Different Taxonomic and Functional Indices Complement the Understanding of Herb-Layer Community Assembly Patterns in a Southern-Limit Temperate Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Vegetation Sampling
2.3. Trait Measurement
2.4. Environmental Variables Measurement
2.5. Calculation of Functional Indices
2.6. Data Analysis
3. Results
3.1. Patterns in Taxonomic Composition and Richness
3.2. Patterns in Functional Composition, Richness, and Diversity
3.3. Taxonomic vs. Functional Composition, Richness, and Diversity
4. Discussion
4.1. Patterns in Taxonomic Composition and Richness
4.2. Patterns in Functional Composition, Richness, and Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.; Butchart, S. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES Secretariat): Bonn, Germany, 2019. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Aranda, I.; Gil, L.; Pardos, J.A. Water relations and gas exchange in Fagus sylvatica L. and Quercus petraea (Mattuschka) Liebl. in a mixed stand at their southern limit of distribution in Europe. Trees 2000, 14, 344–352. [Google Scholar] [CrossRef]
- Jump, A.S.; Hunt, J.M.; Peñuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol 2006, 12, 2163–2174. [Google Scholar] [CrossRef]
- Landuyt, D.; De Lombaerde, E.; Perring, M.P.; Hertzog, L.R.; Ampoorter, E.; Maes, S.L.; De Frenne, P.; Ma, S.; Proesmans, W.; Blondeel, H.; et al. The functional role of temperate forest understorey vegetation in a changing world. Glob. Chang. Biol 2019, 25, 3625–3641. [Google Scholar] [CrossRef]
- Dorado-Liñán, I.; Akhmetzyanov, L.; Menzel, A. Climate threats on growth of rear-edge European beech peripheral populations in Spain. Int J. Biometeorol. 2017, 61, 2097–2110. [Google Scholar] [CrossRef] [PubMed]
- Gracia, M.; Montané, F.; Piqué, J.; Retana, J. Overstory structure and topographic gradients determining diversity and abundance of understory shrub species in temperate forests in central Pyrenees (NE Spain). For. Ecol. Manag. 2007, 242, 391–397. [Google Scholar] [CrossRef]
- Gilliam, F.S. The Ecological Significance of the Herbaceous Layer in Temperate Forest Ecosystems. BioScience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. On the formation of dense understory layers in forests worldwide: Consequences and implications for forest dynamics, biodiversity, and succession. Can. J. For. Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- Landuyt, D.; Perring, M.P.; Seidl, R.; Taubert, F.; Verbeeck, H.; Verheyen, K. Modelling understorey dynamics in temperate forests under global change–Challenges and perspectives. Perspect Plant. Ecol. Evol. Syst. 2018, 31, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Small, C.J.; Mccarthy, B.C. Spatial and temporal variability of herbaceous vegetation in an eastern deciduous forest. Plant. Ecol. 2002, 164, 37–48. Available online: https://www.jstor.org/stable/20146339 (accessed on 5 September 2022).
- Díaz, S.; Cabido, M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef]
- De Pauw, K.; Meeussen, C.; Govaert, S.; Sanczuk, P.; Vanneste, T.; Bernhardt-Römermann, M.; Bollmann, K.; Brunet, J.; Calders, K.; Cousins, S.A.O.; et al. Taxonomic, phylogenetic and functional diversity of understorey plants respond differently to environmental conditions in European forest edges. J. Ecol. 2021, 109, 2629–2648. [Google Scholar] [CrossRef]
- Weiher, E.; Keddy, P.A. Assembly rules, null models, and trait dispersion: New questions from old patterns. Oikos 1995, 74, 159–164. [Google Scholar] [CrossRef]
- Botta-Dukát, Z.; Czúcz, B. Testing the ability of functional diversity indices to detect trait convergence and divergence using individual-based simulation. Methods Ecol. Evol. 2016, 7, 114–126. [Google Scholar] [CrossRef]
