Characterization of Microbial Decay and Microbial Communities in Waterlogged Archaeological Rosewood (Dalbergia Species)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microscopy
2.3. DNA Extraction, Amplification, and Sequencing
3. Results
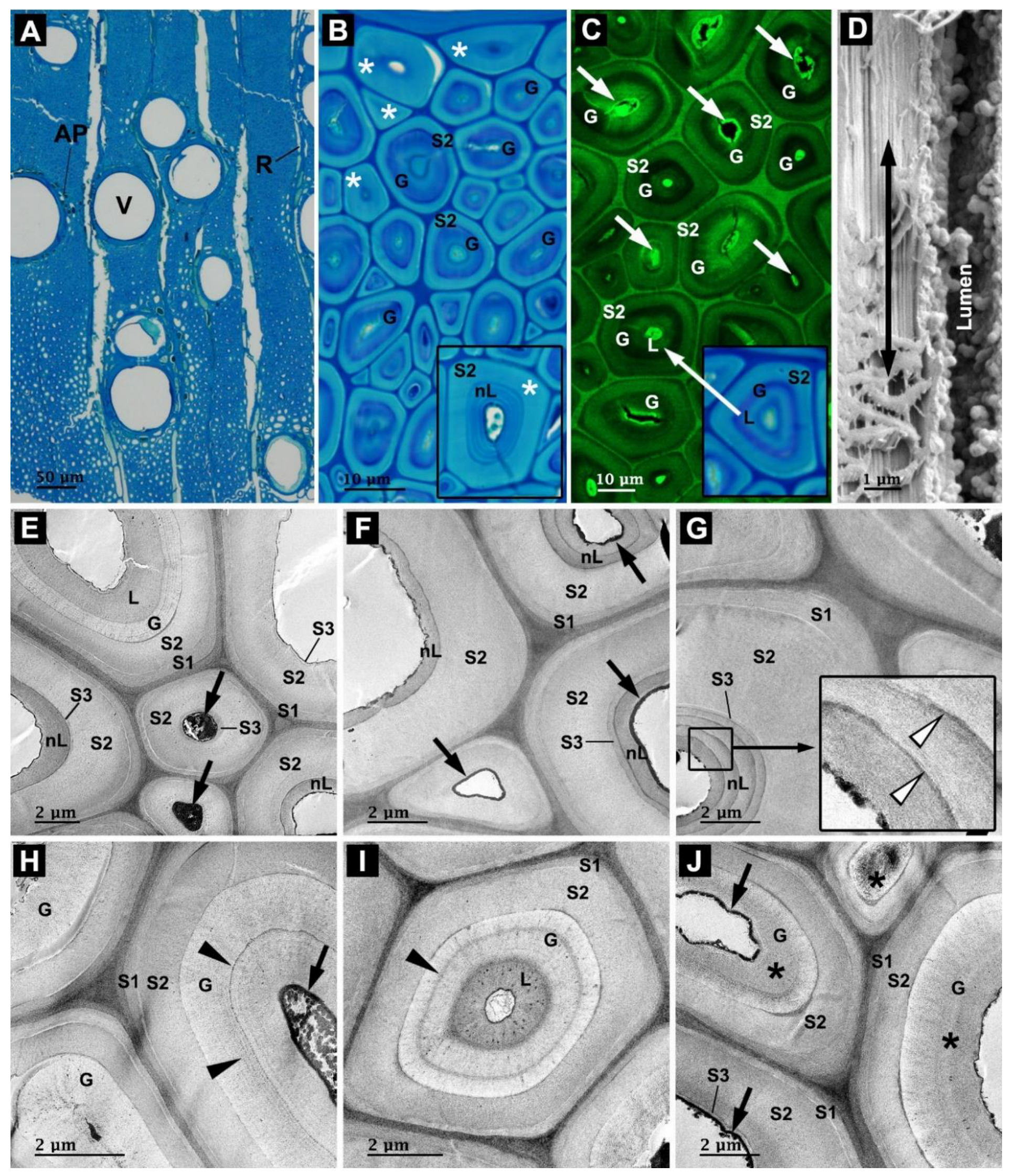
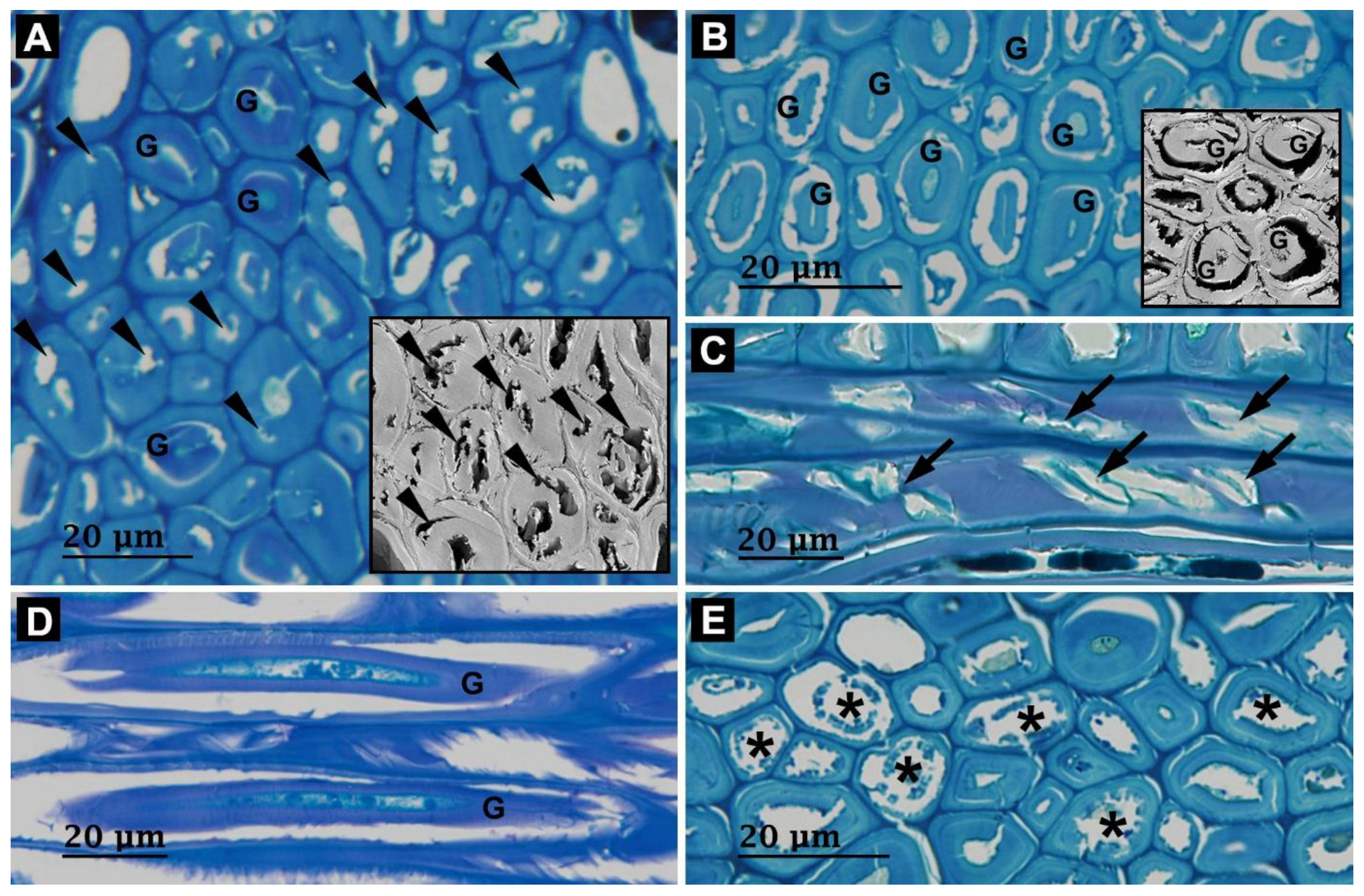
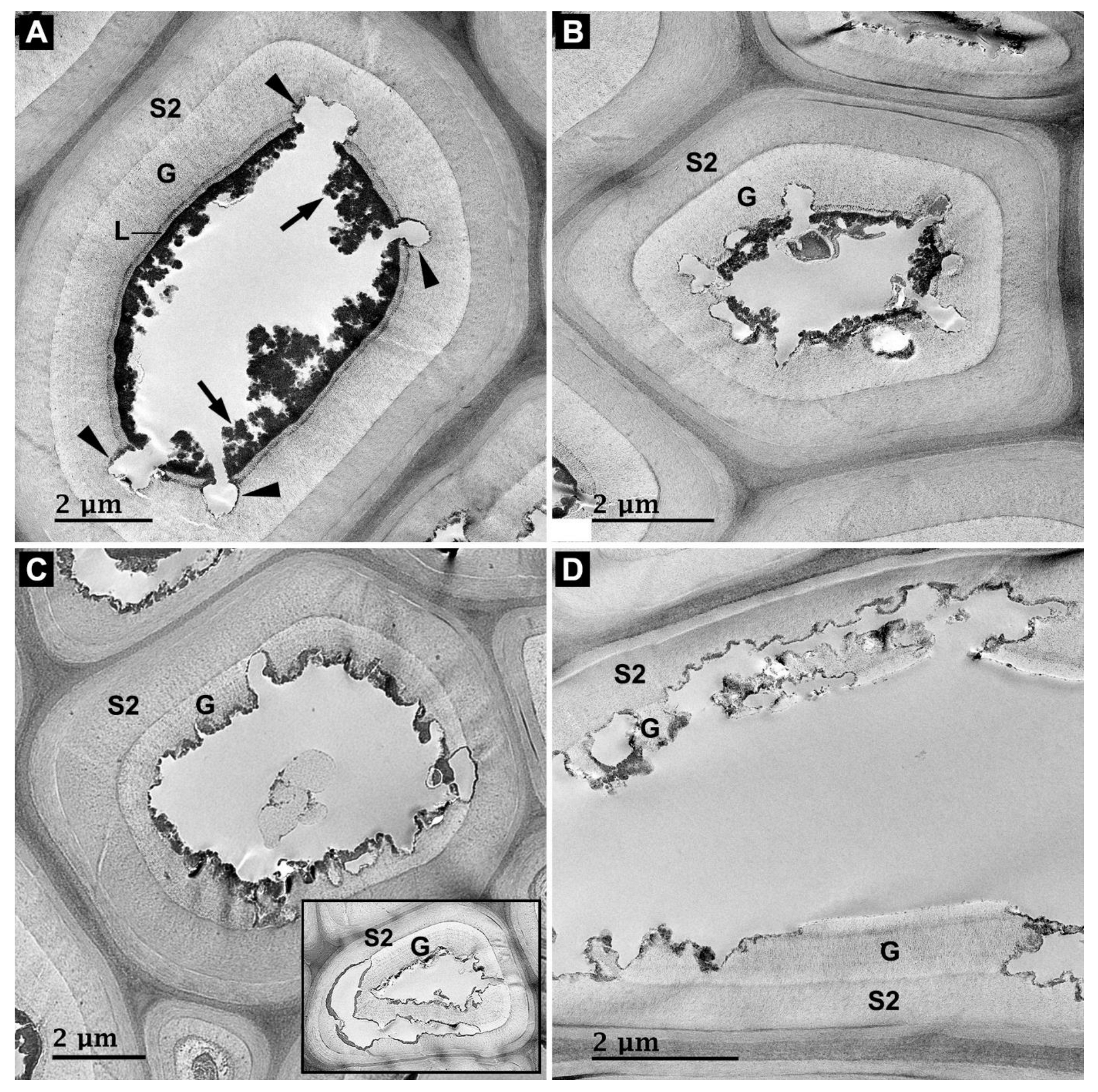
3.1. Anatomy of Rosewood
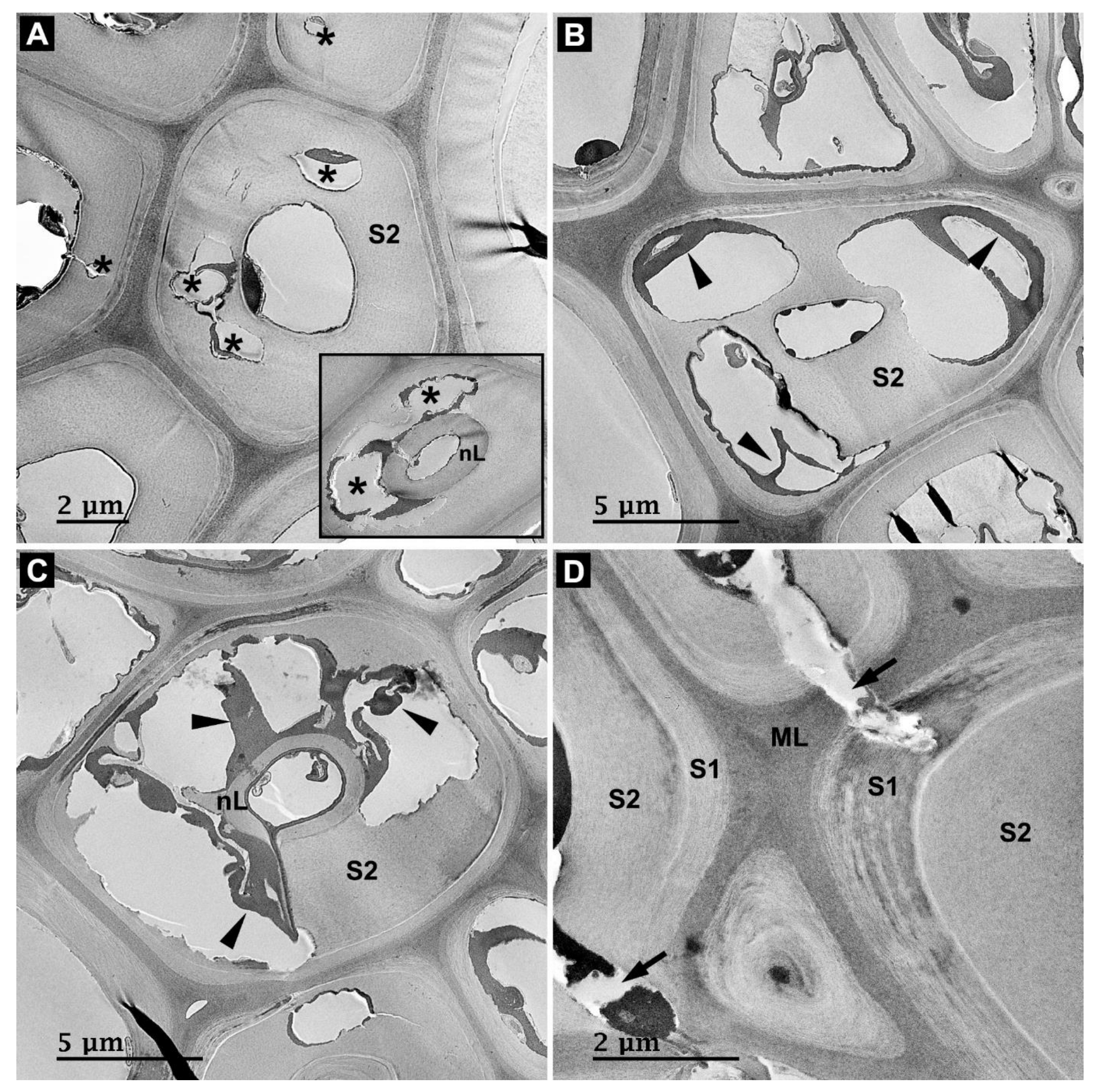
3.2. Microbial Degradation of Wood Fibers
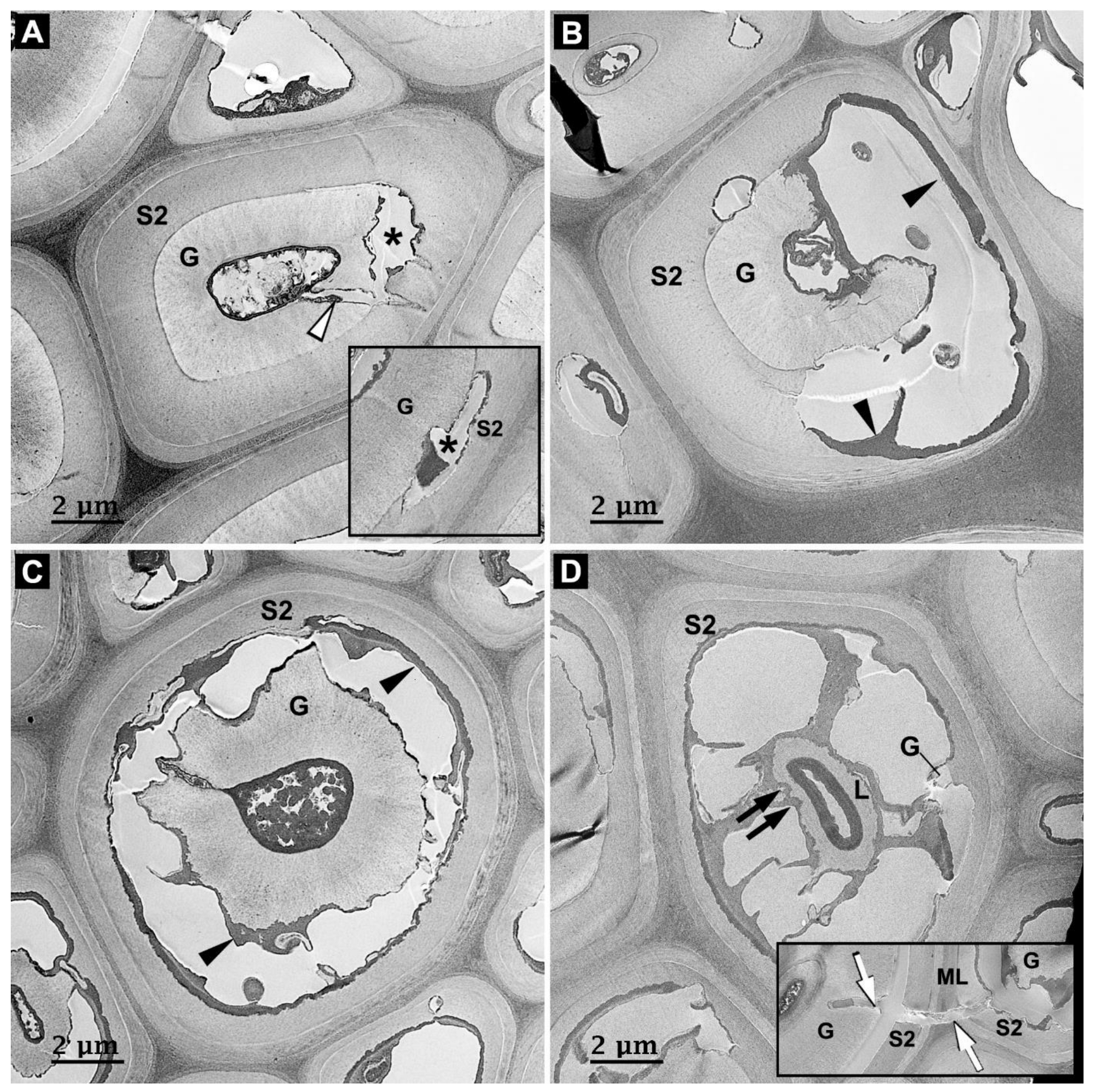
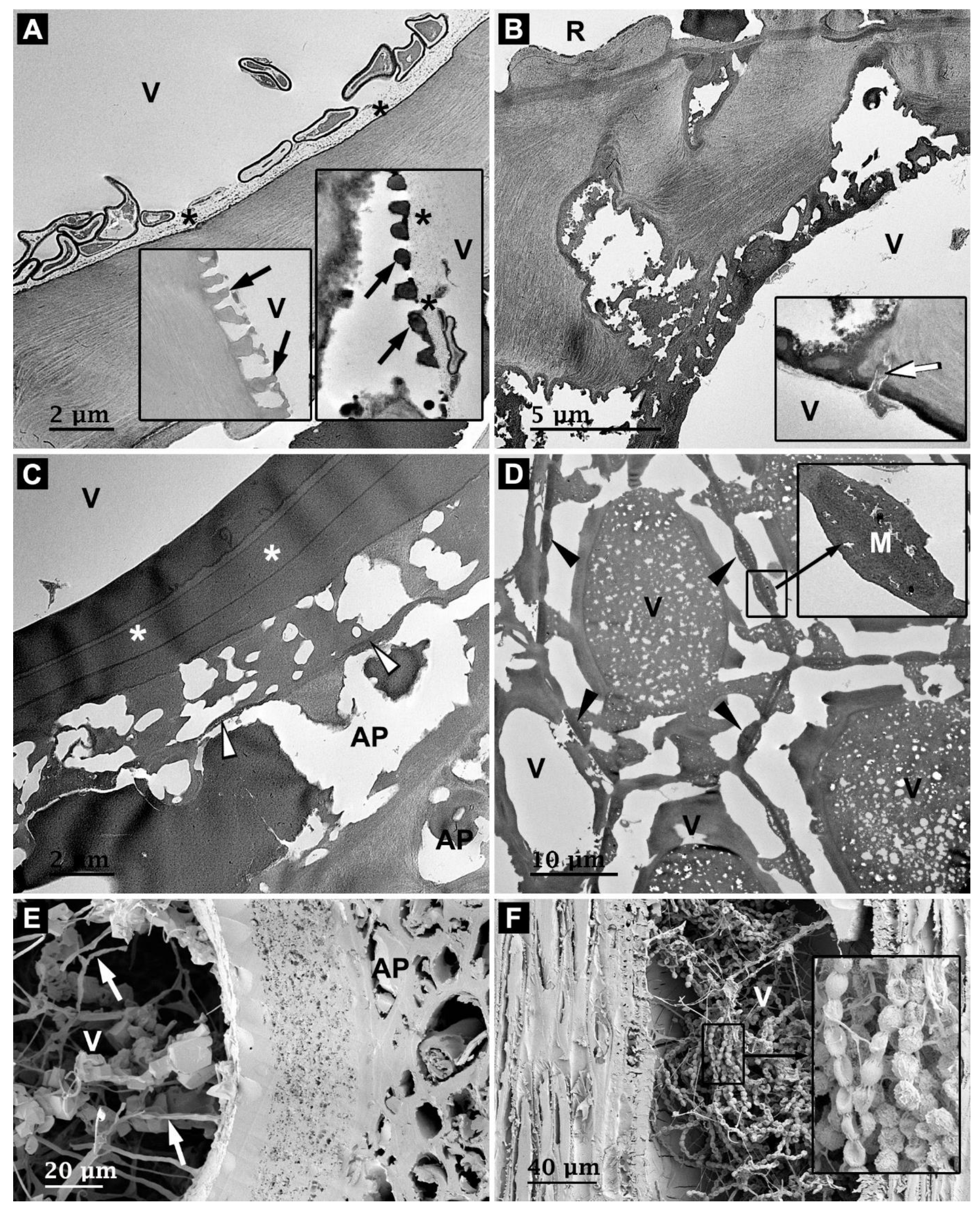
3.3. Microbial Degradation of Vessels
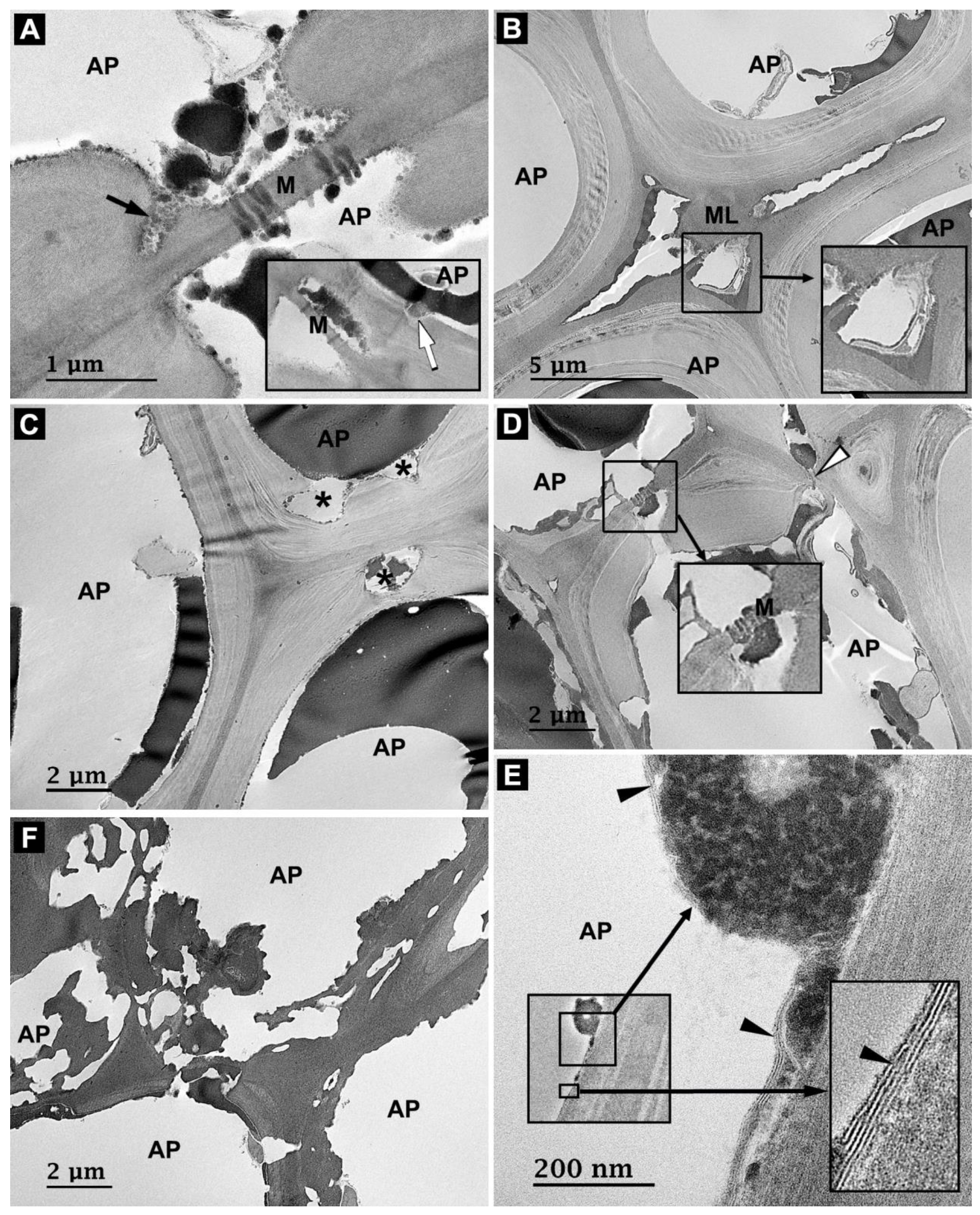
