Localization of TWISTED NEEDLES Locus on Linkage Map of Japanese Cedar (Cryptomeria japonica D. Don)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mapping Family and Evaluation of Twisted Trait
2.2. DNA Extraction
2.3. Identification of Linkage Group Including TWISTED NEEDLES Locus
2.4. Construction of a Linkage Map Using ddRAD-Seq
2.5. Identification of Markers Which Sandwich the TWISTED NEEDLES Locus
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, R.L.; Keeling, C.I.; Yuen, M.M.; Raymond, A.; Taylor, G.A.; Vandervalk, B.P.; Mohamadi, H.; Paulino, D.; Chiu, R.; Jackman, S.D.; et al. Improved white spruce (Picea glauca) genome assemblies and annotation of large gene families of conifer terpenoid and phenolic defense metabolism. Plant J. 2016, 83, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.A.; Wegrzyn, J.L.; Zimin, A.; Puiu, D.; Crepeau, M.; Cardeno, C.; Paul, R.; Conzalez-Ibeas, D.; Koriabine, M.; Holtz-Morris, A.E.; et al. Sequence of the sugar pine megagenome. Genetics 2016, 204, 1613–1626. [Google Scholar] [CrossRef]
- Neal, D.B.; McGuire, P.E.; Wheeler, N.C.; Stevens, K.A.; Crepeau, M.W.; Cardeno, C.; Zimin, A.V.; Puiu, D.; Pertea, G.M.; Sezen, U.U.; et al. The Douglas-Fir genome sequence reveals specialization of the photosynthetic apparatus in Pinaceae. G3 (Bethesda) 2017, 7, 3157–3167. [Google Scholar] [CrossRef] [PubMed]
- Mosca, E.; Cruz, F.; Gómez-Garrido, J.; Bianco, L.; Rellstab, C.; Brodbeck, S.; Csilléry, K.; Fady, B.; Fladung, M.; Fussi, B.; et al. A reference genome sequence for the European silver fir (Abies alba Mill.): A community-generated genomic resource. G3 (Bethesda) 2019, 9, 2039–2049. [Google Scholar] [CrossRef]
- Scott, A.D.; Zimin, A.V.; Puiu, D.; Workman, R.; Britton, M.; Zaman, S.; Caballero, M.; Read, A.C.; Bogdanove, A.J.; Burns, M.; et al. A reference genome sequence for giant sequoia. G3 (Bethesda) 2020, 10, 3907–3919. [Google Scholar] [CrossRef] [PubMed]
- Forestry Agency. Statistical Handbook of Forest and Forestry; Forestry Agency, Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2014; pp. 8–9. (In Japanese) [Google Scholar]
- Tsumura, Y. Cryptomeria. In Wild Crop Relatives: Genomics and Breeding Resources, Forest Trees; Kole, C., Ed.; Springer: Berlin, Germany, 2011; pp. 49–64. [Google Scholar]
- Ujino-Ihara, T.; Yoshimura, K.; Ugawa, Y.; Yoshimaru, H.; Nagasaka, K.; Tsumura, Y. Expression analysis of ESTs derived from the inner bark of Cryptomeria japonica. Plant Mol. Biol. 2000, 43, 451–457. [Google Scholar] [CrossRef]
- Ujino-Ihara, T.; Taguchi, Y.; Yoshimura, K.; Tsumura, Y. Analysis of expressed sequence tags derived from developing seed and pollen cones of Cryptomeria japonica. Plant Biol. 2003, 5, 600–607. [Google Scholar] [CrossRef]
- Ujino-Ihara, T.; Kanamori, H.; Yamane, H.; Taguchi, Y.; Namiki, N.; Mukai, Y.; Yoshimura, K.; Tsumura, Y. Comparative analysis of expressed sequence tags of conifers and angiosperms reveals sequences specifically conserved in conifers. Plant Mol. Biol. 2005, 59, 895–907. [Google Scholar] [CrossRef]
- Futamura, N.; Ujino-Ihara, T.; Nishiguchi, M.; Kanamori, H.; Yoshimura, K.; Sakaguchi, M.; Shinohara, K. Analysis of expressed sequence tags from Cryptomeria japonica pollen reveals novel pollen-specific transcripts. Tree Physiol. 2006, 26, 1517–1528. [Google Scholar] [CrossRef]