- MacArthur, R.; Levins, R. The Limiting Similarity, Convergence, and Divergence of Coexisting Species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Ackerly, D.D. Assembly of plant communities. In Ecology and the Environment; Monson, R.K., Ed.; Springer: New York, NY, USA, 2014; pp. 67–88. [Google Scholar] [CrossRef]
- Spasojevic, M.J.; Suding, K.N. Inferring community assembly mechanisms from functional diversity patterns: The importance of multiple assembly processes. J. Ecol. 2012, 100, 652–661. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Girardello, M.; Chytrý, M.; Svenning, J.-C.; Willner, W.; Gégout, J.-C.; Agrillo, E.; Campos, J.A.; Jandt, U.; Kącki, Z. History and environment shape species pools and community diversity in European beech forests. Nat. Ecol. Evol. 2018, 2, 483–490. [Google Scholar] [CrossRef]
- Adler, P.B.; Fajardo, A.; Kleinhesselink, A.R.; Kraft, N.J.B. Trait-based tests of coexistence mechanisms. Ecol. Lett. 2013, 16, 1294–1306. [Google Scholar] [CrossRef]
- Loughnan, D.; Gilbert, B. Trait-mediated community assembly: Distinguishing the signatures of biotic and abiotic filters. Oikos 2017, 126, 1112–1122. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Lhotsky, B.; Kovács, B.; Ónodi, G.; Csecserits, A.; Rédei, T.; Lengyel, A.; Kertész, M.; Botta-Dukát, Z. Changes in assembly rules along a stress gradient from open dry grasslands to wetlands. J. Ecol. 2016, 104, 507–517. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, J.; Tan, S.; Cadotte, M.W.; Xu, K.; Gao, L.; Li, D. Trait variation and functional diversity maintenance of understory herbaceous species coexisting along an elevational gradient in Yulong Mountain, Southwest China. Plant. Divers. 2016, 38, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Gillison, A.N. Plant Functional Types and Traits at the Community, Ecosystem and World Level. In Vegetation Ecology, 2nd ed.; van der Maarel, E., Franklin, J., Eds.; Wiley-Blackwell: Chichester, UK, 2013; pp. 347–386. [Google Scholar] [CrossRef]
- Hodgson, J.G.; Montserrat-Martí, G.; Charles, M.; Jones, G.; Wilson, P.; Shipley, B.; Sharafi, M.; Cerabolini, B.E.L.; Cornelissen, J.H.C.; Band, S.R.; et al. Is leaf dry matter content a better predictor of soil fertility than specific leaf area? Ann. Bot. 2011, 108, 1337–1345. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Van der Plas, F.; Janzen, T.; Ordonez, A.; Fokkema, W.; Reinders, J.; Etienne, R.S.; Olff, H. A new modeling approach estimates the relative importance of different community assembly processes. Ecology 2015, 96, 1502–1515. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaced framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Rao, C.R. Diversity and dissimilarity coefficients: A unified approach. Theor. Popul. Biol. 1982, 21, 24–43. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Mandle, L.; Ticktin, T. Moderate land use changes plant functional composition without loss of functional diversity in India’s Western Ghats. Ecol. Appl. 2015, 25, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, F.M.; Jiménez-Alfaro, B.; Burrascano, S.; Blasi, C. Drivers of herb-layer species diversity in two unmanaged temperate forests in northern Spain. Community Ecol. 2014, 15, 147–157. [Google Scholar] [CrossRef]
- Rozas, O.V.; González García, A.; Lamas Pose, S.; García González, I. Estudio Dendrocronológico en el Parque Natural Señorío de Bertiz, Navarra. 2008. Available online: http://www.parquedebertiz.es/imgx_new/bertiz/bertiz/documentos_pdf/Estudio%20dendrocronol%C3%B3gico%20en%20el%20Parque%20Natural%20Se%C3%B1or%C3%ADo%20de%20Bertiz.pdf (accessed on 5 July 2022).
