Potential Distribution and Suitable Habitat for Chestnut (Castanea sativa)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Chestnut Occurrence Data
2.3. Climatic Data
2.4. Chestnut Suitable Habitats Modeling
2.5. Comparison of the Climate Datasets
3. Results
3.1. Comparison of the Models
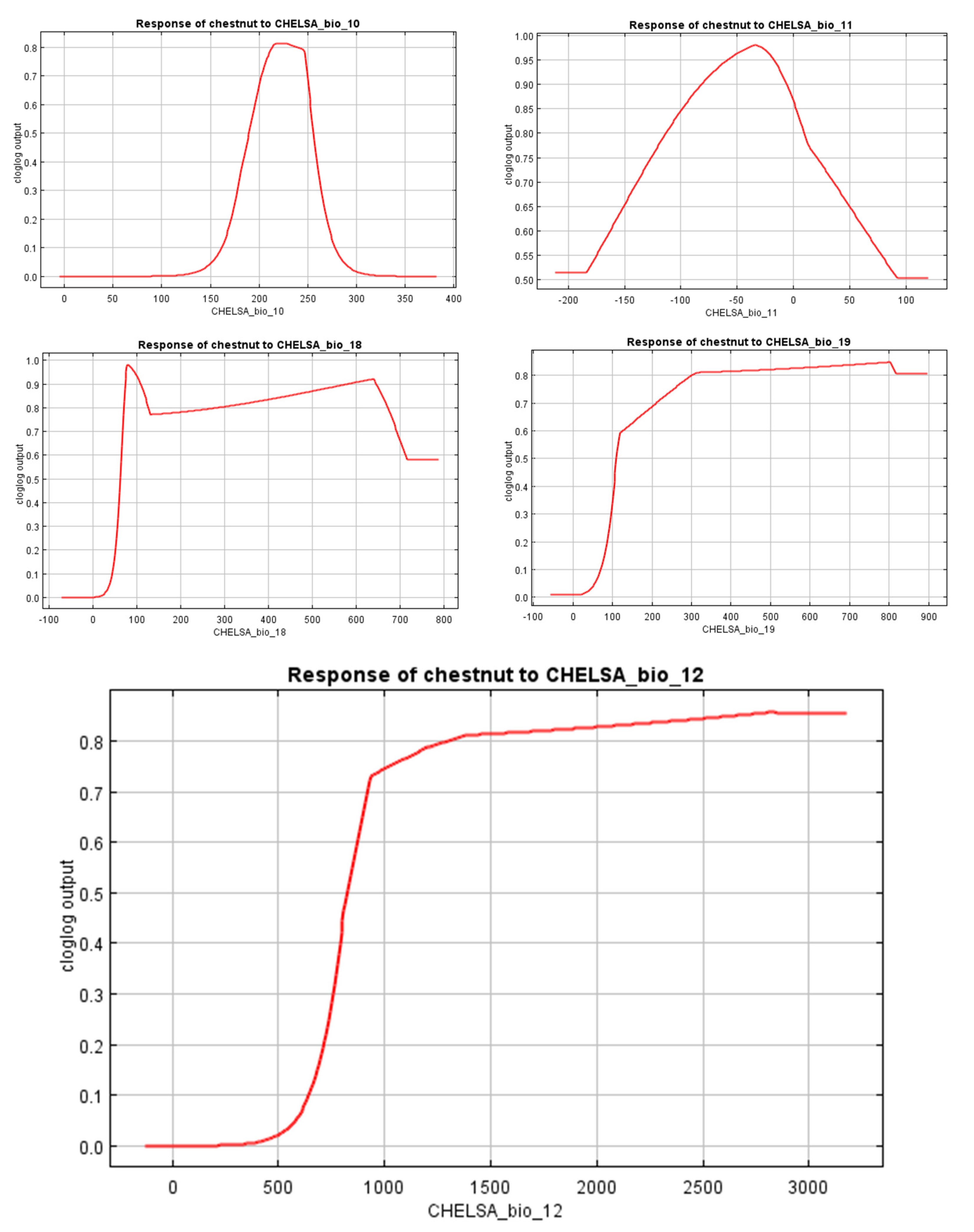
3.2. The Significance and Performance of the Variables
3.3. Suitable Habitats Model in the Caucasus
3.4. Suitable Habitats Model in Europe
4. Discussion
4.1. Comparison of the Models
4.2. The Significance and Performance of Variables
4.3. Suitable Habitats Model
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konstantinidis, P.; Tsiourlis, G.; Xofis, P.; Buckley, G.P. Taxonomy and Ecology of Castanea sativa Mill. Forests in Greece. Plant Ecol. 2008, 195, 235–256. [Google Scholar] [CrossRef]
- Dolukhanov, A. Forest Vegetation of Georgia; Universal: Tbilisi, GA, USA, 2010. [Google Scholar]
- Nakhutsrishvili, G. The Vegetation of Georgia (South Caucasus); Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-29914-8. [Google Scholar]
- Conedera, M.; Tinner, W.; Krebs, P.; de Rigo, D.; Caudullo, G. Castanea sativa in Europe: Distribution, Habitat, Usage and Threats; European Atlas of Forest Tree Species. EU, Luxembourg. 2016, pp. 78–79. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://ies-ows.jrc.ec.europa.eu/efdac/download/Atlas/pdf/Castanea_sativa.pdf (accessed on 2 June 2023).
