The Effect of Elevation on the Population Structure, Spatial Patterning and Intraspecific Interactions of Picea schrenkiana in the Eastern Tianshan Mountains: A Test of the Stress Gradient Hypothesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
2.2.1. Point Pattern Analysis and Null Model (Completely Spatial Randomness, CSR)
2.2.2. Growth Rate and Correlation Analysis
3. Results
3.1. Population Age Structure and Growth
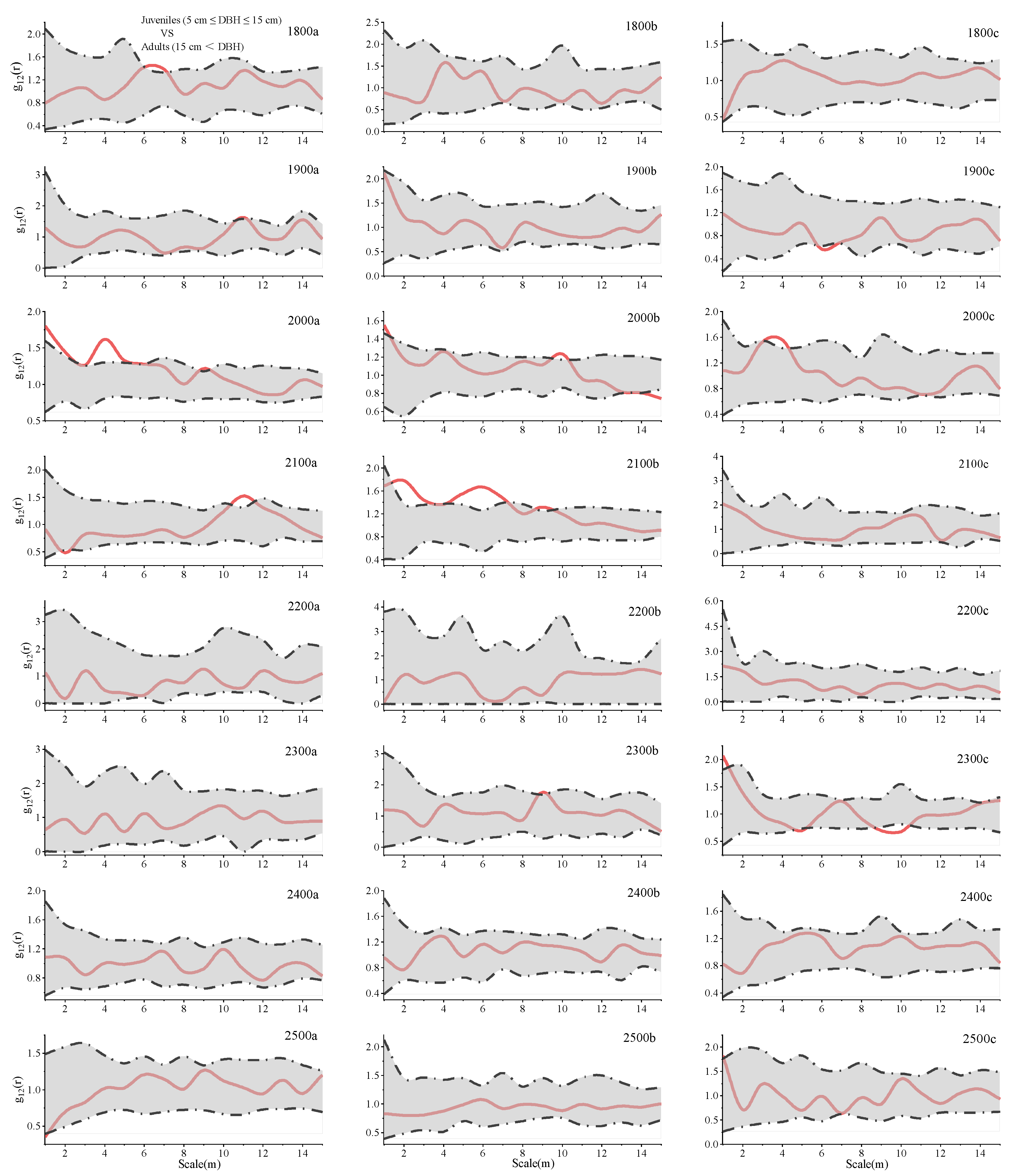
3.2. Spatial Patterns and Intraspecific Interaction
3.3. Correlation Analysis of Different Habitat Factors
4. Discussion
4.1. Population Age Structure and Growth
4.2. Spatial Distribution Pattern
4.3. Intraspecific Interactions and SGH
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmesan, C. Metapopulation ecology. Nature 1999, 399, 747. [Google Scholar] [CrossRef]
- Kubota, Y. Spatial pattern and regeneration dynamics in a temperate Abies-Tsuga forest in southwestern Japan. J. For. Res. 2006, 11, 191–201. [Google Scholar] [CrossRef]
- le Roux, P.C.; McGeoch, M.A. Spatial variation in plant interactions across a severity gradient in the sub-Antarctic. Oecologia 2008, 155, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Salas-Eljatib, C.; Riquelme-Alarcon, J.; Donoso, P.J.; Ponce, D.; Soto, D.P. Analysing changes in spatial point patterns: A proposal using data from a forest thinning experiment. For. Ecosyst. 2022, 9, 100081. [Google Scholar] [CrossRef]
- Aubin, I.; Messier, C.; Kneeshaw, D. Population structure and growth acclimation of mountain maple along a successional gradient in the southern boreal forest. Ecoscience 2005, 12, 540–548. [Google Scholar] [CrossRef]
- Johnson, J.B. Stand structure and vegetation dynamics of a subalpine wooded fen in Rocky Mountain National Park, Colorado. J. Veg. Sci. 1997, 8, 337–342. [Google Scholar] [CrossRef]
- Yusup, A.; Halik, U.; Abliz, A.; Aishan, T.; Keyimu, M.; Wei, J. Population Structure and Spatial Distribution Pattern of Populus euphratica Riparian Forest Under Environmental Heterogeneity Along the Tarim River, Northwest China. Front. Plant. Sci. 2022, 13, 844819. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, M.; Pampuch, T.; Heer, K.; Avanzi, C.; Wuerth, D.G.; Trouillier, M.; Bog, M.; Wilmking, M.; Schnittler, M. Population structure and the influence of microenvironment and genetic similarity on individual growth at Alaskan white spruce treelines. Sci. Total Environ. 2021, 798, 149267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Bai, Z.; Zhang, Z.; Guo, D.; Li, J.; Xu, Z.; Pan, Z. Population structure and spatial distributions patterns of 17 years old plantation in a reclaimed spoil of Pingshuo opencast mine, China. Ecol. Eng. 2012, 44, 147–151. [Google Scholar] [CrossRef]