- Futamura, N.; Totoki, Y.; Toyoda, A.; Igasaki, T.; Nanjo, T.; Seki, M.; Sakaki, Y.; Mari, A.; Shinozaki, K.; Shinohara, K. Characterization of expressed sequence tags from a full-length enriched cDNA library of Cryptomeria japonica male strobili. BMC Genom. 2008, 9, 383. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Nishiguchi, M.; Futamura, N.; Nanjo, T. Expressed sequence tags from Cryptomeria japonica sapwood during the drying process. Tree Physiol. 2007, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, Y.; Suyama, Y.; Yoshimura, K.; Shirato, N.; Mukai, Y. Sequence-tagged-sites (STSs) of cDNA clones in Cryptomeria japonica and their evaluation as molecular markers in conifers. Theor. Appl. Genet. 1997, 94, 764–772. [Google Scholar] [CrossRef]
- Nikaido, A.M.; Ujino, T.; Iwata, H.; Yoshimura, K.; Yoshimura, H.; Suyama, Y.; Murai, M.; Nagasaka, K.; Tsumura, Y. AFLP and CAPS linkage maps of Cryptomeria japonica. Theor. Appl. Genet. 2000, 100, 825–831. [Google Scholar] [CrossRef]
- Iwata, H.; Ujino-Ihara, T.; Yoshimura, K.; Nagasaka, K.; Mukai, Y.; Tsumura, Y. Cleaved amplified polymorphic sequence markers in sugi, Cryptomeria japonica D. Don, and their locations on a linkage map. Theor. Appl. Genet. 2001, 103, 881–895. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Iwata, H.; Ihara, T.; Yoshimura, K.; Taira, H.; Tsumura, Y. Development and characterization of microsatellite markers for Cryptomeria japonica D. Don. Theor. Appl. Genet. 2003, 106, 751–758. [Google Scholar] [CrossRef]
- Tani, N.; Takahashi, T.; Ujino-Ihara, T.; Iwata, H.; Yoshimura, K.; Tsumura, Y. Development and characteristics of microsatellite markers for sugi (Cryptomeria japonica D. Don) from microsatellite enriched libraries. Ann. For. Sci. 2004, 61, 569–575. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Ueno, S.; Ujino-Ihara, T.; Futamura, N.; Matsumoto, A.; Shinohara, K.; Tsumura, Y. Characterization of EST-SSRs from Cryptomeria japonica. Conserv. Genet. Resour. 2009, 1, 373–376. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Ujino-Ihara, T.; Uchiyama, K.; Futamura, N.; Saito, M.; Ueno, S.; Matsumoto, A.; Tani, N.; Taira, H.; Shinohara, K.; et al. The construction of a high-density linkage map for identifying SNP markers that are tightly linked to a nuclear-recessive major gene for male sterility in Cryptomeria japonica D. Don. BMC Genom. 2012, 13, 95. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Ueno, S.; Higuchi, Y.; Miyajima, D.; Itoo, S.; Futamura, N.; Tsumura, Y. Establishment of a microsatellite panel covering the sugi (Cryptomeria japonica) genome, and its application for localization of a male sterile gene (ms2). Mol. Breed. 2014, 33, 315–325. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Uchiyama, K.; Ueno, S.; Ujino-Ihara, T.; Matsumoto, A.; Iwai, J.; Miyajima, D.; Saito, M.; Sato, M.; Tsumura, Y. A high-density linkage map with 2560 markers and its application for the localization of the male-sterile genes ms3 and ms4 in Cryptomeria japonica D. Don. Tree Genet. Genomes 2016, 12, 57. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Ueno, S.; Matsumoto, A.; Ujino-Ihara, T.; Uchiyama, K.; Totsuka, S.; Iwai, J.; Hakamata, T.; Moriguchi, Y. Fine mapping of the male-sterile genes (MS1, MS2, MS3, and MS4) and development of SNP markers for marker-assisted selection in Japanese cedar (Cryptomeria japonica D. Don). PLoS ONE 2018, 13, e0206695. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, Y.; Ueno, S.; Hasegawa, Y.; Tadama, T.; Watanabe, M.; Saito, R.; Hirayama, S.; Iwai, J.; Konno, Y. Marker-assisted selection of trees with MALE STERILITY 1 in Cryptomeria japonica D. Don. Forests 2020, 11, 734. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Totsuka, S.; Iwai, J.; Matsumoto, A.; Ueno, S.; Tsumura, Y. Pyramiding of male-sterile genes in Cryptomeria japonica D. Don with the aid of closely linked markers. Tree Genet. Genomes 2017, 13, 61. [Google Scholar] [CrossRef]