- Mattioni, C.; Cherubini, M.; Micheli, E.; Villani, F.; Bucci, G. Role of Domestication in Shaping Castanea sativa Genetic Variation in Europe. Tree Genet. Genomes 2008, 4, 563–574. [Google Scholar] [CrossRef]
- Kvavadze, E. Identification of Anthropological Landscapes and Human Activity in Georgia in Correlation with Holocene Black Sea Level Fluctuations. Earth 2015, 4, 120. [Google Scholar] [CrossRef]
- Krebs, P.; Conedera, M.; Pradella, M.; Torriani, D.; Felber, M.; Tinner, W. Quaternary Refugia of the Sweet Chestnut (Castanea sativa Mill.): An Extended Palynological Approach. Veget. Hist. Archaeobot. 2004, 13, 145–160. [Google Scholar] [CrossRef]
- Broadmeadow, M.S.J.; Ray, D.; Samuel, C.J.A. Climate Change and the Future for Broadleaved Tree Species in Britain. For. Int. J. For. Res. 2005, 78, 145–161. [Google Scholar] [CrossRef]
- Conedera, M.; Krebs, P.; Gehring, E.; Wunder, J.; Hülsmann, L.; Abegg, M.; Maringer, J. How Future-Proof Is Sweet Chestnut (Castanea sativa) in a Global Change Context. For. Ecoly Manag. 2021, 494, 119320. [Google Scholar] [CrossRef]
- Gulisashvili, V. Chestnut in the Caucasus; Nature: Tbilisi, Georgia, 1967. [Google Scholar]
- Tugushi, L. Chestnut forests of Abkhazia and ways to improve them, Forest Institute of Tbilisi. Ph.D. Thesis, Ochamchire, 1965; pp. 33–37. [Google Scholar]
- Velizarova, E. Physico-Chemical and Morphological Properties of Soils in Chestnut (Castanea sativa Mill.) Habitats of Belasitsa Mountain. Silva Balc. 2015, 16, 60–70. [Google Scholar]
- Badenes, M.L.; Byrne, D.H. (Eds.) Fruit Breeding; Springer US: Boston, MA, USA, 2012; ISBN 978-1-4419-0762-2. [Google Scholar]
- Gomes-Laranjo, J.; Dinis, L.-T.; Martins, L.; Portela, E.; Pinto, T.; Ciordia, M.; Feito, I.; Majada, J.; Peixoto, F.; Pereira, S.; et al. Characterization of Chestnut Behavior with Photosynthetic Traits. In Applied Photosynthesis; Najafpour, M., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0061-4. [Google Scholar]
- Vettraino, A.M.; Morel, O.; Perlerou, C.; Robin, C.; Diamandis, S.; Vannini, A. Occurrence and Distribution of Phytophthora Species in European Chestnut Stands, and Their Association with Ink Disease and Crown Decline. Eur. J. Plant Pathol. 2005, 111, 169–180. [Google Scholar] [CrossRef]
- Beridze, B.; Dering, M. Problems and Threats to the Caucasus Forest Ecosystems on the Example of Castanea sativa. Kosmos 2021, 70, 19–26. [Google Scholar] [CrossRef]
- Heiniger, U.; Rigling, D. Biological Control of Chestnut Blight in Europe. Annu. Rev. Phytopathol. 1994, 32, 581–599. [Google Scholar] [CrossRef]
- Anagnostakis, S.L. American Chestnut Sprout Survival with Biological Control of the Chestnut-Blight Fungus Population. For. Ecol. Manag. 2001, 152, 225–233. [Google Scholar] [CrossRef]
- Venanzi, R.; Picchio, R.; Piovesan, G. Silvicultural and Logging Impact on Soil Characteristics in Chestnut (Castanea sativa Mill.) Mediterranean Coppice. Ecol. Eng. 2016, 92, 82–89. [Google Scholar] [CrossRef]
- Newbigin, M.I. Man and the Forest in Europe: The Pre-Industrial Period. Emp. For. J. 1928, 7, 209–224. [Google Scholar]
- Conedera, M.; Manetti, M.C.; Giudici, F.; Amorini, E. Distribution and economic potential of the Sweet chestnut (Castanea sativa Mill.) in Europe. Ecmed 2004, 30, 179–193. [Google Scholar] [CrossRef]
