Predicting the Potential Distribution of the Endangered Plant Cremastra appendiculata (Orchidaceae) in China under Multiple Climate Change Scenarios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Species Distribution Point Data
2.3. Filtering of Environment Variables
2.4. Model Building, Optimization, and Evaluation
2.5. Model Reliability Test and Suitable Habitats Classification
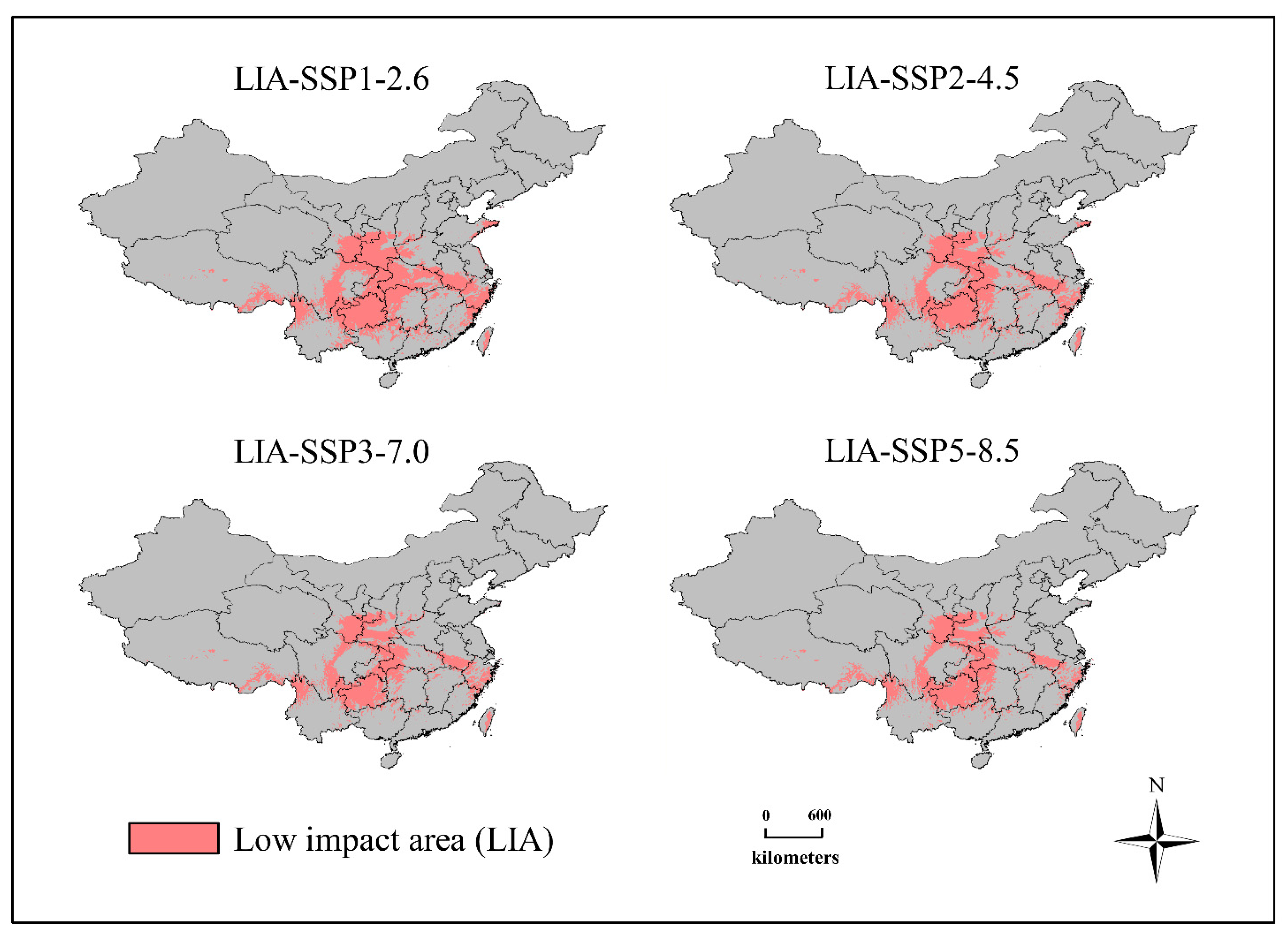
2.6. Analysis of Relatively Stable Suitable Habitats and Changes in Spatial Patterns
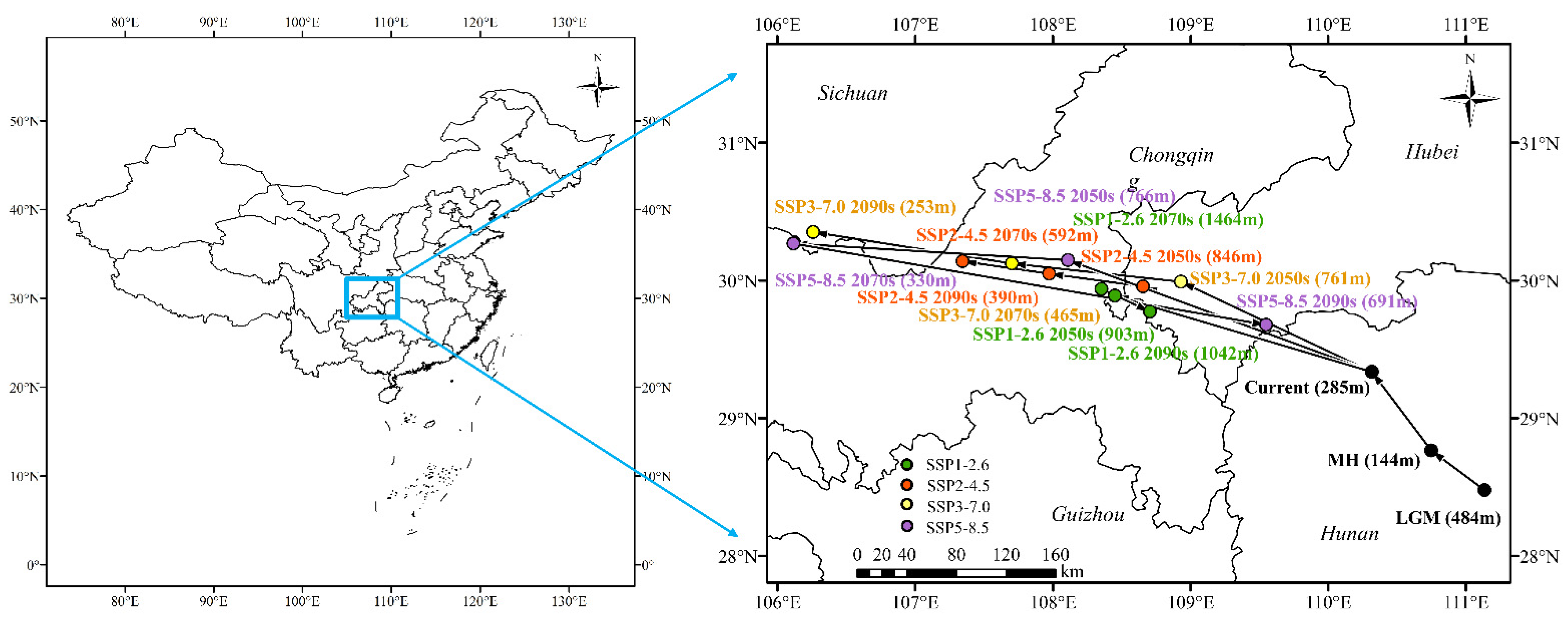
2.7. Core Distributional Shifts
3. Results
3.1. Model Parameter Optimization and Accuracy Analysis
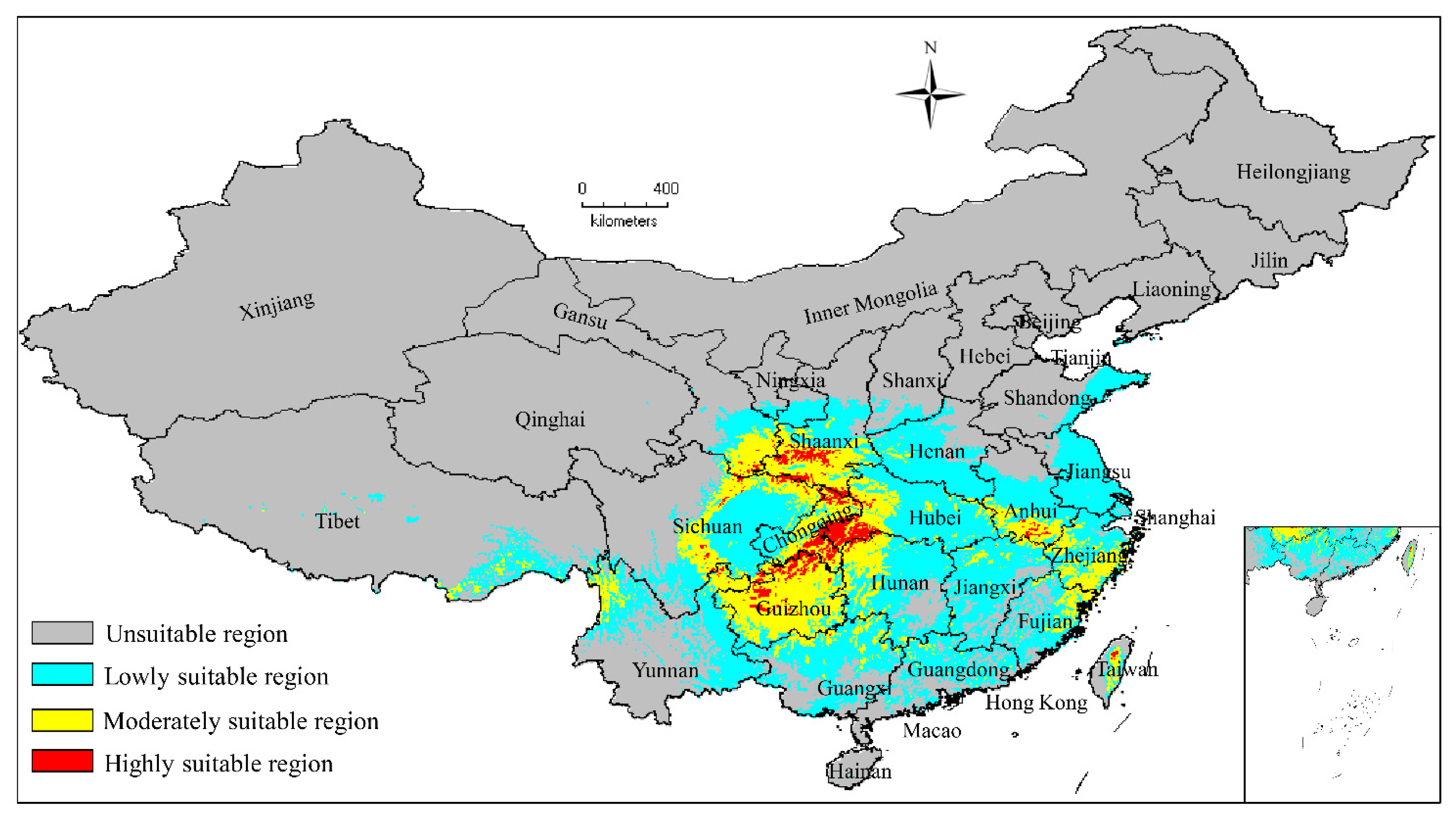
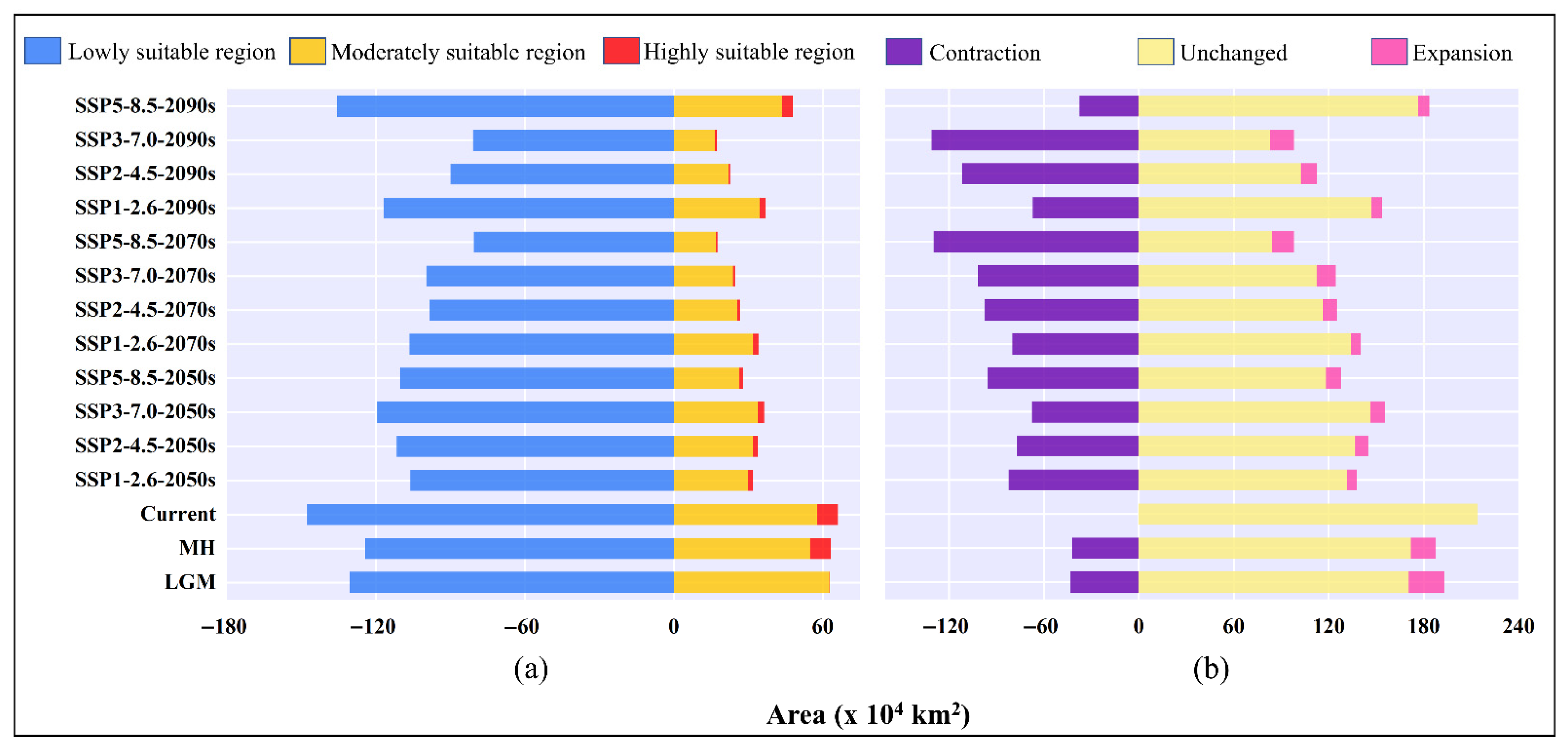
3.2. Current Potentially Suitable Regions
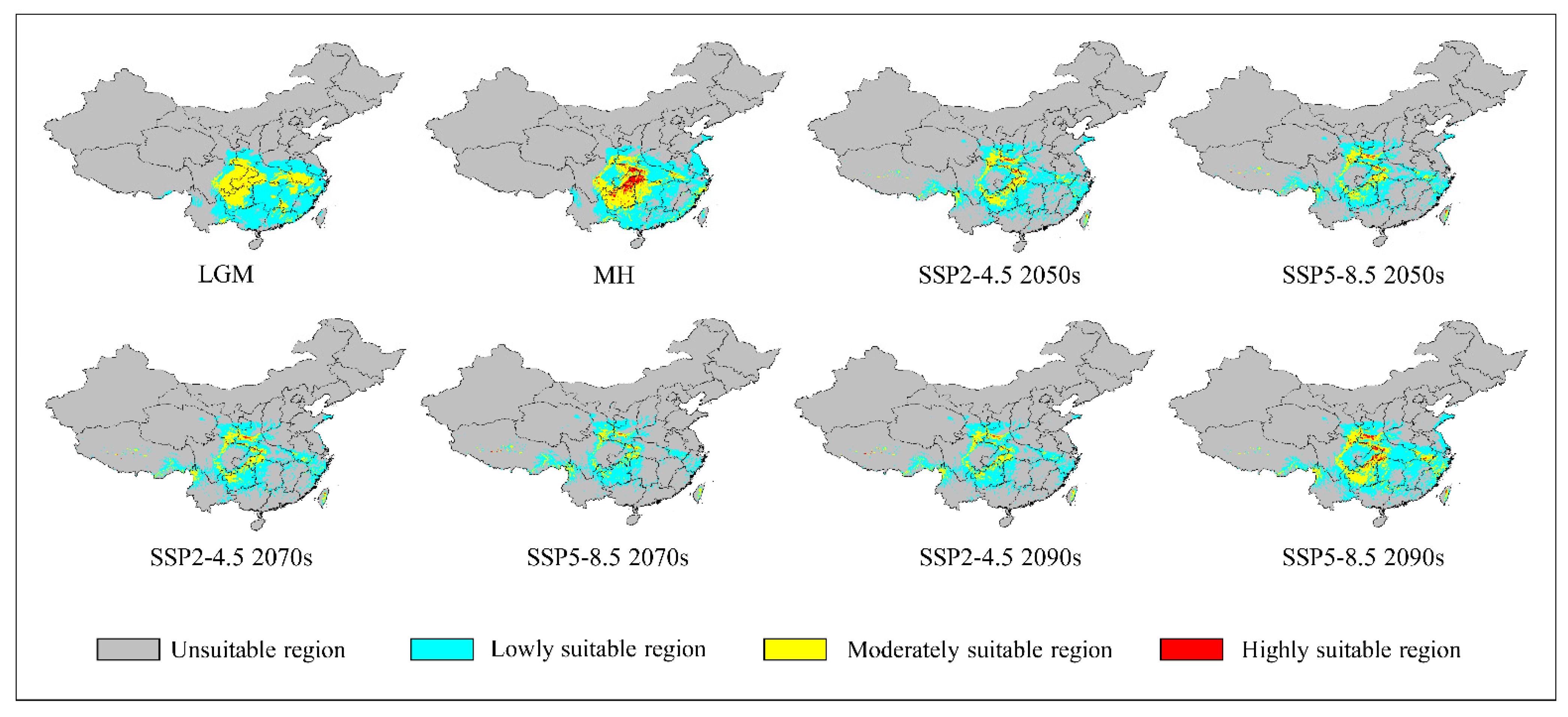
3.3. Potential Past Suitable Regions
3.4. Potentially Suitable Areas in the Future
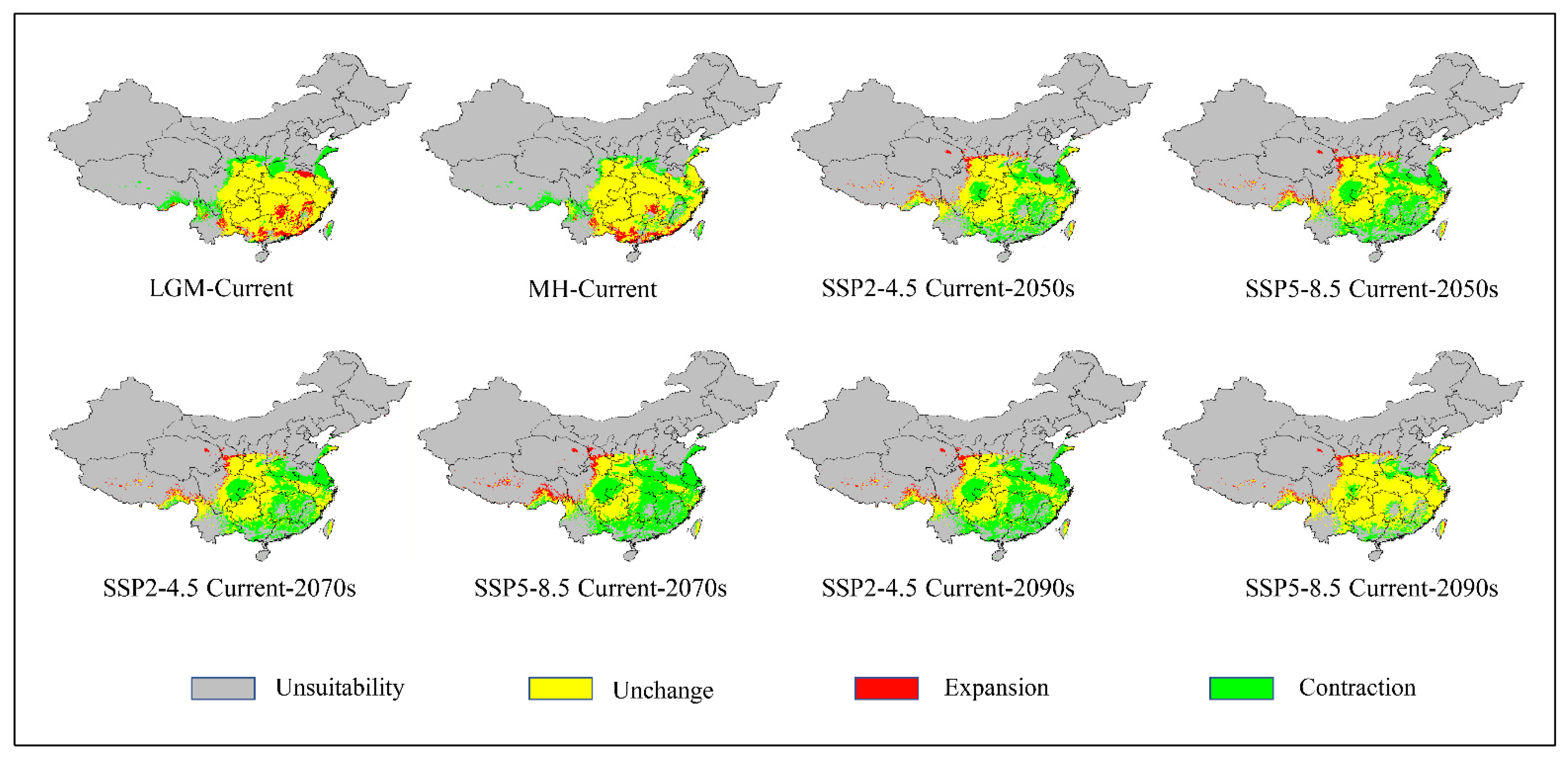
3.5. Relatively Stable Habitat
3.6. Shifts in the Distribution Center of Suitable Habitats
4. Discussion
4.1. Impacts of Environmental Variables on Species Distribution
4.2. Impacts of Climate Change on Species Distribution Dynamics and Migration Trends
4.3. Guiding Significance for Resource Protection and Artificial Cultivation of C. appendiculata
4.4. Model Limitations and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sambaraju, K.R.; Carroll, A.L.; Zhu, J.; Stahl, K.; Moore, R.D.; Aukema, B.H. Climate change could alter the distribution of mountain pine beetle outbreaks in western Canada. Ecography 2012, 35, 211–223. [Google Scholar] [CrossRef]
- Biber-Freudenberger, L.; Ziemacki, J.; Tonnang, H.E.; Borgemeister, C. Future Risks of Pest Species under Changing Climatic Conditions. PLoS ONE 2016, 11, e0153237. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.S.; Kim, H.-Y.; Kim, B.J. Climate change alters diffusion of forest pest: A model study. J. Korean Phys. Soc. 2017, 70, 108–115. [Google Scholar] [CrossRef]
- Xu, W.; Sun, H.; Jin, J.; Cheng, J. Predicting the Potential Distribution of Apple Canker Pathogen (Valsa mali) in China under Climate Change. Forests 2020, 11, 1126. [Google Scholar] [CrossRef]
- Feng, L.; Sun, J.; Shi, Y.; Wang, G.; Wang, T. Predicting Suitable Habitats of Camptotheca acuminata Considering Both Climatic and Soil Variables. Forests 2020, 11, 891. [Google Scholar] [CrossRef]
- Li, X.; Mao, F.; Du, H.; Zhou, G.; Xing, L.; Liu, T.; Han, N.; Liu, Y.; Zhu, D.; Zheng, J.; et al. Spatiotemporal evolution and impacts of climate change on bamboo distribution in China. J. Environ. Manag. 2019, 248, 265. [Google Scholar] [CrossRef]