- Bertness, M.D.; Callaway, R. Positive interactions in communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef]
- Getzin, S.; Wiegand, T.; Wiegand, K.; He, F. Heterogeneity influences spatial patterns and demographics in forest stands. J. Ecol. 2008, 96, 807–820. [Google Scholar] [CrossRef]
- Chaneton, E.J.; Noemi Mazía, C.; Kitzberger, T.J.J.o.E. Facilitation vs. apparent competition: Insect herbivory alters tree seedling recruitment under nurse shrubs in a steppe–woodland ecotone. J. Ecol. 2010, 98, 488–497. [Google Scholar] [CrossRef]
- Kothari, S.; Montgomery, R.A.; Cavender-Bares, J. Physiological responses to light explain competition and facilitation in a tree diversity experiment. J. Ecol. 2021, 109, 2000–2018. [Google Scholar] [CrossRef]
- Callaway, R.M.; Walker, L.R. Competition and facilitation: A synthetic approach to interactions in plant communities. Ecology 1997, 78, 1958–1965. [Google Scholar] [CrossRef]
- Choler, P.; Michalet, R.; Callaway, R.M. Facilitation and competition on gradients in alpine plant communities. Ecology 2001, 82, 3295–3308. [Google Scholar] [CrossRef]
- Maestre, F.T.; Cortina, J. Do positive interactions increase with abiotic stress? A test from a semi-arid steppe. Proc. Biol. Sci. 2004, 271, S331–S333. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Bertness, M.D. Extreme stresses, niches, and positive species interactions along stress gradients. Ecology 2014, 95, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Sun, T.; Xue, S.; Yang, W.; Shao, D.; Martinez-Lopez, J. Competitive ability, stress tolerance and plant interactions along stress gradients. Ecology 2018, 99, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Ziffer-Berger, J.; Weisberg, P.J.; Cablk, M.E.; Osem, Y. Spatial patterns provide support for the stress-gradient hypothesis over a range-wide aridity gradient. J. Arid Environ. 2014, 102, 27–33. [Google Scholar] [CrossRef]
- Malkinson, D.; Tielboerger, K. What does the stress-gradient hypothesis predict? Resolving the discrepancies. Oikos 2010, 119, 1546–1552. [Google Scholar] [CrossRef]
- Michalet, R.; Brooker, R.W.; Cavieres, L.A.; Kikvidze, Z.; Lortie, C.J.; Pugnaire, F.I.; Valiente-Banuet, A.; Callaway, R.M. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol. Lett. 2006, 9, 767–773. [Google Scholar] [CrossRef]