- Akhalkatsi, M. Forest Habitat Restoratio in Georgia, Caucaus Ecoregion; Mtsigobari: Tbilisi, GA, USA, 2015; ISBN 978-9941-450-68-6. [Google Scholar]
- Quinn, J. Gatekhili Mountains, Gatekhili State: Fractured Alpine Forest Governance and Post-Soviet Development in the Republic of Georgia. Rga 2017, 105, 1–14. [Google Scholar] [CrossRef]
- Prospero, S.; Lutz, A.; Tavadze, B.; Supatashvili, A.; Rigling, D. Discovery of a New Gene Pool and a High Genetic Diversity of the Chestnut Blight Fungus Cryphonectria Parasitica in Caucasian Georgia. Infect. Genet. Evol. 2013, 20, 131–139. [Google Scholar] [CrossRef]
- Beridze, B.; Sękiewicz, K.; Walas, Ł.; Thomas, P.A.; Danelia, I.; Fazaliyev, V.; Kvartskhava, G.; Sós, J.; Dering, M. Biodiversity Protection against Anthropogenic Climate Change: Conservation Prioritization of Castanea sativa in the South Caucasus Based on Genetic and Ecological Metrics. Ecol. Evol. 2023, 13, e10068. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.; Miller, J.A. Mapping Species Distributions: Spatial Inference and Prediction; Ecology, biodiversity and conservation; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2009; ISBN 978-0-521-87635-3. [Google Scholar]
- Araújo, M.B.; Guisan, A. Five (or so) Challenges for Species Distribution Modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Luoto, M.; Virkkala, R.; Heikkinen, R.K. The Role of Land Cover in Bioclimatic Models Depends on Spatial Resolution. Glob. Ecol. Biogeogr. 2006, 16, 34–42. [Google Scholar] [CrossRef]
- Soria-Auza, R.W.; Kessler, M.; Bach, K.; Barajas-Barbosa, P.M.; Lehnert, M.; Herzog, S.K.; Böhner, J. Impact of the Quality of Climate Models for Modelling Species Occurrences in Countries with Poor Climatic Documentation: A Case Study from Bolivia. Ecol. Model. 2010, 221, 1221–1229. [Google Scholar] [CrossRef]
- Bakuradze, G. Assessment of Above-Ground Carbon Stocks in the Forest Ecosystem of Georgia; Ilia State University: Tbilisi, GA, USA, 2023. [Google Scholar]
- Kurdadze, T. Influence of Competition on Beech Growth in Mtskheta-Mtianeti Region Based on National Forest Inventory Data; Ilia State University: Tbilisi, GA, USA, 2020. [Google Scholar]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth’s Land Surface Areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Thuiller, W.; Richardson, D.M.; Pyšek, P.; Midgley, G.F.; Hughes, G.O.; Rouget, M. Niche-based Modelling as a Tool for Predicting the Risk of Alien Plant Invasions at a Global Scale. Glob. Chang. Biol. 2005, 11, 2234–2250. [Google Scholar] [CrossRef] [PubMed]
- Tarkhnishvili, D.; Gavashelishvili, A.; Mumladze, L. Palaeoclimatic Models Help to Understand Current Distribution of Caucasian Forest Species: Modeling West Asian Forest Refugia. Biol. J. Linn. Soc. 2012, 105, 231–248. [Google Scholar] [CrossRef]
- Tielidze, L.G.; Wheate, R.D. The Greater Caucasus Glacier Inventory (Russia, Georgia and Azerbaijan). Cryosphere 2018, 12, 81–94. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global Biodiversity Conservation: The Critical Role of Hotspots. In Biodiversity Hotspots; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. ISBN 978-3-642-20991-8. [Google Scholar]
- Zazanashvili, N.; Mallon, D. Status and Protection of Globally Threatened Species in the Caucasus; CEPF, WWF: Tbilisi, GA, USA, 2009; Volume 232, ISBN 978-9941-0-2203-6. [Google Scholar]