- Costa, M.; Morla, C.; Sainz, H. Los Bosques Ibéricos: Una Interpretación Geobotánica; Editorial Planeta: Barcelona, Spain, 1998. [Google Scholar]
- Valladares, F. La disponibilidad de luz bajo el dosel de los bosques y matorrales ibéricos estimada mediante fotografía hemisférica. Ecología 2006, 20, 11–30. [Google Scholar]
- Kermavnar, J.; Kutnar, L.; Marinšek, A. Variation in floristic and trait composition along environmental gradients in the herb layer of temperate forests in the transition zone between Central and SE Europe. Plant. Ecol. 2022, 223, 229–242. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Burrascano, S.; Tuomisto, H.; Blasi, C. Ground layer plant species turnover and beta diversity in southern-European old-growth forests. PLoS ONE 2014, 9, e95244. [Google Scholar] [CrossRef]
- Tinya, F.; Kovács, B.; Bidló, A.; Dima, B.; Király, I.; Kutszegi, G.; Lakatos, F.; Mag, Z.; Márialigeti, S.; Nascimbene, J. Environmental drivers of forest biodiversity in temperate mixed forests—A multi-taxon approach. Sci. Total Environ. 2021, 795, 148720. [Google Scholar] [CrossRef]
- Márialigeti, S.; Tinya, F.; Bidló, A.; Ódor, P. Environmental drivers of the composition and diversity of the herb layer in mixed temperate forests in Hungary. Plant. Ecol. 2016, 217, 549–563. [Google Scholar] [CrossRef] [Green Version]
- Gobierno de Navarra. Meteorología y Climatología de Navarra. 2019. Available online: http://meteo.navarra.es (accessed on 30 May 2019).
- Bermejo, R.; Garrigó, J.; Gazol, A.; Ibáñez, R.; Ederra, A.; Ariño, A.; Santamaría, J.M. Report on national ICP IM activities in Spain. The. Finn. Environ. 2008, 28, 106–108. [Google Scholar]
- Ariño, A.; Bermejo, R.; Ibáñez, R.; Santamaría, C.; Elustondo, D.; Lasheras, E.; Santamaría, J.M. Report on national ICP IM activities in Spain. The. Finn. Environ. 2011, 18, 53–57. [Google Scholar]
- Gazol, A.; Ibáñez, R. Different response to environmental factors and spatial variables of two attributes (cover and diversity) of the understorey layers. For. Ecol. Manag. 2009, 258, 1267–1274. [Google Scholar] [CrossRef]
- Castroviejo, S. (Coord.); Flora Iberica. Plantas Vasculares de la Península Ibérica e Islas Baleares; Real Jardín Botánico CSIC: Madrid, Spain, 2019; pp. 1986–2021. [Google Scholar]
- Carmona, C.P.; Mason, N.W.H.; Azcárate, F.M.; Peco, B. Inter-annual fluctuations in rainfall shift the functional structure of Mediterranean grasslands across gradients of productivity and disturbance. J. Veg. Sci. 2015, 26, 538–551. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ. National Institute of Health Bethesda: Maryland, USA, 1997–2019. Available online: https://imagej.nih.gov/ij/ (accessed on 30 May 2019).
- Gobierno de Navarra. Mapa Topográfico 1:5.000, hoja 90 1-6. 2004. Available online: http://www.navarra.es/appsext/tiendacartografia/ (accessed on 5 November 2008).
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring Functional Diversity From Multiple Traits, and Other Tools for Functional Ecology. 2014. R Package Version 1.0–12. Available online: https://cran.r-project.org/web/packages/FD/FD.pdf (accessed on 5 July 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 5 July 2022).
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Gotelli, N.J.; McCabe, D.J. Species Co-Occurrence: A Meta-Analysis of J. M. Diamond’s Assembly Rules Model. Ecology 2002, 83, 2091–2096. [Google Scholar] [CrossRef]
- Wilson, J. Assembly rules in plant communities. In Ecological Assembly Rules: Perspectives, Advances, Retreats; Weiher, E., Keddy, P., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 130–164. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier Science BV: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn Minchin, D.P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P. Vegan: Community Ecology Package. 2019. R Package Version 2.5-6. Available online: https://CRAN.R-project.org/package=vegan (accessed on 5 July 2022).
- Zelený, D. Which results of the standard test for community-weighted mean approach are too optimistic? J. Veg. Sci. 2018, 29, 953–966. [Google Scholar] [CrossRef]
- Cormont, A.; Vos, C.C.; van Turnhout, C.A.M.; Foppen, R.P.B.; ter Braak, C.J.F. Using life-history traits to explain bird population responses to changing weather variability. Clim. Res. 2011, 49, 59–71. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Peres-Neto, P.; Dray, S. A critical issue in model-based inference for studying trait-based community assembly and a solution. PeerJ 2017, 5, e2885. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Dray, S.; ter Braak, C.J.F. Linking trait variation to the environment: Critical issues with community-weighted mean correlation resolved by the fourth-corner approach. Ecography 2017, 40, 806–816. [Google Scholar] [CrossRef]
- Zelený, D. weimea: Weighted Mean Analysis, R package version 0.1.18; R Package: Madison, WI, USA, 2020.