- Maestre, F.T.; Valladares, F.; Reynolds, J.F. The stress-gradient hypothesis does not fit all relationships between plant-plant interactions and abiotic stress: Further insights from arid environments. J. Ecol. 2006, 94, 17–22. [Google Scholar] [CrossRef]
- Malanson, G.P. Diversity differs among three variations of the stress gradients hypothesis in two representations of niche space. J. Theor. Biol. 2015, 384, 121–130. [Google Scholar] [CrossRef]
- Viejo, R.M.; Arenas, F.; Fernandez, C.; Gomez, M. Mechanisms of succession along the emersion gradient in intertidal rocky shore assemblages. Oikos 2008, 117, 376–389. [Google Scholar] [CrossRef]
- Pablo Lopez, R.; Squeo, F.A.; Armas, C.; Kelt, D.A.; Gutierrez, J.R. Enhanced facilitation at the extreme end of the aridity gradient in the Atacama Desert: A community-level approach. Ecology 2016, 97, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.; He, F. Spatial point-pattern analysis for detecting density-dependent competition in a boreal chronosequence of Alberta. For. Ecol. Manag. 2009, 259, 98–106. [Google Scholar] [CrossRef]
- Liu, P.; Wang, W.; Bai, Z.; Guo, Z.; Ren, W.; Huang, J.; Xu, Y.; Yao, J.; Ding, Y.; Zang, R. Competition and facilitation co-regulate the spatial patterns of boreal tree species in Kanas of Xinjiang, northwest China. For. Ecol. Manag. 2020, 467, 118167. [Google Scholar] [CrossRef]
- Martinez, I.; Wiegand, T.; Gonzalez-Taboada, F.; Ramon Obeso, J. Spatial associations among tree species in a temperate forest community in North-western Spain. For. Ecol. Manag. 2010, 260, 456–465. [Google Scholar] [CrossRef]
- Miyamoto, K.; Sato, T.; Arana Olivos, E.A.; Clostre Orellana, G.; Rohner Stornaiuolo, C.M. Variation in Tree Community Composition and Carbon Stock under Natural and Human Disturbances in Andean Forests, Peru. Forests 2018, 9, 390. [Google Scholar] [CrossRef]
- Kuennecke, B.H. Temperate Forest Biomes; Bloomsbury Publishing USA: New York, NY, USA, 2008. [Google Scholar]
- Xie, Y.; Yang, T.; Wang, X.; Chen, X.; Pang, S.; Hu, J.; Wang, A.; Chen, L.; Shen, Z. Applying a Portable Backpack Lidar to Measure and Locate Trees in a Nature Forest Plot: Accuracy and Error Analyses. Remote Sens. 2022, 14, 1806. [Google Scholar] [CrossRef]
- Wang, T.; Liang, Y.; Ren, H.; Yu, D.; Ni, J.; Ma, K. Age structure of Picea schrenkiana forest along an altitudinal gradient in the central Tianshan Mountains, northwestern China. For. Ecol. Manag. 2004, 196, 267–274. [Google Scholar] [CrossRef]