- Akhalkatsi, M. Pine Forest on Tree-Line Ecotone in the Mountain Kazbegi in the Georgia (South Caucasus). Agri. Res. Technol. Open Access J. 2019, 21, 556149. [Google Scholar] [CrossRef]
- Tarkhnishvili, D.N. Historical Biogeography of the Caucasus; Wildlife protection, destruction and extinction; Nova Publishers: New York, NY, USA, 2014; ISBN 978-1-63321-910-6. [Google Scholar]
- Aoyama, H. A Study of the Stratified Random Sampling. Ann. Inst. Stat. Math. 1954, 6, 1–36. [Google Scholar] [CrossRef]
- Hayek, L.-A.C.; Buzas, M.A. Surveying Natural Populations: Quantitative Tools for Assessing Biodiversity; Columbia University Press: New York, NY, USA, 2010; ISBN 978-0-231-14620-3. [Google Scholar]
- Iachan, R. Systematic Sampling: A Critical Review. Int. Stat. Rev. Rev. Int. Stat. 1982, 50, 293. [Google Scholar] [CrossRef]
- Dormann, C.F.; McPherson, J.M.; Araújo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetz, W.; Daniel Kissling, W.; et al. Methods to Account for Spatial Autocorrelation in the Analysis of Species Distributional Data: A Review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Veloz, S.D. Spatially Autocorrelated Sampling Falsely Inflates Measures of Accuracy for Presence-Only Niche Models. J. Biogeogr. 2009, 36, 2290–2299. [Google Scholar] [CrossRef]
- Occurrence data of Castanea sativa, Global Biodiversity Information Facility (GBIF), 2023. Available online: https://doi.org/10.15468/dl.k6d6hn (accessed on 25 February 2023).
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Duarte Ritter, C.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. CoordinateCleaner: Standardized Cleaning of Occurrence Records from Biological Collection Databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Friedl, M.; Sulla-Menashe, D. MODIS/Terra+Aqua Land Cover Type Yearly L3 Global 500m SIN Grid MCD12Q1. NASA EOSDIS Land Processes Distributed Active Archive Center, South Dakota, USA, 2019. Available online: https://search.earthdata.nasa.gov/search (accessed on 20 May 2023).
- Maldonado, C.; Molina, C.I.; Zizka, A.; Persson, C.; Taylor, C.M.; Albán, J.; Chilquillo, E.; Rønsted, N.; Antonelli, A. Estimating Species Diversity and Distribution in the Era of B Ig D Ata: To What Extent Can We Trust Public Databases? Glob. Ecol. Biogeogr. 2015, 24, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Bivand, R.; Keitt, T.; Rowlingson, B.; Pebesma, E.; Sumner, M.; Hijmans, R.; Rouault, E.; Maintainer, R.B. Package ‘rgdal’. Bindings for the Geospatial Data Abstraction Library. 2022, p. 172. Available online: https://cran.r-project.org/web/packages/rgdal/index.html (accessed on 15 May 2022).
- Hijmans, R. Raster package in R, The Comprehensive R Archive Network, 2023. p. 172. Available online: https://rspatial.org/raster (accessed on 8 February 2022).
- Frank, E.H., Jr. Package ‘Hmisc’, The Comprehensive R Archive Network, 2023. Available online: https://hbiostat.org/R/Hmisc/ (accessed on 10 May 2022).
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Warnes, G.R.; Bolker, B.; Lumley, T.; Warnes, M.G.R. Package‘Gtools’, The Comprehensive R Archive Network, R Package version, 3(1), 2021. Available online: https://github.com/r-gregmisc/gtools (accessed on 5 April 2022).
