen shape | “oblong”; “elliptical”; “lanceolate”; “ovate”; “obovate”; “oblanceolate”; “falciform” |
| Asymmetric leaflet base | “yes”; “no” |
| Tertiary venation | “tight and prominent”; “discrete” |
| Translucent dots | “yes”; “no” |
| Terminal leaflet lamina shape | “no acumen”; “sharp”; “tapered”; “retuse”; “obtuse”; “truncated”; “emarginated” |
| Terminal leaflet lamina length | Mm |
| Terminal leaflet lamina width | mm |
| Basal leaflet lamina shape | “no acumen”; “sharp”; “tapered”; “retuse”; “obtuse”; “truncated”; “emarginated” |
| Basal leaflet lamina length | Mm |
| Basal leaflet lamina width | Mm |
| Coriaceous lamina | “yes”; “no” |
| Twisted lamina | “yes”; “no” |
| Petiolule length | mm |
| Ratio petiole/rachis | - |
| Ratio length/width of terminal leaflet | - |
| Ratio length/width of basal leaflet | - |
| Ratio basal leaflet length/terminal leaflet length | - |
| Ratio terminal leaflet length/petiolule length | - |
| Species | Tree | Position | Altitude | Slope | p | hp | h1 | h2 | ht |
|---|---|---|---|---|---|---|---|---|---|
| D. pachyphyllum | 1 | Em | 369 | 0 | 3.30 | 2.2 | 1.3 | 12.0 | 43.0 |
| 2 | Can | 358 | 5 | 3.10 | 3.1 | 1.6 | 10.2 | 41.0 | |
| 3 | Em | 360 | 0 | 2.90 | 6.1 | 2.0 | 17.1 | 42.0 | |
| 4 | Em | 365 | 0 | 2.86 | 1.3 | 1.7 | 14.0 | 46.5 | |
| D. sp. nov. | 1 | Can | 348 | 0 | 3.60 | 5.1 | 2.7 | 18.9 | 43.0 |
| 2 | Em | 343 | 0 | 2.60 | 3.3 | 1.9 | 19.6 | 39.0 | |
| 3 | Em | 347 | 30 | 2.65 | 2.4 | 2.5 | 21.0 | 42.0 | |
| 4 | Can | 345 | 0 | 2.55 | 2.8 | 1.8 | 16.7 | 44.5 |
| Experimental Repetition (n) | |||||
|---|---|---|---|---|---|
| Tree | Hemicellulose | Cellulose | Lignin | Nitrogen | Ash |
| 1 | 2 | 2 | 2 | 3 | 3 |
| 2 | 2 | 2 | 2 | 3 | 3 |
| 3 | 2 | 2 | 2 | - | - |
| 4 | 2 | 2 | 2 | - | - |
| D. pachyphyllum | D. sp. nov. | ||
|---|---|---|---|
| Predicted | D. pachyphyllum | 15 | 0 |
| D. sp. nov. | 1 | 14 |
| W | Δ | VIP Scores | Chemical Bond Assignment | Mol | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LV1 | LV2 | LV3 | LV4 | LV5 | LV6 | LV7 | |||||
| 1714 | 38 | 1.47 | 1.19 | 1.14 | 1.12 | 1.10 | 1.08 | 1.09 | 1712 cm−1: C=O groups | L | [43] |
| 1585 | 45 | 1.62 | 1.28 | 1.25 | 1.22 | 1.21 | 1.20 | 1.18 | 1585 cm−1: Aromatic ring stretching | L | [44] |
| 1519 | 41 | 1.56 | 1.29 | 1.22 | 1.20 | 1.19 | 1.16 | 1.15 | 1517 cm−1: Aromatic skeletal vibration | L | [43] |
| 1511 | 44 | 1.37 | 1.13 | 1.07 | 1.05 | 1.04 n | 1.02 n | 1.05 | 1508–1513 cm−1: Aromatic skeletal vibration | L, T | [42,43] |
| 1494 | 40 | 1.60 | 1.31 | 1.24 | 1.23 | 1.22 | 1.19 | 1.19 | 1495 cm−1: aromatic vibration | P, T | [45] |
| 1471 | 58 | 1.77 | 1.40 | 1.33 | 1.32 | 1.30 | 1.28 | 1.26 | 1460–1470 cm−1: C-H vibration | L | [46,47] |
| 1413 | 83 | 1.93 | 1.53 | 1.46 | 1.43 | 1.41 | 1.38 | 1.37 | 1413 cm−1: C=C aromatic | L | [48] |
| 1384 | 31 | 1.49 | 1.20 | 1.14 | 1.13 | 1.14 | 1.11 | 1.10 | 1384 cm−1: -OH bending of phenolic bond | L | [49] |
| 1270 | 32 | 1.09 n | 1.14 | 1.09 | 1.07 | 1.05 n | 1.03 n | 1.01 n | 1265 cm−1: C-O vibration | L | [50,51] |
| 1253 | 33 | 1.07 | 0.89 n | 0.85 n | 0.84 n | 0.83 n | 0.82 n | 0.81 n | 1250 cm−1: C-O-C asymmetric stretch | L | [34] |
| 1209 | 62 | 1.50 | 1.21 | 1.15 | 1.13 | 1.11 | 1.10 | 1.09 | 1203–1210 cm−1: O-H bending | C | [52] |
| 1133 | 57 | 1.18 | 0.94 n | 1.07 n | 1.11 | 1.10 | 1.09 | 1.08 | 1134 cm−1: C-O-C glycosidic vibration of xylan | H | [50] |
| 1114 | 37 | 1.54 | 1.64 | 1.63 | 1.62 | 1.60 | 1.56 | 1.54 | 1112–1113 cm−1: C-H bending in plane | T | [42] |
| 1051 | 98 | 1.73 | 1.64 | 1.56 | 1.53 | 1.51 | 1.50 | 1.48 | 1050 cm−1: C-H stretching in plane | T | [42] |
| 958 | 42 | 1.33 | 1.12 n | 1.12 | 1.13 | 1.11 | 1.09 | 1.08 n | 957–961 cm−1: C-H aromatic out of plane deformation | - | [45] |
| 1. |
| 2. |
| D. pachyphyllum | |
| 2. |
| 3. |
| D. lopense | |
| 3. |
| D. sp. nov. |
| D. lopense |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doucet, R.; Bibang Bengono, G.; Ruwet, M.; Van De Vreken, I.; Lecart, B.; Doucet, J.-L.; Fernandez Pierna, J.A.; Lejeune, P.; Jourez, B.; Souza, A.; et al. Highlighting a New Morphospecies within the Dialium Genus Using Leaves and Wood Traits. Forests 2022, 13, 1339. https://doi.org/10.3390/f13081339
Doucet R, Bibang Bengono G, Ruwet M, Van De Vreken I, Lecart B, Doucet J-L, Fernandez Pierna JA, Lejeune P, Jourez B, Souza A, et al. Highlighting a New Morphospecies within the Dialium Genus Using Leaves and Wood Traits. Forests. 2022; 13(8):1339. https://doi.org/10.3390/f13081339
Chicago/Turabian StyleDoucet, Robin, Gaël Bibang Bengono, Marius Ruwet, Isabelle Van De Vreken, Brieuc Lecart, Jean-Louis Doucet, Juan Antonio Fernandez Pierna, Philippe Lejeune, Benoit Jourez, Alain Souza, and et al. 2022. "Highlighting a New Morphospecies within the Dialium Genus Using Leaves and Wood Traits" Forests 13, no. 8: 1339. https://doi.org/10.3390/f13081339