- Zelený, D.; Schaffers, A.P. Too good to be true: Pitfalls of using mean Ellenberg indicator values in vegetation analyses. J. Veg. Sci. 2012, 23, 419–431. [Google Scholar] [CrossRef]
- Gazol, A.; Ibáñez, R. Plant species composition in a temperate forest: Multi-scale patterns and determinants. Acta Oecol. 2010, 36, 634–644. [Google Scholar] [CrossRef]
- Härdtle, W.; von Oheimb, G.; Westphal, C. Relationships between the vegetation and soil conditions in beech and beech-oak forests of northern Germany. Plant Ecol. 2005, 177, 113–124. [Google Scholar] [CrossRef]
- Verheyen, K.; Baeten, L.; De Frenne, P.; Bernhardt-Römermann, M.; Brunet, J.; Cornelis, J.; Decocq, G.; Dierschke, H.; Eriksson, O.; Hédl, R.; et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. J. Ecol. 2012, 100, 352–365. [Google Scholar] [CrossRef]
- Verstraeten, G.; Baeten, L.; Van den Broeck, T.; De Frenne, P.; Demey, A.; Tack, W.; Muys, B.; Verheyen, K. Temporal changes in forest plant communities at different site types. Appl. Veg. Sci. 2013, 16, 237–247. [Google Scholar] [CrossRef]
- Graae, B.J.; Økland, R.H.; Petersen, P.M.; Jensen, K.; Fritzbøger, B. Influence of historical, geographical and environmental variables on understorey composition and richness in Danish forests. J. Veg. Sci. 2004, 15, 465–474. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Gawroński, S. Effect of litter removal on species richness and acidification of a mixed oak-pine woodland. Biol. Conserv. 2002, 106, 389–398. [Google Scholar] [CrossRef]
- North, M.; Oakley, B.; Fiegener, R.; Gray, A.; Barbour, M. Influence of light and soil moisture on Sierran mixed-conifer understory communities. Plant Ecol. 2005, 177, 13–24. [Google Scholar] [CrossRef]
- Facelli, J.M.; Picket, S.T.A. Plant litter: Its dynamics and effects on plant community structure. Bot. Rev. 1991, 57, 1–32. [Google Scholar] [CrossRef]