- Cuena-Lombrana, A.; Fois, M.; Fenu, G.; Cogoni, D.; Bacchetta, G. The impact of climatic variations on the reproductive success of Gentiana lutea L. in a Mediterranean mountain area. Int. J. Biometeorol. 2018, 62, 1283–1295. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Sgrò, C.M. Climate change and evolutionary adaptation. Nature 2011, 470, 479–485. [Google Scholar] [CrossRef]
- Razgour, O.; Taggart, J.B.; Manel, S.; Juste, J.; Ibanez, C.; Rebelo, H.; Alberdi, A.; Jones, G.; Park, K. An integrated framework to identify wildlife populations under threat from climate change. Mol. Ecol. Resour. 2018, 18, 18–31. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J. Projected changes in climate extremes in China in a 1.5 °C warmer world. Int. J. Climatol. 2018, 38, 3607–3617. [Google Scholar] [CrossRef]
- Waldvogel, A.M.; Feldmeyer, B.; Rolshausen, G.; Exposito-Alonso, M.; Rellstab, C.; Kofler, R.; Mock, T.; Schmid, K.; Schmitt, I.; Bataillon, T.; et al. Evolutionary genomics can improve prediction of species’ responses to climate change. Evol. Lett. 2020, 4, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.; Price, J.; VanDerWal, J.; Cornelius, S.; Sohl, H. The implications of the United Nations Paris Agreement on climate change for globally significant biodiversity areas. Clim. Change 2018, 147, 395–409. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.-z.; Zhao, G.-h.; Zhang, M.-z.; Cui, X.-y.; Fan, H.-h.; Liu, B. Distribution Pattern of Endangered Plant Semiliquidambar cathayensis (Hamamelidaceae) in Response to Climate Change after the Last Interglacial Period. Forests 2020, 11, 434. [Google Scholar] [CrossRef]
- Clark, P.U.; Shakun, J.D.; Baker, P.A.; Bartlein, P.J.; Brewer, S.; Brook, E.; Carlson, A.E.; Cheng, H.; Kaufman, D.S.; Liu, Z.; et al. Global climate evolution during the last deglaciation. Proc. Natl. Acad. Sci. USA 2012, 109, E1134–E1142. [Google Scholar] [CrossRef]
- Martínez-Freiría, F.; Velo-Antón, G.; Brito, J.C.; Green, D. Trapped by climate: Interglacial refuge and recent population expansion in the endemic Iberian adder Vipera seoanei. Divers. Distrib. 2015, 21, 331–344. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhu, R.W.; Zhong, D.L.; Zhang, J.Q. Nunataks or massif de refuge? A phylogeographic study of Rhodiola crenulata (Crassulaceae) on the world’s highest sky islands. BMC Evol. Biol. 2018, 18, 154. [Google Scholar] [CrossRef]