- Maruyama, E.; Tanaka, T.; Hosoi, Y.; Ishii, K.; Morohoshi, N. Embryogenic cell culture, protoplast regeneration, cryopreservation, biolistic gene transfer and plant regeneration in Japanese cedar (Cryptomeria japonica D. Don). Plant Biotechnol. 2000, 17, 281–296. [Google Scholar] [CrossRef]
- Maruyama, E.T.; Hosoi, Y.; Futamura, N.; Saito, M. Initiation of embryogenic cultures from immature seeds of pollen-free sugi (Cryptomeria japonica). Kanto Shinrin Kenkyu 2014, 65, 107–110, (In Japanese with English abstract). [Google Scholar]
- Taniguchi, T.; Konagaya, K.; Nanasato, Y. Somatic embryogenesis in artificially pollinated seed families of 2nd generation plus trees and cryopreservation of embryogenic tissue in Cryptomeria japonica D. Don (sugi). Plant Biotechnol. 2020, 37, 239–245. [Google Scholar] [CrossRef]
- Igasaki, T.; Sato, T.; Akashi, N.; Mohri, T.; Maruyama, E.; Kinoshita, I.; Walter, C.; Shinohara, K. Somatic embryogenesis and plant regeneration from immature zygotic embryos of Cryptomeria japonica D. Don. Plant Cell Rep. 2003, 22, 239–243. [Google Scholar] [CrossRef]
- Igasaki, T.; Akashi, N.; Ujino-Ihara, T.; Matsubayashi, Y.; Sakagami, Y.; Shinohara, K. Phytosulfokine stimulates somatic embryogenesis in Cryptomeria japonica. Plant Cell Physiol. 2003, 44, 1412–1416. [Google Scholar] [CrossRef]
- Nakagawa, R.; Ogita, S.; Kubo, T.; Funada, R. Effect of polyamines and L-ornithine on the development of proembryogenic masses of Cryptomeria japonica. Plant Cell Tissue Organ. Cult. 2006, 85, 229–234. [Google Scholar] [CrossRef]
- Maruyama, E.; Hosoi, Y. Polyethylene glycol enhance somatic embryo production in Japanese cedar (Cryptomeria japonica D. Don). Propag. Ornam. Plants 2007, 7, 57–61. [Google Scholar]
- Taniguchi, T.; Ohmiya, Y.; Kurita, M.; Tsubomura, M.; Kondo, T. Regeneration of transgenic Cryptomeria japonica D. Don after Agrobacterium tumefaciens-mediated transformation of embryogenic tissue. Plant Cell Rep. 2008, 27, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Konagaya, K.; Kurita, M.; Taniguchi, T. High-efficiency Agrobacterium-mediated transformation of Cryptomeria japonica D. Don by co-cultivation on filter paper wicks followed by meropenem treatment to eliminate Agrobacterium. Plant Biotechnol. 2013, 30, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Konagaya, K.; Nagasato, Y.; Taniguchi, T. A protocol for Agrobacterium-mediated transformation of Japanese cedar, Sugi (Cryptomeria japonica D. Don) using embryogenic tissue explants. Plant Biotechnol. 2020, 37, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Nanasato, Y.; Mikami, M.; Futamura, N.; Endo, M.; Nishiguchi, M.; Ohmiya, Y.; Konagaya, K.; Taniguchi, T. CRISPR/Cas9-mediated targeted mutagenesis in Japanese cedar (Cryptomeria japonica D. Don). Sci. Rep. 2021, 11, 16186. [Google Scholar] [CrossRef]
- Yonekura, K.; Kajita, T.; BG Plants. YList. Available online: http://bean.bio.chibau.jp/bgplants/ylist_main.html (accessed on 11 November 2021).