- Gazol, A.; Ibáñez, R. Variation of plant diversity in a temperate unmanaged forest in northern Spain: Behind the environmental and spatial explanation. Plant. Ecol. 2010, 207, 1–11. [Google Scholar] [CrossRef]
- Kermavnar, J.; Marinšek, A.; Eler, K.; Kutnar, L. Evaluating short-term impacts of forest management and microsite conditions on understory vegetation in temperate fir-beech forests: Floristic, ecological, and trait-based perspective. Forests 2019, 10, 909. [Google Scholar] [CrossRef] [Green Version]
- Worrall, J.J.; Lee, T.D.; Harrington, T.C. Forest dynamics and agents that initiate and expand canopy gaps in Picea-Abies forests of Crawford Notch, New Hampshire, USA. J. Ecol. 2005, 93, 178–190. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Kern, C.C.; Montgomery, R.A.; Reich, P.B.; Strong, T.F. Canopy gap size influences niche partitioning of the ground-layer plant community in a northern temperate forest. J. Plant. Ecol. 2013, 6, 101–112. [Google Scholar] [CrossRef]
- Valerio, M.; Ibáñez, R.; Gazol, A. The role of canopy cover dynamics over a decade of changes in the understory of an Atlantic beech-oak forest. Forests 2021, 12, 938. [Google Scholar] [CrossRef]
- Laanisto, L.; Urbas, P.; Pärtel, M. Why does the unimodal species richness-productivity relationship not apply to woody species: A lack of clonality or a legacy of tropical evolutionary history? Glob. Ecol. Biogeogr. 2008, 17, 320–326. [Google Scholar] [CrossRef]
- Peet, R.K.; Christensen, N.L.; Gilliam, F.S. Temporal patterns in Herbaceous layer communities of the North Carolina piedmont. In The Herbaceous Layer in Forests of Eastern North America, 2nd ed.; Gilliam, F.S., Ed.; Oxford University Press: New York, NY, USA, 2014; pp. 277–293. [Google Scholar] [CrossRef]