- Wiegand, T.; Moloney, K.A. Rings, circles, and null-models for point pattern analysis in ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Ripley, B.D. Modelling spatial patterns. J. R. Stat. Soc. Ser. B 1977, 39, 172–192. [Google Scholar] [CrossRef]
- Ramsay, P.M. Handbook of Spatial Point-Pattern Analysis in Ecology; Taylor & Francis: Abingdon, UK, 2015. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Y.; Guan, W.; Cao, X.; Li, Z.; Ding, J. Using unmanned aerial vehicles to quantify spatial patterns of dominant vegetation along an elevation gradient in the typical Gobi region in Xinjiang, Northwest China. Glob. Ecol. Conserv. 2021, 27, e01571. [Google Scholar] [CrossRef]
- Porte, A.; Trichet, P.; Bert, D.; Loustau, D. Allometric relationships for branch and tree woody biomass of Maritime pine (Pinus pinaster Aıt.). For. Ecol. Manag. 2002, 158, 71–83. [Google Scholar] [CrossRef]
- Mottet, M.-J.; Lambert, M.-C.; DeBlois, J. Natural regeneration of Norway spruce, an introduced species, in and around plantations in Quebec, Canada. For. Ecol. Manag. 2021, 498, 119–553. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Jiao, D.Y.; Tang, S.X.; Dao, X.S.; Lei, Y.B.; Cai, C.T. Leaf Photosynthesis, Growth, and Seed Chemicals of Sacha Inchi Plants Cultivated Along an Altitude Gradient. Crop Sci. 2012, 52, 1859–1867. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Zhao, S.; Dong, M.; Chen, H.Y.H.; Kang, X. Different responses of the radial growth of conifer species to increasing temperature along altitude gradient: Pinus tabulaeformis in the Helan Mountains (Northwestern China). Pol. J. Ecol. 2016, 64, 509–525. [Google Scholar] [CrossRef]
- Lieberman, D.; Lieberman, M.; Peralta, R.; Hartshorn, G.S. Tropical forest structure and composition on a large-scale altitudinal gradient in Costa Rica. J. Ecol. 1996, 84, 137–152. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, H.; Wu, X.; Hao, Q. Climate-driven speedup of alpine treeline forest growth in the Tianshan Mountains, Northwestern China. Glob. Change Biol. 2015, 21, 816–826. [Google Scholar] [CrossRef]
- Kang, D.; Guo, Y.; Ren, C.; Zhao, F.; Feng, Y.; Han, X.; Yang, G. Population structure and spatial pattern of main tree species in secondary Betula platyphylla forest in Ziwuling Mountains, China. Sci. Rep. 2014, 4, 6873. [Google Scholar] [CrossRef]
- Li, W.; Zhang, G.-F. Population structure and spatial pattern of the endemic and endangered subtropical tree Parrotia subaequalis (Hamamelidaceae). Flora 2015, 212, 10–18. [Google Scholar] [CrossRef]