- Ohba, K.; Maeda, T.; Fukuhara, N. Inheritance of twisted-leaf sugi, Cryptomeria japonica D. DON and linkage between the twisted-leaf gene and two recessive genes, albino and green (midori sugi). J. Jpn. For. Soc. 1974, 56, 276–281, (In Japanese with English abstract). [Google Scholar]
- Buschmann, H.; Borchers, A. Handedness in plant cell expansion: A mutant perspective on helical growth. New Phytol. 2020, 225, 53–69. [Google Scholar] [CrossRef]
- Kuromaru, M. Time serial changes of isozyme patterns of primary leaves on sugi (Cryptomeria japonica D. DON) seedlings. J. Jpn. For. Soc. 1983, 65, 73–81, (In Japanese with English abstract). [Google Scholar]
- Kuromaru, M.; Kawasaki, H.; Ohba, K. Genetic analysis of the peroxidase isozyme of seedlings derived from a twisted-leaf sugi (Cryptomeria japonica D. Don). J. Jpn. For. Soc. 1983, 65, 253–257, (In Japanese with English abstract). [Google Scholar]
- Hasegawa, Y.; Ueno, S.; Fu-Jin, W.; Matsumoto, A.; Ujino-Ihara, T.; Uchiyama, K.; Moriguchi, Y.; Kasahara, M.; Fujino, T.; Shigenobu, S.; et al. Development of diagnostic PCR and LAMP markers for MALE STERILITY 1 (MS1) in Cryptomeria japonica D Don. BMC Res. Note 2020, 13, 457. [Google Scholar] [CrossRef]