- Frey, S.J.K.; Hadley, A.S.; Johnson, S.L.; Schulze, M.; Jones, J.A.; Betts, M.G. Spatial models reveal the microclimatic buffering capacity of old-growth forests. Sci. Adv. 2016, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lin, K.; Vadeboncoeur, M.A.; Chen, M.; Huang, M.; Lin, T. Understorey plant community and light availability in conifer plantations and natural hardwood forests in Taiwan. Appl. Veg. Sci. 2015, 18, 591–602. [Google Scholar] [CrossRef]
- Gazol, A.; Ibáñez, R. Scale-specific determinants of a mixed beech and oak seedling-sapling bank under different environmental and biotic conditions. Plant Ecol. 2010, 211, 37–48. [Google Scholar] [CrossRef]
- Rozas, V. Regeneration patterns, dendroecology, and forest-use history in an old-growth beech-oak lowland forest in Northern Spain. For. Ecol. Manag. 2003, 182, 175–194. [Google Scholar] [CrossRef]
- Van Hees, A. Growth and morphology of pedunculate oak (Quercus robur L.) and beech (Fagus sylvatica L.) seedlings in relation to shading and drought. Ann. Sci. For. 1997, 54, 9–18. [Google Scholar] [CrossRef]
- Dwyer, J.M.; Laughlin, D.C. Selection on trait combinations along environmental gradients. J. Veg. Sci. 2017, 28, 672–673. [Google Scholar] [CrossRef] [Green Version]

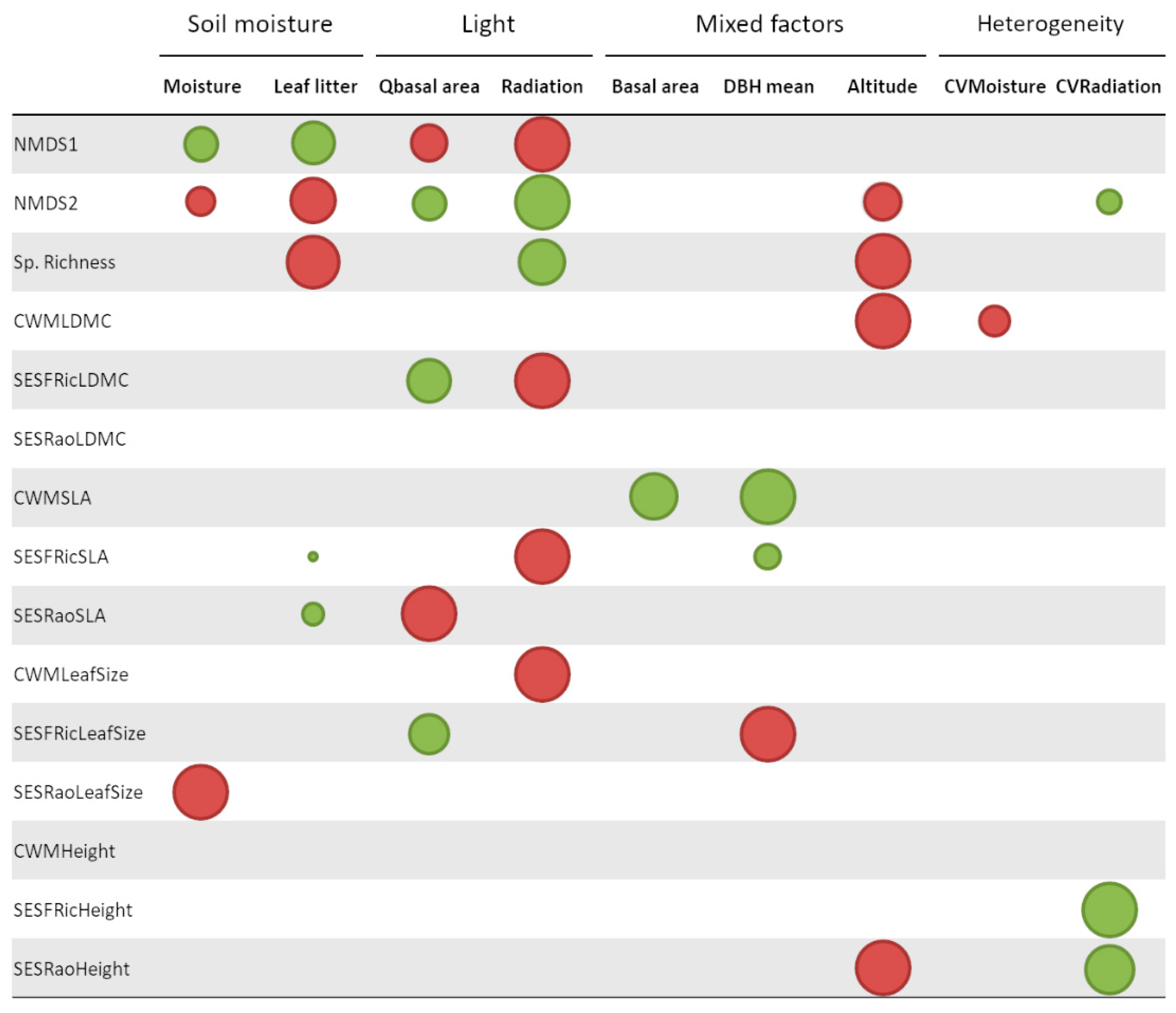
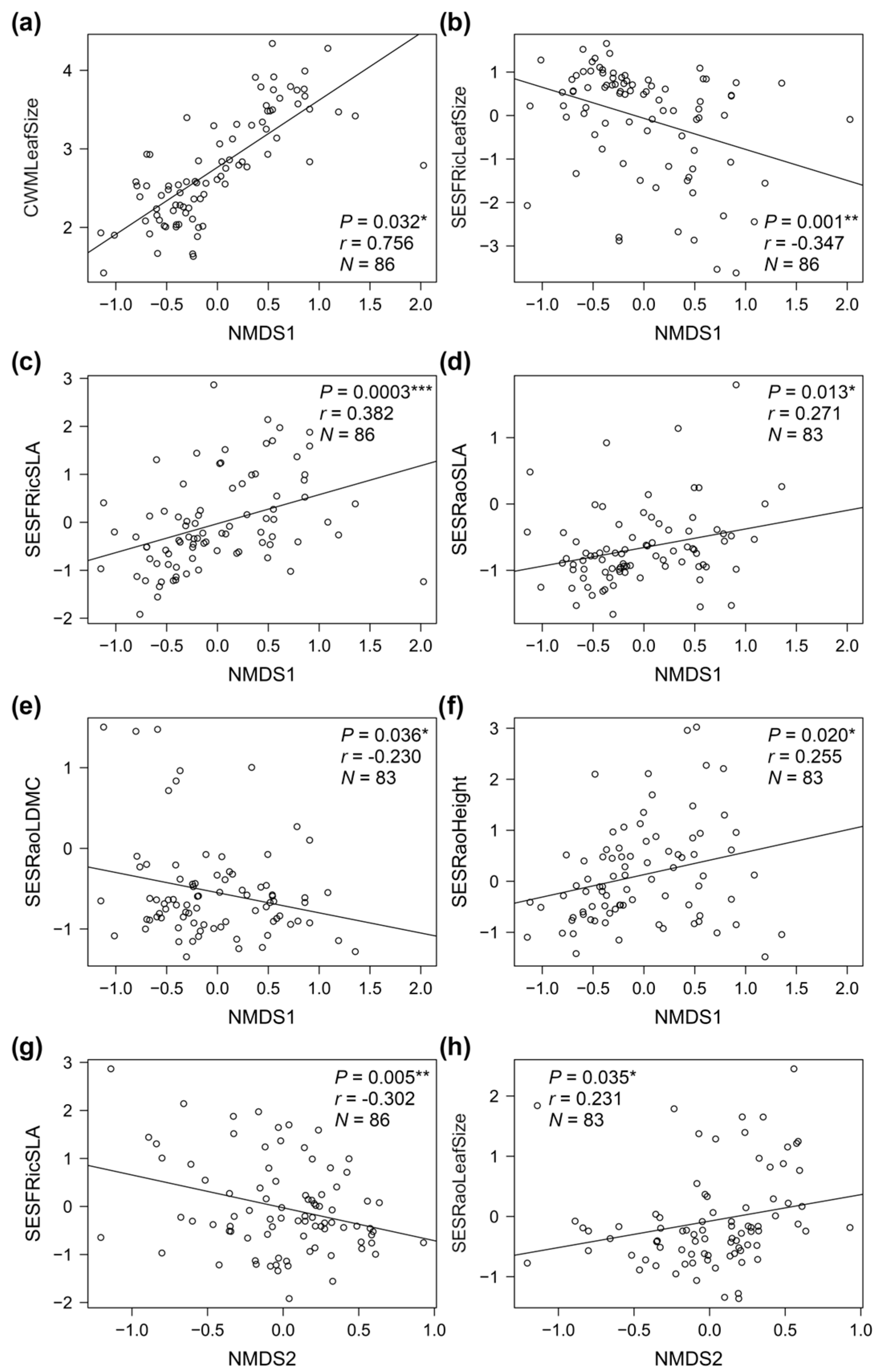
| Trait | Index | Predictor | Estimate | Standard Error | F Value | p-Value | df |
|---|---|---|---|---|---|---|---|
| Leaf Dry Matter Content (LDMC) | |||||||
| CWM | 83 | ||||||
| altitude | −10.781 | 2.556 | 17.786 | 0.012 * | |||
| CV moisture | −6.029 | 2.736 | 4.856 | 0.018 * | |||
| SESFRic | 83 | ||||||
| radiation | −0.281 | 0.104 | 4.041 | 0.048 * | |||
| Quercus basal area | 0.224 | 0.104 | 4.618 | 0.035 * | |||
| SESRao | 81 | ||||||
| altitude | −0.130 | 0.066 | 3.826 | 0.054. | |||
| Specific Leaf Area (SLA) | |||||||
| CWM | 83 | ||||||
| basal area | 0.865 | 0.407 | 4.506 | 0.038 * | |||