- Calviño-Cancela, M.J.J.o.E. Spatial patterns of seed dispersal and seedling recruitment in Corema album (Empetraceae): The importance of unspecialized dispersers for regeneration. J. Ecol. 2002, 90, 775–784. [Google Scholar] [CrossRef]
- Chu, C.J.; Maestre, F.T.; Xiao, S.; Weiner, J.; Wang, Y.S.; Duan, Z.H.; Wang, G. Balance between facilitation and resource competition determines biomass–density relationships in plant populations. Ecol. Lett. 2008, 11, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Resource competition between plankton algae: An experimental and theoretical approach. Ecology 1977, 58, 338–348. [Google Scholar] [CrossRef]
- Thakur, S.; Dhyani, R.; Negi, V.S.; Patley, M.; Rawal, R.; Bhatt, I.; Yadava, A. Spatial forest vulnerability profile of major forest types in Indian Western Himalaya. For. Ecol. Manag. 2021, 497, 119527. [Google Scholar] [CrossRef]
- Raath-Kruger, M.J.; Schob, C.; McGeoch, M.A.; le Roux, P.C. Interspecific facilitation mediates the outcome of intraspecific interactions across an elevational gradient. Ecology 2021, 102, e03200. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Wang, Y.; Han, B.; Mi, X.; Chen, L.; Liang, Y.; Ma, K. Effects of functional phylogeny of light-response-related orthologous genes on seedling survival in a subtropical forest. For. Ecosyst. 2023, 10, 100087. [Google Scholar] [CrossRef]
- Muhamed, H.; Maalouf, J.-P.; Michalet, R. Summer drought and canopy opening increase the strength of the oak seedlings–shrub spatial association. Ann. For. Sci. 2013, 70, 345–355. [Google Scholar] [CrossRef]
- Zanini, L.; Ganade, G.; Hubel, I. Facilitation and competition influence succession in a subtropical old field. Plant Ecol. 2006, 185, 179–190. [Google Scholar] [CrossRef]
- Silvertown, J. Dorothy’s Dilemma and the unification of plant population biology. Trends Ecol. Evol. 1991, 6, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Nabel, M.R.; Newton, M.; Cole, E.C. Abundance of natural regeneration and growth comparisons with planted seedlings 10–13 years after commercial thinning in 50-year-old Douglas-fir, Douglas-fir/western hemlock, Oregon Coast Range. For. Ecol. Manag. 2013, 292, 96–110. [Google Scholar] [CrossRef]
- Omelko, A.; Ukhvatkina, O.; Zhmerenetsky, A.; Sibirina, L.; Petrenko, T.; Bobrovsky, M. From young to adult trees: How spatial patterns of plants with different life strategies change during age development in an old-growth Korean pine-broadleaved forest. For. Ecol. Manag. 2018, 411, 46–66. [Google Scholar] [CrossRef]