- Cubry, P.; De Bellis, F.; Pot, D.; Musoli, P.; Leroy, T. Global analysis of Coffea canephora Pierre ex Froehner (Rubiaceae) from the Guineo-Congolese region reveals impacts from climatic refuges and migration effects. Genet. Resour. Crop Evol. 2012, 60, 483–501. [Google Scholar] [CrossRef]
- Chen, D.-M.; Kang, H.-Z.; Liu, C.-J. An Overview on the Potential Quaternary Glacial Refugia of Plants in China Mainland. Bull. Bot. Res. 2011, 31, 623–632. [Google Scholar]
- Zhao, P.; Zhou, H.J.; Potter, D.; Hu, Y.H.; Feng, X.J.; Dang, M.; Feng, L.; Zulfiqar, S.; Liu, W.Z.; Zhao, G.F.; et al. Population genetics, phylogenomics and hybrid speciation of Juglans in China determined from whole chloroplast genomes, transcriptomes, and genotyping-by-sequencing (GBS). Mol. Phylogenet. Evol. 2018, 126, 250–265. [Google Scholar] [CrossRef]
- Schipper, A.M.; Posthuma, L.; de Zwart, D.; Huijbregts, M.A. Deriving field-based species sensitivity distributions (f-SSDs) from stacked species distribution models (S-SDMs). Environ. Sci. Technol. 2014, 48, 14464–14471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyner, T.A.; Lukhnova, L.; Pazilov, Y.; Temiralyeva, G.; Hugh-Jones, M.E.; Aikimbayev, A.; Blackburn, J.K. Modeling the potential distribution of Bacillus anthracis under multiple climate change scenarios for Kazakhstan. PLoS ONE 2010, 5, e9596. [Google Scholar] [CrossRef] [PubMed]
- Steven, J.; Phillips, M.D. Modeling of species distributions with Maxent: New extensions and acomprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Changjun, G.; Yanli, T.; Linshan, L.; Bo, W.; Yili, Z.; Haibin, Y.; Xilong, W.; Zhuoga, Y.; Binghua, Z.; Bohao, C. Predicting the potential global distribution of Ageratina adenophora under current and future climate change scenarios. Ecol. Evol. 2021, 11, 12092–12113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Mi, Z.Y.; Lu, C.; Zhang, X.F.; Chen, L.J.; Wang, S.Q.; Niu, J.F.; Wang, Z.Z. Predicting potential distribution of Ziziphus spinosa (Bunge) H.H. Hu ex F.H. Chen in China under climate change scenarios. Ecol. Evol. 2022, 12, e8629. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, C.; Tang, Y.; Liu, W.; Xu, Y.; Li, Z.; Qin, X.; Jin, S. A review of Cremastra appendiculata (D.Don) Makino as a traditional herbal medicine and its main components. J. Ethnopharmacol. 2021, 279, 114357. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.Y.; Chung, M.G. The breeding systems of Cremastra appendiculata and Cymbidium goeringii: High levels of annual fruit failure in two self-compatible orchids. Ann. Bot. Fenn. 2003, 40, 81–85. [Google Scholar]