- Peterson, B.K.; Weber, J.N.; Kay, E.H.; Fisher, H.S.; Hoekstra, H.E. Double digest RADseq: An inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 2012, 7, e37135. [Google Scholar]
- Van Ooijen, J.W.; Voorrips, R.E. JoinMap: Version 3.0: Software for the Calculation of Genetic Linkage Maps; University and Research Center: Wageningen, The Netherlands, 2001. [Google Scholar]
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1944, 12, 172–175. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Heun, M.; Kennedy, A.E.; Anderson, J.A.; Lapitan, N.L.V.; Sorrells, M.E.; Tanksley, S.D. Construction of a restriction fragment length polymorphism map for barley (Hordeum vulgare). Genome 1991, 34, 437–447. [Google Scholar] [CrossRef]
- Kiss, G.B.; Csanadi, G.; Kalman, K.; Kalo, P.; Okresz, L. Construction of a basic genetic map for alfalfa using RFLP, RAPD, isozyme and morphological markers. Mol. Gen. Gent. 1993, 238, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Nodari, R.O.; Tsai, S.M.; Gilbertson, R.L.; Gepts, P. Towards an integrated linkage map of common bean 2. Development of an RFLP-based linkage map. Theor. Appl. Genet. 1993, 85, 513–520. [Google Scholar] [CrossRef]