3.4. Microbial Degradation of Axial Parenchyma Cells
3.5. Microbial Community Analysis
3.5.1. Fungal Community Analysis
3.5.2. Bacterial Community Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanchette, R.A.; Nilsson, T.; Daniel, G. Biological degradation of wood. In Archaeological Wood: Properties, Chemistry and Preservation, Advances in Chemistry; Rowel, R.M., Barbour, J., Eds.; American Chemical Society: Washington, DC, USA, 1990; pp. 141–174. [Google Scholar]
- Daniel, G. Fungal degradation of wood cell walls. In Secondary Xylem; Kim, Y.S., Funada, R., Singh, A.P., Eds.; Academic Press: London, UK, 2016; pp. 131–167. [Google Scholar]
- Kim, Y.S.; Singh, A.P. Micromorphological characteristics of wood biodegradation in wet environments: A review. IAWA J. 2000, 21, 135–155. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Singh, T. Bacterial degradation of wood. In Secondary Xylem; Kim, Y.S., Funada, R., Singh, A.P., Eds.; Academic Press: London, UK, 2016; pp. 169–190. [Google Scholar]
- Pournou, A. Wood deterioration by aquatic microorganisms. In Biodeterioration of Wooden Cultural Heritage, Organisms and Decay Mechanisms in Aquatic and Terrestrial Ecosystems; Pournou, A., Ed.; Springer: Cham, Switzerland, 2020; pp. 177–260. [Google Scholar]