- Zhao, Q.; Zhang, Y.; Li, W.-N.; Hu, B.-W.; Zou, J.-B.; Wang, S.-Q.; Niu, J.-F.; Wang, Z.-Z. Predicting the Potential Distribution of Perennial Plant Coptis chinensis Franch. in China under Multiple Climate Change Scenarios. Forests 2021, 12, 1464. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Tao, J. Predicting the Potential Distribution of Paeonia veitchii (Paeoniaceae) in China by Incorporating Climate Change into a Maxent Model. Forests 2019, 10, 190. [Google Scholar] [CrossRef]
- Poirazidis, K.; Bontzorlos, V.; Xofis, P.; Zakkak, S.; Xirouchakis, S.; Grigoriadou, E.; Kechagioglou, S.; Gasteratos, I.; Alivizatos, H.; Panagiotopoulou, M. Bioclimatic and environmental suitability models for capercaillie (Tetrao urogallus) conservation: Identification of optimal and marginal areas in Rodopi Mountain-Range National Park (Northern Greece). Glob. Ecol. Conserv. 2019, 17, e00526. [Google Scholar] [CrossRef]
- Yu, W.; Wen, J.; Zhang, Z.; Chen, X.; Zhang, Y. Spatio-temporal variations in the potential habitat of a pelagic commercial squid. J. Mar. Syst. 2020, 206, 339. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Wang, J.; Wang, W.; Niyati, N.; Guo, Y.; Wang, X. Chinese caterpillar fungus (Ophiocordyceps sinensis) in China: Current distribution, trading, and futures under climate change and overexploitation. Sci. Total Environ. 2021, 755, 142548. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Heidy Kikillus, K.; Hare, K.M.; Hartley, S. Minimizing false-negatives when predicting the potential distribution of an invasive species: A bioclimatic envelope for the red-eared slider at global and regional scales. Anim. Conserv. 2010, 13, 5–15. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, Y.; Huang, D.; Wang, H.; Cao, Q.; Fan, P.; Yang, N.; Zheng, P.; Wang, R. The effect of climate change on the richness distribution pattern of oaks (Quercus L.) in China. Sci. Total Environ. 2020, 744, 140786. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Gonçalves, J.F.; Moreno, M.; Villar, R. Projected climate changes are expected to decrease the suitability and production of olive varieties in southern Spain. Sci. Total Environ. 2020, 709, 136161. [Google Scholar] [CrossRef]
- Akpoti, K.; Kabo-Bah, A.T.; Dossou-Yovo, E.R.; Groen, T.A.; Zwart, S.J. Mapping suitability for rice production in inland valley landscapes in Benin and Togo using environmental niche modeling. Sci. Total Environ. 2020, 709, 136165. [Google Scholar] [CrossRef]