- Mukai, Y.; Suyama, Y.; Tsumura, Y.; Kawahara, T.; Yoshimaru, H.; Kondo, T.; Tomaru, N.; Kuramoto, N.; Mukai, M. A linkage map for sugi (Cryptomeria japonica) based on RFLP, RAPD, and isozyme loci. Theor. Appl. Genet. 1995, 90, 835–840. [Google Scholar] [CrossRef]
- Guan, L.; Shiraishi, S. Tetranucleotide microsatellite markers in Cryptomeria japonica. Conserv. Genet. Resour. 2011, 3, 283–285. [Google Scholar] [CrossRef]
- Ueno, S.; Moriguchi, Y.; Uchiyama, K.; Ujino-Ihara, T.; Futamura, N.; Sakurai, T.; Shinohara, K.; Tsumura, Y. A second generation framework for the analysis of microsatellites in expressed sequence tags and the development of EST-SSR markers for a conifer, Cryptomeria japonica. BMC Genom. 2012, 13, 136. [Google Scholar] [CrossRef] [Green Version]

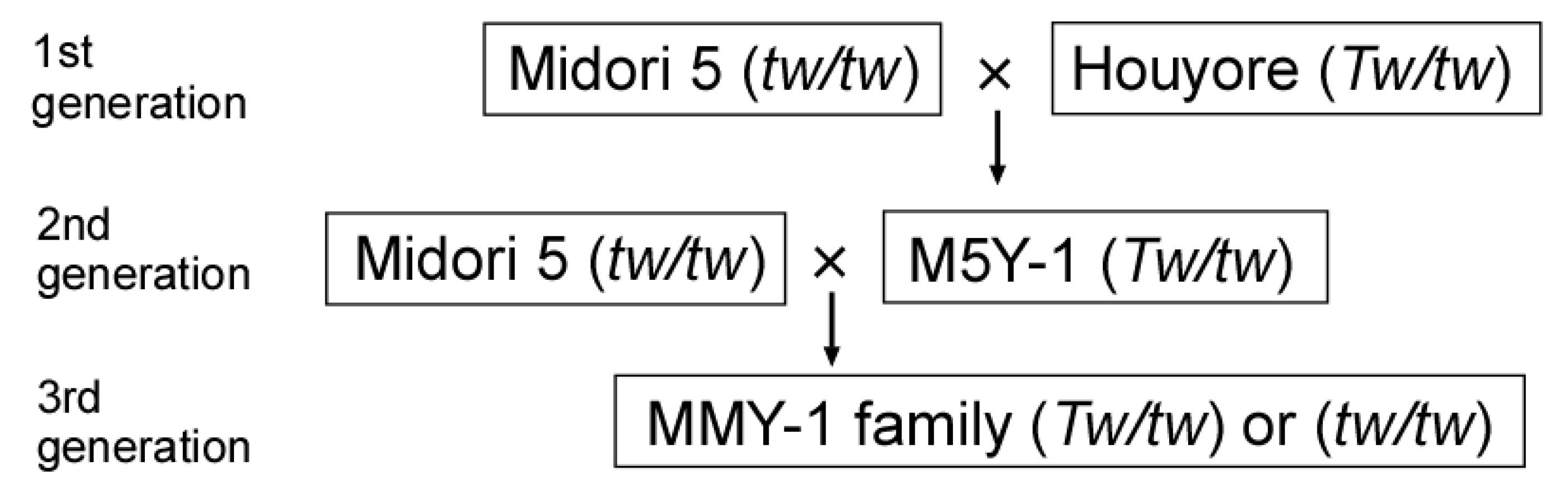
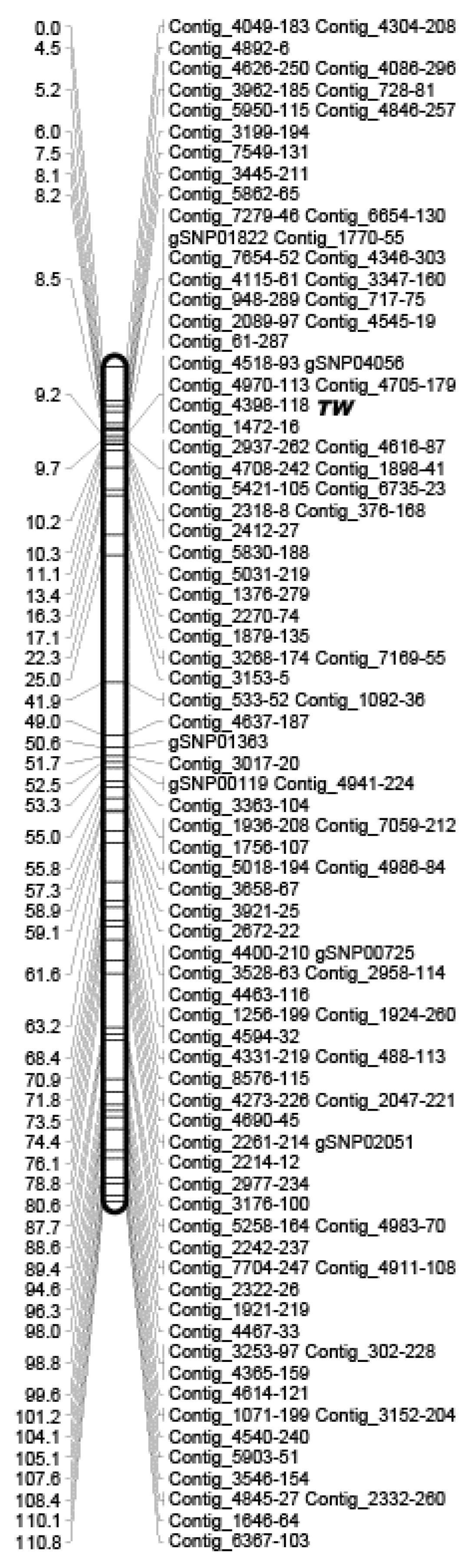
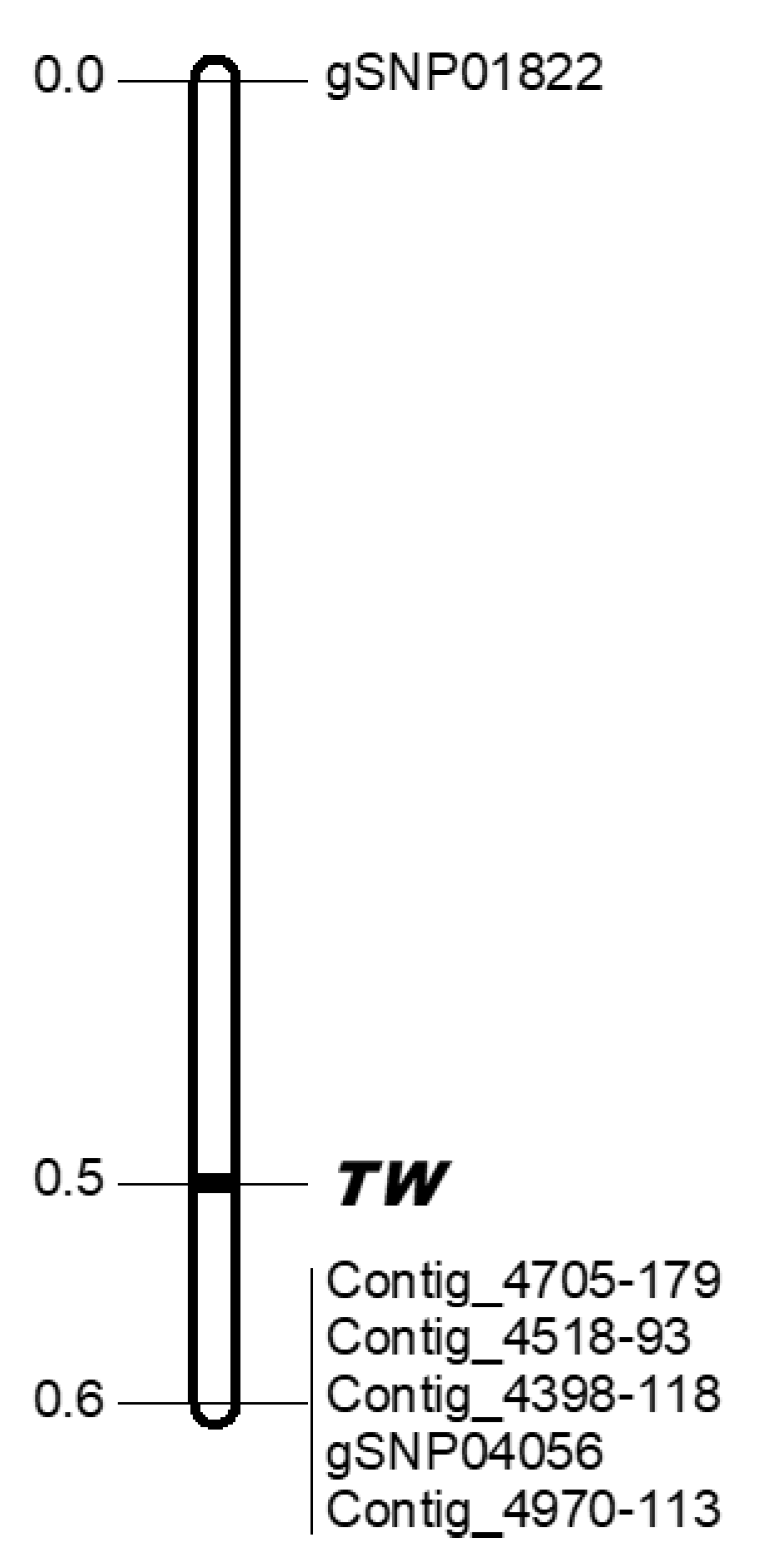
| Linkage Group | Marker Name | χ2 | P | Number of Analyzed Individuals |
|---|---|---|---|---|
| LG1 | HS4_c16648_ES | 0.35 | 0.56 | 32 |
| CS2169_S | 3.13 | 0.08 | 32 | |
| Cjgssr175_S | 0.50 | 0.48 | 32 | |
| LG2 | BY893784_ES | 1.13 | 0.29 | 32 |
| LG3 | S4049_S | 2.42 | 0.12 | 32 |
| Cjgssr77_S | 0.00 | 0.96 | 32 | |
| LG4 | CJS0333_S | 2.42 | 0.12 | 32 |
| Cjgssr121_S | 0.50 | 0.48 | 32 | |
| Cjgssr123_S | 0.07 | 0.80 | 32 | |
| LG5 | CS0038FC_S | 3.13 | 0.08 | 32 |
| Cjgssr181_S | 0.03 | 0.85 | 32 | |