- Evans, T.G.; Diamond, S.E.; Kelly, M.W. Mechanistic Species Distribution Modelling as a Link between Physiology and Conservation. Conserv. Physiol. 2015, 3, cov056. [Google Scholar] [CrossRef] [PubMed]
- Gurney, K.M.; Schaberg, P.G.; Hawley, G.J.; Shane, J.B. Inadequate Cold Tolerance as a Possible Limitation to American Chestnut Restoration in the Northeastern United States. Restor. Ecol. 2011, 19, 55–63. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Vera, F.W.M.; Bakker, E.S.; Olff, H. Large Herbivores: Missing Partners of Western European Light-demanding Tree and Shrub Species? In Large Herbivore Ecology, Ecosystem Dynamics and Conservation; Danell, K., Bergström, R., Duncan, P., Pastor, J., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 203–231. ISBN 978-0-521-83005-8. [Google Scholar]
- Meier, E.S.; Kienast, F.; Pearman, P.B.; Svenning, J.-C.; Thuiller, W.; Araújo, M.B.; Guisan, A.; Zimmermann, N.E. Biotic and Abiotic Variables Show Little Redundancy in Explaining Tree Species Distributions. Ecography 2010, 33, 1038–1048. [Google Scholar] [CrossRef]
- VanDerWal, J.; Shoo, L.P.; Graham, C.; Williams, S.E. Selecting Pseudo-Absence Data for Presence-Only Distribution Modeling: How Far Should You Stray from What You Know? Ecol. Model. 2009, 220, 589–594. [Google Scholar] [CrossRef]
- Bleyhl, B.; Arakelyan, M.; Askerov, E.; Bluhm, H.; Gavashelishvili, A.; Ghasabian, M.; Ghoddousi, A.; Heidelberg, A.; Khorozyan, I.; Malkhasyan, A.; et al. Assessing Niche Overlap between Domestic and Threatened Wild Sheep to Identify Conservation Priority Areas. Divers. Distrib. 2019, 25, 129–141. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Beck, J.; Böller, M.; Erhardt, A.; Schwanghart, W. Spatial Bias in the GBIF Database and Its Effect on Modeling Species’ Geographic Distributions. Ecol. Inform. 2014, 19, 10–15. [Google Scholar] [CrossRef]
- Jacobs, J. Quantitative Measurement of Food Selection: A Modification of the Forage Ratio and Ivlev’s Electivity Index. Oecologia 1974, 14, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Lechowicz, M.J. The Sampling Characteristics of Electivity Indices. Oecologia 1982, 52, 22–30. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, Z. Predicting the Potential Distribution of the Endangered Przewalski’s Gazelle. J. Zool. 2010, 282, 54–63. [Google Scholar] [CrossRef]
- Bobrowski, M.; Schickhoff, U. Why Input Matters: Selection of Climate Data Sets for Modelling the Potential Distribution of a Treeline Species in the Himalayan Region. Ecol. Model. 2017, 359, 92–102. [Google Scholar] [CrossRef]
- Hijmans, R.J. Cross-Validation of Species Distribution Models: Removing Spatial Sorting Bias and Calibration with a Null Model. Ecology 2012, 93, 679–688. [Google Scholar] [CrossRef]
- Johansen, J. Pollen Diagrams From the Shetland and Faroe Islands. New Phytol. 1975, 75, 369–387. [Google Scholar] [CrossRef]
- Akobia, I.; Janiashvili, Z.; Metreveli, V.; Zazanashvili, N.; Batsatsashvili, K.; Ugrekhelidze, K. Modelling the Potential Distribution of Subalpine Birches (Betula Spp.) in the Caucasus. Community Ecol. 2022, 23, 209–218. [Google Scholar] [CrossRef]
- Bowler, D.E.; Callaghan, C.T.; Bhandari, N.; Henle, K.; Benjamin Barth, M.; Koppitz, C.; Klenke, R.; Winter, M.; Jansen, F.; Bruelheide, H.; et al. Temporal Trends in the Spatial Bias of Species Occurrence Records. Ecography 2022, 2022, e06219. [Google Scholar] [CrossRef]
- Gavashelishvili, A.; Tarkhnishvili, D. Biomes and Human Distribution during the Last Ice Age: Biomes and Humans during the Ice Age. Glob. Ecol. Biogeogr. 2016, 25, 563–574. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Abd_Allah, E.F.; Alqarawi, A.A.; Calixto, E.S.; Ali, N.; Ijaz, F.; Kausar, R.; Alsubeie, M.S.; et al. Role of Multivariate Approaches in Floristic Diversity of Manoor Valley (Himalayan Region), Pakistan. Appl. Ecol. Env. Res. 2019, 17, 1475–1498. [Google Scholar] [CrossRef]