- Liu, B.; Gao, X.; Ma, J.; Jiao, Z.; Xiao, J.; Hayat, M.A.; Wang, H. Modeling the present and future distribution of arbovirus vectors Aedes aegypti and Aedes albopictus under climate change scenarios in Mainland China. Sci. Total Environ. 2019, 664, 203–214. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Zhao, Z.; Nawaz, Z. Predicting the impacts of climate change, soils and vegetation types on the geographic distribution of Polyporus umbellatus in China. Sci. Total Environ. 2019, 648, 1–11. [Google Scholar] [CrossRef]
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Dong, Y.F.; Momohara, A.; Herrando-Moraira, S.; Qian, S.; Yang, Y.; Ohsawa, M.; Luu, H.T.; et al. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 2018, 9, 4488. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Yu, X.; Yu, C.; Tayibazhaer, A.; Xu, F.; Skidmore, A.K.; Wang, T. Impacts of future climate and land cover changes on threatened mammals in the semi-arid Chinese Altai Mountains. Sci. Total Environ. 2018, 612, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Lei, Y.; Ren, Z.; Chen, H.; Li, D. Predicting the potential risk area of illegal vaccine trade in China. Sci. Rep. 2017, 7, 3883. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-M.; Shen, X.-L.; Tong, L.; Lei, F.-W.; Mu, X.-Y.; Zhang, Z.-X. Impact of Past and Future Climate Change on the Potential Distribution of an Endangered Montane Shrub Lonicera oblata and Its Conservation Implications. Forests 2021, 12, 125. [Google Scholar] [CrossRef]
- Santos-Hernández, A.F.; Monterroso-Rivas, A.I.; Granados-Sánchez, D.; Villanueva-Morales, A.; Santacruz-Carrillo, M. Projections for Mexico’s Tropical Rainforests Considering Ecological Niche and Climate Change. Forests 2021, 12, 119. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Václavík, T.; Meentemeyer, R.K. Invasive species distribution modeling (iSDM): Are absence data and dispersal constraints needed to predict actual distributions? Ecol. Model. 2009, 220, 3248–3258. [Google Scholar] [CrossRef]
- Smith, A.B.; Godsoe, W.; Rodríguez-Sánchez, F.; Wang, H.H.; Warren, D. Niche Estimation Above and Below the Species Level. Trends Ecol. Evol. 2019, 34, 260–273. [Google Scholar] [CrossRef]