- Daniel, G.; Nilsson, T. Developments in the study of soft rot and bacterial decay. In Forest Products Biotechnology; Bruce, A., Palfreyman, J.W., Eds.; Taylor & Francis: London, UK, 1998; pp. 37–62. [Google Scholar]
- Björdal, C.G. Evaluation of microbial degradation of shipwrecks in the Baltic sea. Int. Biodeterior. Biodegrad. 2012, 70, 126–140. [Google Scholar] [CrossRef]
- Mouzouras, R. Soft rot decay of wood by marine microfungi. J. Inst. Wood Sci. 1989, 11, 193–201. [Google Scholar]
- Santhakumaran, L.N.; Singh, A.P. Destruction of two tropical timbers by marine borers and microorganisms in Goa water (India). In Proceedings of the 23rd Annual Meeting of the International Research Group on Wood Preservation (Document No. IRG/WP/4176-92), Harrogate, UK, 10–15 May 1992. [Google Scholar]
- Takahashi, M.; Kishima, T. Decay resistance of sixty-five southeast Asian timber specimens in accelerated laboratory tests. Tonan Ajia Kenkyu 1973, 10, 525–541. [Google Scholar] [CrossRef]
- Tsunoda, K. The natural resistance of tropical woods against biodeterioration. Wood Res. 1990, 77, 18–27. Available online: http://hdl.handle.net/2433/53275 (accessed on 27 September 2023).