| Cjgssr125_S | 0.50 | 0.48 | 32 | |
| LG6 | BY898881_ES | 0.00 | 1.00 | 32 |
| HS4_rep_c13952_ES | 0.13 | 0.72 | 32 | |
| Cjs1817FC_S | 0.00 | 1.00 | 32 | |
| LG7 | Cjgssr13_S | 0.00 | 0.96 | 32 |
| LG8 | CS1200FC_S | 0.45 | 0.50 | 32 |
| HS4_rep_c17715_ES | 0.00 | 1.00 | 32 | |
| LG9 | S4050_S | 0.14 | 0.71 | 32 |
| LG10 | CJS0201_S | 0.00 | 1.00 | 32 |
| HS4_rep_c39488_ES | 0.27 | 0.61 | 32 | |
| LG11 | Cjgssr124_S | 28.13 | <0.0001 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriguchi, Y.; Saito, R.; Ueno, S.; Hasegawa, Y.; Kakui, H.; Matsumoto, A. Localization of TWISTED NEEDLES Locus on Linkage Map of Japanese Cedar (Cryptomeria japonica D. Don). Forests 2022, 13, 1524. https://doi.org/10.3390/f13091524
Moriguchi Y, Saito R, Ueno S, Hasegawa Y, Kakui H, Matsumoto A. Localization of TWISTED NEEDLES Locus on Linkage Map of Japanese Cedar (Cryptomeria japonica D. Don). Forests. 2022; 13(9):1524. https://doi.org/10.3390/f13091524
Chicago/Turabian StyleMoriguchi, Yoshinari, Ryunosuke Saito, Saneyoshi Ueno, Yoichi Hasegawa, Hiroyuki Kakui, and Asako Matsumoto. 2022. "Localization of TWISTED NEEDLES Locus on Linkage Map of Japanese Cedar (Cryptomeria japonica D. Don)" Forests 13, no. 9: 1524. https://doi.org/10.3390/f13091524