- Ashcroft, M.B.; French, K.O.; Chisholm, L.A. A simple post-hoc method to add spatial context to predictive species distribution models. Ecol. Model. 2012, 228, 17–26. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Thuiller, W.; Jiguet, F. The fate of European breeding birds under climate, land-use and dispersal scenarios. Glob. Change Biol. 2011, 18, 881–890. [Google Scholar] [CrossRef]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2019, 50, 68–75. [Google Scholar] [CrossRef]
- Wei, H.B.; Lv, X.; Gao, X.F.; Zhang, M. Advances in Orchidacea Medicinal Plant Cremastra appendiculata. Guizhou Agric. Sci. 2017, 45, 88–92. [Google Scholar]
- An-min, M. Description on Wild Flower Resources of Orchidaceae (Cremastra, Dendrobium, Spiranthes, Cymbidium) in Western Henan Province. Hortic. Seed 2021, 41, 23–24. [Google Scholar] [CrossRef]
- Zhang, S.L.; Zhang, W.; Zhang, S. Preliminary study on relationship between fruiting phenology of Orchidaceae in China and major climatic factors. J. Plant Resour. Environ. 2021, 30, 1–9. [Google Scholar]
- Zahoor, B.; Liu, X.; Songer, M. The impact of climate change on three indicator Galliformes species in the northern highlands of Pakistan. Environ. Sci. Pollut. Res. Int. 2022, 29, 54330–54347. [Google Scholar] [CrossRef]
- Guo, Y.-L.; Wei, H.-Y.; Lu, C.-Y.; Zhang, H.-L.; Gu, W. Predictions of potential geographical distribution of Sinopodophyllum hexandrum under climate change. Chin. J. Plant Ecol. 2014, 38, 249–261. [Google Scholar] [CrossRef]
- Kumar, D.; Rawat, S. Modeling the effect of climate change on the distribution of threatened medicinal orchid Satyrium nepalense D. Don in India. Environ. Sci. Pollut. Res. Int. 2022, 412, 1–14. [Google Scholar] [CrossRef]
- Feng, G.; Mao, L.; Sandel, B.; Swenson, N.G.; Svenning, J.C. High plant endemism in China is partially linked to reduced glacial-interglacial climate change. J. Biogeogr. 2015, 43, 145–154. [Google Scholar] [CrossRef]
- Charitonidou, M.; Kougioumoutzis, K.; Halley, J.M. An Orchid in Retrograde: Climate-Driven Range Shift Patterns of Ophrys helenae in Greece. Plants 2021, 10, 470. [Google Scholar] [CrossRef]
- Chen, D.-L.; Zhong, C.; Jian, S.; Li, L.-X.; Wei, K.-H.; Miu, J.-H. Conservation Research on Endangered Medicinal Plant Dysosma versipellis (Hance) M. Cheng ex Ying. Mod. Chin. Med. 2022, 24, 395–402. [Google Scholar]
- Zhang, X.; Bi, C.; Shi, H.; Li, X. Structural studies of a mannoglucan from Cremastra appendiculata (Orchidaceae) by chemical and enzymatic methods. Carbohydr. Polym. 2021, 272, 118524. [Google Scholar] [CrossRef]
- Adhikari, D.; Barik, S.K.; Upadhaya, K. Habitat distribution modelling for reintroduction of Ilex khasiana Purk., a critically endangered tree species of northeastern India. Ecol. Eng. 2012, 40, 37–43. [Google Scholar] [CrossRef]