- Wong, A.H.H.; Kim, Y.S.; Singh, A.P.; Ling, C.W. Natural durability of tropical species with emphasis on Malaysian hardwoods—Variations and prospects. In Proceedings of the 35th Annual Meeting of the International Research Group on Wood Preservation, Bangalore, India, 24–28 April 2005. Document No. IRG/WP 05-10568. [Google Scholar]
- Daniel, G.; Nilsson, T. Polylaminate concentric cell wall layering in fibers of Homalium foetidum and its effects on degradation by soft rot fungi. In Recent Advances in Wood Anatomy; Donaldson, L.A., Singh, A.P., Butterfield, B.G., Whitehouse, L.J., Eds.; New Zealand Forest Research Institute: Rotorua, New Zealand, 1998; pp. 369–372. [Google Scholar]
- Singh, A.P.; Wong, A.H.H.; Kim, Y.S.; Wi, S.G. The relationship of fiber cell wall ultrastructure to soft rot decay in kempas (Koompassia malaccensis) heartwood. In Proceedings of the 36th Annual Meeting of the International Research Group on Wood Preservation, Ljubljana, Slovenia, 6–10 June 2004. Document No. IRG/WP 04-10541. [Google Scholar]
- National Research Institute of Maritime Cultural Heritage. Underwater Archaeology in Korea; Gongmyoung: Seoul, Republic of Korea, 2016; pp. 55–77. [Google Scholar]
- National Research Institute of Maritime Cultural Heritage. The Shinan Wreck; National Maritime Museum of Korea: Mokpo, Republic of Korea, 2016; pp. 3–4. [Google Scholar]
- Fonseka, D.L.C.K.; Wickramaarachchi, W.W.U.I. Rosewood (Dalbergia spp.): A luxury timber. SSRG–IJAES 2018, 5, 25–28. [Google Scholar] [CrossRef]
- ICRAF Database—Wood Density. Available online: http://db.worldagroforestry.org/wd (accessed on 18 August 2023).
- Antonelli, F.; Exposito, A.; Galotta, G.; Petriaggi, B.D.; Piazza, S.; Romagnoli, M.; Guerrieri, F. Microbiota in waterlogged archaeological wood: Use of next-generation sequencing to evaluate the risk of biodegradation. App. Sci. 2020, 10, 4636. [Google Scholar] [CrossRef]
- Jia, Y.; Yin, L.; Zhang, F.; Wang, M.; Sun, M.; Hu, C.; Liu, Z.; Chen, Y.; Liu, J.; Pan, J. Fungal community analysis and biodeterioration of waterlogged wooden lacquerware from the Nanhai no. 1 shipwreck. App. Sci. 2020, 10, 3797. [Google Scholar] [CrossRef]
- Landy, E.T.; Mitchell, J.I.; Hotchkiss, S.; Eaton, R.A. Bacterial diversity associated with archaeological waterlogged wood: Ribosomal RNA clone libraries and denaturing gradient gel electrophoresis (DGGE). Int. Biodeter. Biodegr. 2007, 61, 106–116. [Google Scholar] [CrossRef]
- Preston, J.; Watts, J.E.M.; Jones, M. Novel bacterial community associated with 500-year-old unpreserved archaeological wood from King Henry VIII’s Tudor warship the Mary Rose. AEM 2012, 78, 8822–8828. [Google Scholar] [CrossRef]
- Li, Q.; Cao, L.; Wang, W.; Tan, H.; Jin, T.; Wang, G.; Lin, G.; Xu, R. Analysis of the bacterial communities in the waterlogged wooden cultural relics of the Xiaobaijiao No. 1 shipwreck via high-throughput sequencing technology. Holzforschung 2018, 72, 609–619. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, T.; Hu, C.; Shen, D.; Macchioni, N.; Sozzi, L.; Chen, Y.; Liu, J.; Tian, X.; Ge, Q.; et al. Microbial community analysis and biodeterioration of waterlogged archaeological wood from the Nanhai No. 14 shipwreck during storage. Sci. Rep. 2018, 8, 7170. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-K. Review of the red sandalwood cargo of the Shinan ship. Marit. Cult. Herit. 2013, 6, 37–70. (In Korean) [Google Scholar]
- Yu, Z.; Morrrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2012, 7, 335–336. [Google Scholar] [CrossRef]
- Li, W.; Fu, L.; Niu, B.; Wu, S.; Wooley, J. Ultrafast clustering algorithms for metagenomic sequence analysis. Brief. Bioinform. 2012, 13, 656–668. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Chavan, R.R. Advances in understanding microbial deterioration of buried and waterlogged archaeological woods: A review. Forests 2022, 13, 394. [Google Scholar] [CrossRef]
- Kim, Y.S. Micromorphology of degraded archaeological pine wood in waterlogged situations. Mater. Org. 1989, 24, 271–286. [Google Scholar]
- Blanchette, R.A.; Haight, J.E.; Koestler, R.J.; Hatchfield, P.B.; Arnold, D. Assessment of deterioration in archaeological wood from ancient Egypt. JAIC 1994, 33, 55–70. [Google Scholar] [CrossRef]