- Urbisz, A.; Urbisz, A. European Chestnut (Castanea sativa Mill.)-a Tree Naturalized on the Baltic Sea Coast? Pol. J. Ecol. 2007, 55, 175–179. [Google Scholar]
- Howe, H.F.; Estabrook, G.F. On Intraspecific Competition for Avian Dispersers in Tropical Trees. Am. Nat. 1977, 111, 817–832. [Google Scholar] [CrossRef]
- Hengl, T.; De Jesus, J.M.; MacMillan, R.A.; Batjes, N.H.; Heuvelink, G.B.M.; Ribeiro, E.; Samuel-Rosa, A.; Kempen, B.; Leenaars, J.G.B.; Walsh, M.G.; et al. SoilGrids1km—Global Soil Information Based on Automated Mapping. PLoS ONE 2014, 9, e105992. [Google Scholar] [CrossRef]





| Coded Bioclimatic Variables | Bioclimatic Variables |
|---|---|
| BIO1 | Annual mean temperature |
| BIO2 | Mean diurnal range (mean of monthly (maximum temperature − inimum temperature)) |
| BIO3 | Isothermally (BIO2/BIO7) (×100) |
| BIO4 | Temperature seasonality (standard deviation ×100) |
| BIO5 | Maximum temperature of warmest month |
| BIO6 | Minimum temperature of coldest month |
| BIO7 | Temperature annual range (BIO5–BIO6) |
| BIO8 | Mean temperature of wettest quarter |
| BIO9 | Mean temperature of driest quarter |
| BIO10 | Mean temperature of warmest quarter |
| BIO11 | Mean temperature of coldest quarter |
| BIO12 | Annual precipitation |
| BIO13 | Precipitation of wettest month |
| BIO14 | Precipitation of driest month |
| BIO15 | Precipitation seasonality (coefficient of variation) |
| BIO16 | Precipitation of wettest quarter |
| BIO17 | Precipitation of driest quarter |
| BIO18 | Precipitation of warmest quarter |
| BIO19 | Precipitation of coldest quarter |
| ID | Climate Data | Models | Variables |
|---|---|---|---|
| 1 | CHELSA V2.1 | The model with all variables | All 19 BIO |
| 2 | CHELSA V2.1 | Mechanistic model | BIO_19, BIO_18, BIO_12, BIO_11, BIO_10 |
| 3 | CHELSA V2.1 | The correlative model with the best AUC performances | BIO_19, BIO_18, BIO_16, BIO_15, BIO_11, BIO_10, BIO_09, BIO_08, BIO_07, BIO_06, BIO_04, BIO_03, BIO_02, BIO_01 |
| 4 | Wordclim 2 V2.1 | The model with all variables | All 19 BIO |
| 5 | Wordclim 2 V2.1 | Mechanistic model | BIO_ 19, BIO_18, BIO_12, BIO_11, BIO_10 |
| 6 | Wordclim 2 V2.1 | The correlative model with the best AUC performances | BIO_19, BIO_18, BIO_17, BIO_15, BIO_13, BIO_09, BIO_08, BIO_06, BIO_04, BIO_03, BIO_02 |
| ID | Climate Data | Models | AUC | HEI |
|---|---|---|---|---|
| 1 | CHELSA V2.1 | The model with all variables | 0.9634 | −0.616084571 |
| 2 | CHELSA V2.1 | Mechanistic model | 0.9535 | 0.932336488 |
| 3 | CHELSA V2.1 | The correlative model with the best AUC performances | 0.9625 | −0.382977772 |
| 4 | Wordclim 2 V2.1 | The model with all variables | 0.9625 | 0.956727365 |
| 5 | Wordclim 2 V2.1 | Mechanistic model | 0.9559 | 0.969826464 |
| 6 | Wordclim 2 V2.1 | The correlative model with the best AUC performances | 0.9624 | 0.92322457 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metreveli, V.; Kreft, H.; Akobia, I.; Janiashvili, Z.; Nonashvili, Z.; Dzadzamia, L.; Javakhishvili, Z.; Gavashelishvili, A. Potential Distribution and Suitable Habitat for Chestnut (Castanea sativa). Forests 2023, 14, 2076. https://doi.org/10.3390/f14102076
Metreveli V, Kreft H, Akobia I, Janiashvili Z, Nonashvili Z, Dzadzamia L, Javakhishvili Z, Gavashelishvili A. Potential Distribution and Suitable Habitat for Chestnut (Castanea sativa). Forests. 2023; 14(10):2076. https://doi.org/10.3390/f14102076
Chicago/Turabian StyleMetreveli, Vasil, Holger Kreft, Ilia Akobia, Zurab Janiashvili, Zaza Nonashvili, Lasha Dzadzamia, Zurab Javakhishvili, and Alexander Gavashelishvili. 2023. "Potential Distribution and Suitable Habitat for Chestnut (Castanea sativa)" Forests 14, no. 10: 2076. https://doi.org/10.3390/f14102076