- Blach-Overgaard, A.; Svenning, J.-C.; Dransfield, J.; Greve, M.; Balslev, H. Determinants of palm species distributions across Africa: The relative roles of climate, non-climatic environmental factors, and spatial constraints. Ecography 2010, 33, 380–391. [Google Scholar] [CrossRef]
- Lin, L.; Jin, L.; Wang, Z.H.; Cui, Z.J.; Ma, Y. Prediction of the potential distribution of Tibetan medicinal Lycium ruthenicum in context of climate change. China J. Chin. Mater. Med. 2017, 42, 2659–2669. [Google Scholar] [CrossRef]
- Tupac Otero, J.; Ackerman, J.D.; Bayman, P. Diversity and host specificity of endophytic Rhizoctonia-like fungi from tropical orchids. Am. J. Bot. 2002, 89, 1852–1858. [Google Scholar] [CrossRef]
- Pellegrino, G.; Luca, A.; Bellusci, F. Relationships between orchid and fungal biodiversity: Mycorrhizal preferences in Mediterranean orchids. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2014, 150, 180–189. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Waud, M.; Brys, R.; Lallemand, F.; Courty, P.E.; Robionek, A.; Selosse, M.A. Mycorrhizal Associations and Trophic Modes in Coexisting Orchids: An Ecological Continuum between Auto- and Mixotrophy. Front. Plant Sci. 2017, 8, 1497. [Google Scholar] [CrossRef]
- Reiter, N.; Lawrie, A.C.; Linde, C.C. Matching symbiotic associations of an endangered orchid to habitat to improve conservation outcomes. Ann. Bot. 2018, 122, 947–959. [Google Scholar] [CrossRef]

| Code | Environmental Variable | Percent Contribution | Permutation Importance |
|---|---|---|---|
| BIO02 | Mean diurnal range (Mean of monthly (max temp − min temp)) (°C) | 51.1 | 30.7 |
| BIO11 | Mean temperature of coldest quarter (°C) | 38.5 | 51.4 |
| BIO04 | Temperature seasonality (standard deviation × 100) | 3.8 | 4.7 |
| BIO14 | Precipitation of driest month (mm) | 2.3 | 3.2 |
| BIO08 | Mean temperature of wettest quarter (°C) | 2.3 | 2.7 |
| BIO15 | Precipitation seasonality (Coefficient of Variation) (mm) | 1.2 | 2.8 |
| BIO18 | Precipitation of warmest quarter (mm) | 0.8 | 4.5 |
| Decades | Predicted Area (×104 km2) and % of the Corresponding Current Area | ||||
|---|---|---|---|---|---|
| Total Suitable Region | Lowly Suitable Region | Moderately Suitable Region | Highly Suitable Region | ||
| LGM | 193.49 | 130.53 | 62.69 | 0.28 | |
| (90.46%) | (88.34%) | (108.64%) | (3.26%) | ||
| MH | 187.61 | 124.29 | 54.95 | 8.38 | |