- Kim, Y.S.; Singh, A.P. Wood as cultural heritage material and its deterioration by biotic and abiotic agents. In Secondary Xylem; Kim, Y.S., Funada, R., Singh, A.P., Eds.; Academic Press: London, UK, 2016; pp. 169–190. [Google Scholar]
- Björdal, C.G.; Dayton, P.K. First evidence of microbial wood degradation in the coastal waters of the Antarctic. Sci. Rep. 2020, 10, 12774. [Google Scholar] [CrossRef] [PubMed]
- Björdal, C.G.; Daniel, G.; Nilsson, T. Microbial decay of waterlogged archaeological wood found in Sweden—Applicable to archaeology and conservation. Int. Biodeterior. Biodegrad. 1999, 43, 63–73. [Google Scholar] [CrossRef]
- Blanchette, R.A. A review of microbial deterioration found in archaeological wood from different environments. Int. Biodeter. Biodegr. 2000, 46, 189–204. [Google Scholar] [CrossRef]
- Daniel, G. Fungal and bacteria. In Deterioration and Protection of Sustainable Biomaterials; Schultz, T.P., Goodell, B., Nicholas, D.D., Eds.; American Chemical Society: Washington, DC, USA, 2014; pp. 23–58. [Google Scholar]
- Pilate, G.; Chabbert, B.; Cathala, B.; Yoshinaga, A.; Leplé, J.-C.; Laurans, F.; Lapierre, C.; Ruel, K. Lignification and tension wood. Comptes Rendus Biol. 2004, 327, 889–901. [Google Scholar] [CrossRef]
- Gorshkova, T.A.; Gurjanov, O.P.; Mikshina, P.V.; Ibragimova, N.N.; Mokshina, N.E.; Salnikov, V.V.; Ageeva, M.V.; Amenitskii, S.I.; Chernova, T.E.; Chemikosova, S.B. Specific type of secondary cell wall formed by plant fibers. Russ. J. Plant Physiol. 2010, 57, 328–341. [Google Scholar] [CrossRef]
- Schwarze, F.; Baum, S. Mechanisms of reaction zone penetration by decay fungi in wood of beech (Fagus sylvatica). New Phytol. 2000, 146, 129–140. [Google Scholar] [CrossRef]
- Sachs, I.; Kuntz, J.; Ward, J.; Nair, G.; Schultz, N. Tyloses structure. Wood Fiber Sci. 1970, 2, 259–268. [Google Scholar]
- Nilsson, T.; Singh, A.; Daniel, G. Ultrastructure of the attack of Eusideroxylon zwageri wood by tunneling bacteria. Holzforschung 1992, 46, 361–368. [Google Scholar] [CrossRef]
- Singh, A.P.; Wong, A.H.H.; Kim, Y.S.; Wi, S.G. Soft rot decay of cengal (Neobalanocarpus heimii) heartwood in ground contact in relation to extractive microdistribution. In Proceedings of the 34th Annual Meeting of the International Research Group on Wood Preservation, Brisbane, Australia, 18–23 May 2003. Document No. IRG/WP 03-10501. [Google Scholar]
- Kim, Y.S.; Singh, A.P.; Wong, A.H.H.; Eom, T.J.; Lee, K.H. Micromorphological and chemical characteristics of cengal (Neobalanocarpus heimii) heartwood decayed by soft rot fungi. J. Kor. Wood Sci. Technol. 2006, 34, 68–77. [Google Scholar]
- Singh, A.P.; Nilsson, T.; Daniel, G.F. Alstonia scholaris vestures are resistant to degradation by tunnelling bacteria. IAWA J. 1993, 14, 119–126. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Wi, S.G. Inhomogeneity in the composition of vesture walls in an archaeological wood. IAWA J. 2002, 23, 77–81. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Chavan, R.R. Relationship of wood cell wall ultrastructure to bacterial degradation of wood. IAWA J. 2019, 40, 1–26. [Google Scholar] [CrossRef]
- Worrall, J.J.; Anagnost, S.E.; Zabel, R.A. Comparison of wood decay among diverse lignicolous fungi. Mycologia 1997, 89, 199–219. [Google Scholar] [CrossRef]
- Xiaobin, C.; Rong, J.; Pingsheng, L.; Shiqian, T.; Qin, Z.; Wenzhong, T.; Xudong, L. Purification of a new manganese peroxidase of the white-rot fungus Schizophyllum sp. F17, and decolorization of azo dyes by the enzyme. Enzyme Microb. Technol. 2007, 41, 258–264. [Google Scholar] [CrossRef]
- Gao, D.; Du, L.; Yang, J.; Wu, W.-M.; Liang, H. A critical review of the application of white rot fungus to environmental pollution control. Crit. Rev. Biotechnol. 2010, 30, 70–77. [Google Scholar] [CrossRef]
- Kamei, I.; Yoshida, T.; Enami, D.; Meguro, S. Coexisting Curtobacterium bacterium promotes growth of white-rot fungus Stereum sp. Curr. Microbiol. 2012, 64, 173–178. [Google Scholar] [CrossRef]
- Tovar-Herrera, O.E.; Batista-García, R.A.; Sánchez-Carbente, M.d.R.; Iracheta-Cárdenas, M.M.; Arévalo-Niño, K.; Folch-Mallol, J.L. A novel expansion protein from the white-rot fungus Schizophyllum commune. PLoS ONE 2015, 10, e0122296. [Google Scholar] [CrossRef]
- Corfixen, P.; Parmasto, E. Hymenochaete and Hymenochaetopsis (Basidiomycota) in Europe. Karstenia 2017, 57, 49–80. [Google Scholar] [CrossRef]