| DBH mean | 1.020 | 0.403 | 6.404 | 0.032 * | |||
| SESFRic | 82 | ||||||
| radiation | −0.402 | 0.106 | 15.626 | 0.0002 *** | |||
| leaf litter | 0.063 | 0.106 | 8.866 | 0.004 ** | |||
| DBH mean | 0.188 | 0.091 | 4.262 | 0.042 * | |||
| SESRao | 80 | ||||||
| leaf litter | 0.080 | 0.062 | 5.549 | 0.021 * | |||
| Quercus basal area | −0.201 | 0.062 | 10.385 | 0.002 ** | |||
| Leaf size | |||||||
| CWM | 84 | ||||||
| radiation | −0.355 | 0.064 | 31.234 | 0.006 ** | |||
| SESFRic | 83 | ||||||
| Quercus basal area | 0.306 | 0.123 | 6.180 | 0.015 * | |||
| DBH mean | −0.417 | 0.123 | 13.518 | 0.0004 *** | |||
| SESRao | 81 | ||||||
| moisture | −0.339 | 0.079 | 18.343 | 0.0001 *** | |||
| Plant height | |||||||
| CWM | 84 | ||||||
| slope | 0.044 | 0.015 | 8.206 | 0.208 | |||
| SESFRic | 84 | ||||||
| CV radiation | 0.292 | 0.109 | 7.190 | 0.009 ** | |||
| SESRao | 80 | ||||||
| altitude | −0.313 | 0.099 | 8.297 | 0.005 ** | |||
| CV radiation | 0.281 | 0.099 | 7.987 | 0.006 ** | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valerio, M.; Gazol, A.; Puy, J.; Ibáñez, R. Different Taxonomic and Functional Indices Complement the Understanding of Herb-Layer Community Assembly Patterns in a Southern-Limit Temperate Forest. Forests 2022, 13, 1434. https://doi.org/10.3390/f13091434
Valerio M, Gazol A, Puy J, Ibáñez R. Different Taxonomic and Functional Indices Complement the Understanding of Herb-Layer Community Assembly Patterns in a Southern-Limit Temperate Forest. Forests. 2022; 13(9):1434. https://doi.org/10.3390/f13091434
Chicago/Turabian StyleValerio, Mercedes, Antonio Gazol, Javier Puy, and Ricardo Ibáñez. 2022. "Different Taxonomic and Functional Indices Complement the Understanding of Herb-Layer Community Assembly Patterns in a Southern-Limit Temperate Forest" Forests 13, no. 9: 1434. https://doi.org/10.3390/f13091434