| (87.71%) | (84.11%) | (95.21%) | (99.30%) | ||
| 1970–2000 | 213.90 | 147.76 | 57.71 | 8.44 | |
| SSP1-2.6 | 2050s | 137.87 | 106.12 | 30.07 | 1.68 |
| (64.45%) | (71.82%) | (52.10%) | (19.92%) | ||
| 2070s | 140.54 | 106.40 | 31.98 | 2.17 | |
| (65.70%) | (72.01%) | (55.42%) | (25.69%) | ||
| 2090s | 153.85 | 116.84 | 34.74 | 2.27 | |
| (71.92%) | (79.08%) | (60.19%) | (26.88%) | ||
| SSP2-4.5 | 2050s | 145.42 | 111.56 | 31.75 | 2.11 |
| (67.98%) | (75.50%) | (55.01%) | (24.99%) | ||
| 2070s | 125.32 | 98.45 | 25.57 | 1.30 | |
| (58.59%) | (66.63%) | (44.32%) | (15.36%) | ||
| 2090s | 112.65 | 89.88 | 21.97 | 0.80 | |
| (52.66%) | (60.83%) | (38.08%) | (9.47%) | ||
| SSP3-7.0 | 2050s | 155.87 | 119.44 | 33.88 | 2.55 |
| (72.87%) | (80.84%) | (58.70%) | (30.23%) | ||
| 2070s | 124.61 | 99.65 | 23.83 | 1.12 | |
| (58.25%) | (67.44%) | (41.30%) | (13.31%) | ||
| 2090s | 98.19 | 80.87 | 16.68 | 0.63 | |
| (45.90%) | (54.73%) | (28.91%) | (7.49%) | ||
| SSP5-8.5 | 2050s | 128.16 | 110.10 | 26.57 | 1.48 |
| (59.91%) | (67.75%) | (46.05%) | (17.56%) | ||
| 2070s | 98.14 | 80.54 | 16.93 | 0.67 | |
| (45.88%) | (54.51%) | (29.34%) | (7.91%) | ||
| 2090s | 183.65 | 135.63 | 43.77 | 4.26 | |
| (85.86%) | (91.79%) | (75.84%) | (50.45%) | ||
| LIA Statistics | Shared Socio-Economic Pathways (SSPs) | |||
|---|---|---|---|---|
| SSP1-2.6 | SSP2-4.5 | SSP3-7.0 | SSP5-8.5 | |
| Geographic area (×104 km2) | 130.01 | 102.15 | 82.95 | 84.40 |
| Percentage of current suitable area (%) | 60.78 | 47.75 | 38.78 | 39.46 |
| Percentage of SSP1-2.6 area (%) | 100.00 | 78.57 | 63.80 | 64.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-N.; Zhao, Q.; Guo, M.-H.; Lu, C.; Huang, F.; Wang, Z.-Z.; Niu, J.-F. Predicting the Potential Distribution of the Endangered Plant Cremastra appendiculata (Orchidaceae) in China under Multiple Climate Change Scenarios. Forests 2022, 13, 1504. https://doi.org/10.3390/f13091504
Li W-N, Zhao Q, Guo M-H, Lu C, Huang F, Wang Z-Z, Niu J-F. Predicting the Potential Distribution of the Endangered Plant Cremastra appendiculata (Orchidaceae) in China under Multiple Climate Change Scenarios. Forests. 2022; 13(9):1504. https://doi.org/10.3390/f13091504
Chicago/Turabian StyleLi, Wen-Na, Qian Zhao, Ming-Hao Guo, Chan Lu, Fei Huang, Zhe-Zhi Wang, and Jun-Feng Niu. 2022. "Predicting the Potential Distribution of the Endangered Plant Cremastra appendiculata (Orchidaceae) in China under Multiple Climate Change Scenarios" Forests 13, no. 9: 1504. https://doi.org/10.3390/f13091504