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The genus Cladosporium. Stud. Micol. 2012, 72, 1–401. [Google Scholar] [CrossRef] [PubMed]
- Zabel, R.A.; Wang, C.J.K.; Anagnost, S.E. Soft-rot capabilities of the major microfungi, isolated from Douglas-fir poles in the northeast. Wood Fiber Sci. 1991, 23, 220–237. [Google Scholar]
- Klankeo, P.; Nopcharoenkul, W.; Pinyakong, O. Two novel pyrene-degrading Diaphorobacter sp. and Pseudoxanthomonas sp. isolated from soil. J. Biosci. Bioeng. 2009, 108, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Bugg, T.D.H.; Ahmad, M.; Hardiman, E.M.; Singh, R. The emerging role for bacteria in lignin degradation and bio-product formation. Curr. Opin. Biotechnol. 2011, 22, 394–400. [Google Scholar] [CrossRef]
- Wang, L.; Nie, Y.; Tang, Y.-Q.; Song, X.-M.; Cao, K.; Sun, L.-Z.; Wang, Z.-J.; Wu, X.-L. Diverse bacteria with lignin degrading potentials isolated from two ranks of coal. Front. Microbiol. 2016, 7, 1428. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhao, Y.; Ni, K.; Shi, Y.; Xu, Q. Characterization of ligninolytic bacteria and analysis of alkali-lignin biodegradation products. Poi. J. Microbiol. 2020, 69, 339–347. [Google Scholar] [CrossRef]
- Grgas, D.; Rukavina, M.; Bešlo, D.; Štefanac, T.; Crnek, V.; Šikić, T.; Habuda-Stanić, M.; Dragićević, T.L. The bacterial degradation of lignin-a review. Water 2023, 15, 1272. [Google Scholar] [CrossRef]
- Ali, H.R.K.; Hemeda, N.F.; Abdelaliem, Y.F. Symbiotic cellulolytic bacteria from the gut of the subterranean termite Psammotermes hypostoma Desneux and their role in cellulose digestion. AMB Expr. 2019, 9, 111. [Google Scholar] [CrossRef]
- Cui, J.; Mai, G.; Want, Z.; Liu, Q.; Zhou, Y.; Ma, T.; Liu, C. Metagenomic insights into a cellulose-rich niche reveal microbial cooperation in cellulose degradation. Front. Microbiol. 2016, 10, 618. [Google Scholar] [CrossRef]
- Batubara, U.M.; Mardalisa, M.; Suparjo, S.; Maritsa, H.U.; Pujianto, E.; Herlini, M. Isolation and characterization of cellulolytic bacteria diversity in peatland ecosystem and their cellulolytic activities. IOP Conf. Ser. Earth Environ. Sci. 2021, 934, 12028. [Google Scholar] [CrossRef]
- Froidurot, A.; Julliand, V. Cellulolytic bacteria in the large intestine of mammals. Gut Microbes 2022, 14, e2031694. [Google Scholar] [CrossRef] [PubMed]
- Thu, N.K.; Tanabe, Y.; Yoshida, M.; Matsuura, H.; Watanabe, M.M. Aerosakkonema funiforme gen. et sp. nov. (Oscillatoriales), a new gas-vacuolated oscillatorioid cyanobacterium isolated from a mesotrophic reservoir. Phycologia 2012, 51, 672–683. [Google Scholar] [CrossRef]
- Aguilera, A.; Klemenčič, M.; Sueldo, D.J.; Rzymski, P.; Giannuzzi, L.; Martin, M.V. Cell death in Cyanobacteria: Current understanding and recommendations for a consensus on its nomenclature. Front. Microbiol. 2021, 12, 631654. [Google Scholar] [CrossRef] [PubMed]





| Kingdom | Phylum | Relative Abundance (%) | Number of Reads |
|---|---|---|---|
| Fungi | Ascomycota | 48.0 | 26,971 |
| Basidiomycota | 49.8 | 27,973 | |
| Unclassified | 2.2 | 1254 | |
| Bacteria | Cyanobacteria | 37.7 | 7229 |
| Proteobacteria | 36.9 | 7065 | |
| Firmicutes | 10.1 | 1944 | |
| Actinobacteria | 6.3 | 1212 | |
| Bacteroidetes | 5.6 | 1074 | |
| Acidobacteria | 2.0 | 376 | |
| Deinococcus–Thermus | 0.3 | 64 | |
| Gemmatimonadetes | 0.3 | 64 | |
| Chloroflexi | 0.3 | 56 | |
| Planctomycetes | 0.2 | 35 | |
| Verrucomicrobia | 0.1 | 19 | |
| Ignavibacteriae | 0.1 | 19 | |
| Chlamydiae | 0.0 | 7 | |
| Fusobacteria | 0.0 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.S.; Kim, M.; Lim, J.W.; Cha, M.Y.; Lee, K.H.; Yoon, Y.H.; Kim, Y.S. Characterization of Microbial Decay and Microbial Communities in Waterlogged Archaeological Rosewood (Dalbergia Species). Forests 2023, 14, 1992. https://doi.org/10.3390/f14101992
Kim JS, Kim M, Lim JW, Cha MY, Lee KH, Yoon YH, Kim YS. Characterization of Microbial Decay and Microbial Communities in Waterlogged Archaeological Rosewood (Dalbergia Species). Forests. 2023; 14(10):1992. https://doi.org/10.3390/f14101992
Chicago/Turabian StyleKim, Jong Sik, Minseok Kim, Ju Won Lim, Mi Young Cha, Kwang Ho Lee, Yong Hee Yoon, and Yoon Soo Kim. 2023. "Characterization of Microbial Decay and Microbial Communities in Waterlogged Archaeological Rosewood (Dalbergia Species)" Forests 14, no. 10: 1992. https://doi.org/10.3390/f14101992