- Shen, Z.H.; Fang, J.Y.; Liu, Z.L.; Wu, J. Structure and dynamics of Abies fabri population near the alpine timberline in Hailuo clough of Gongga Mountain. Acta Bot. Sin. 2001, 43, 1288–1293. [Google Scholar] [CrossRef]
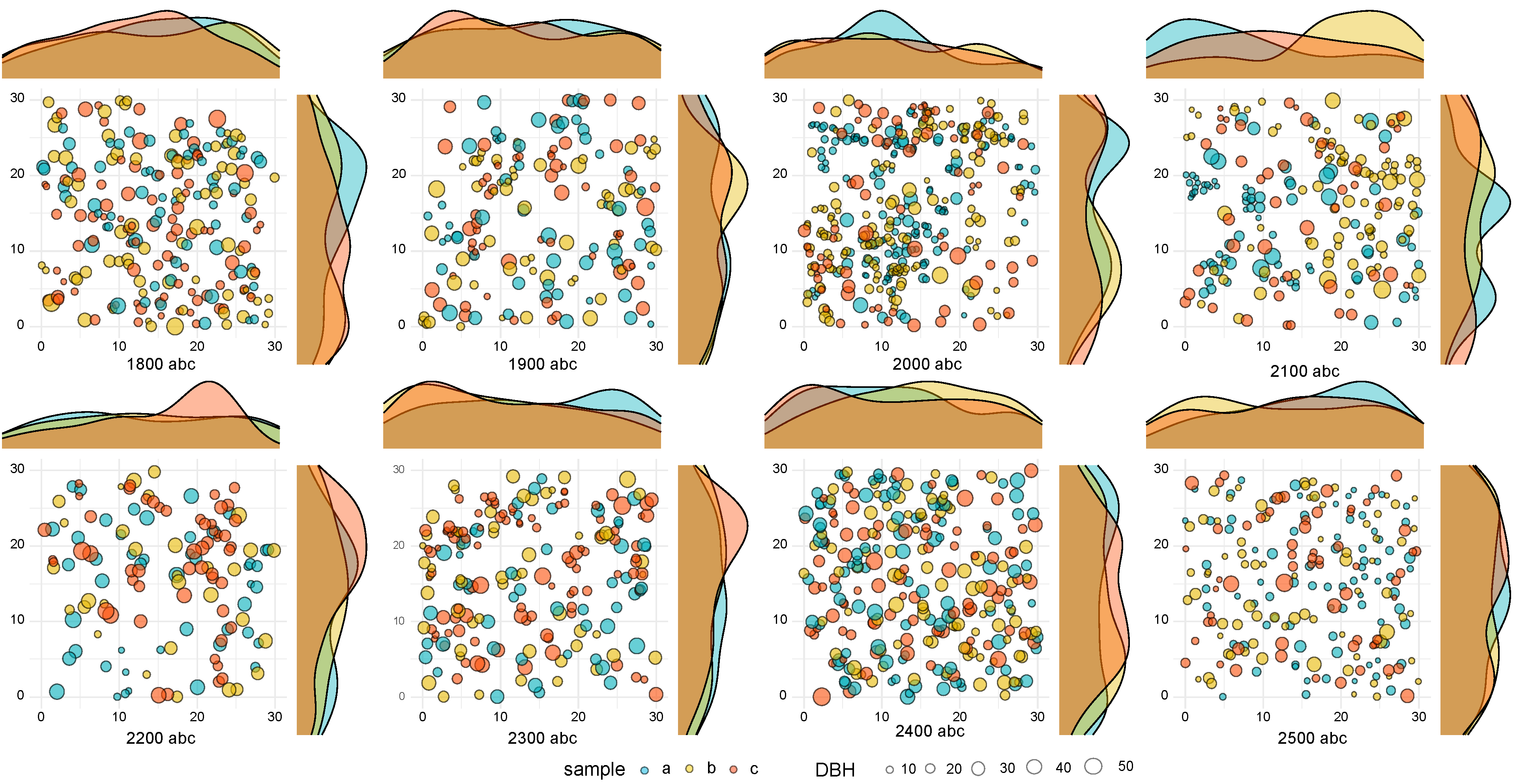



| Elevation (m) | Model | R2 | F | p | Average Growth Rate (cm/a) |
|---|---|---|---|---|---|
| 1800 | D = 0.473 A0.952 | 0.364 | 36.108 | 0.000 | 0.4047 |
| 1900 | D = 2.411 A0.587 | 0.341 | 29.989 | 0.000 | 0.4417 |
| 2000 | D = 0.797 A0.842 | 0.525 | 73.978 | 0.000 | 0.4333 |
| 2100 | D = 0.392 A1.05 | 0.689 | 137.269 | 0.000 | 0.4973 |
| 2200 | D = 0.328 A0.951 | 0.385 | 31.277 | 0.000 | 0.2707 |
| 2300 | D = 0.154 A1.082 | 0.513 | 59.996 | 0.000 | 0.2431 |
| 2400 | D = 10.293 A0.159 | 0.074 | 5.037 | 0.028 | 0.1707 |
| 2500 | D = 0.614 A0.756 | 0.563 | 81 | 0.000 | 0.2004 |
| 2600 | D = 0.961 A0.795 | 0.738 | 87.29 | 0.000 | 0.3991 |
| Juveniles | Adults | Juveniles | Adults | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scale | S | M | L | S | M | L | Plot | S | M | L | S | M | L |
| 1800a | C | C | R | R | R | R | 2200a | — | — | — | R | R | R |
| 1800b | C | C | R | R | R | R | 2200b | — | — | — | R | R | R |
| 1800c | R | R | R | R | R | R | 2200c | — | — | — | C | R | R |
| 1900a | R | R | R | R | R | R | 2300a | — | — | — | R | R | R |
| 1900b | C | R | R | C | R | R | 2300b | — | — | — | R | R | R |
| 1900c | C | R | R | R | R | R | 2300c | C | C | C | R | R | R |
| 2000a | C | C | R | R | R | R | 2400a | C | R | R | R | R | R |
| 2000b | C | C | R | C | R | R | 2400b | R | C | R | R | R | R |
| 2000c | C | C | R | R | R | R | 2400c | R | R | R | R | C | R |
| 2100a | C | C | C | R | R | R | 2500a | R | C | R | R | R | R |
| 2100b | C | C | C | R | R | C | 2500b | R | R | R | R | R | R |
| 2100c | — | — | — | R | R | R | 2500c | R | R | R | R | R | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Ning, C.; Zhang, W.; Halik, Ü.; Shen, Z. The Effect of Elevation on the Population Structure, Spatial Patterning and Intraspecific Interactions of Picea schrenkiana in the Eastern Tianshan Mountains: A Test of the Stress Gradient Hypothesis. Forests 2023, 14, 2092. https://doi.org/10.3390/f14102092
He J, Ning C, Zhang W, Halik Ü, Shen Z. The Effect of Elevation on the Population Structure, Spatial Patterning and Intraspecific Interactions of Picea schrenkiana in the Eastern Tianshan Mountains: A Test of the Stress Gradient Hypothesis. Forests. 2023; 14(10):2092. https://doi.org/10.3390/f14102092
Chicago/Turabian StyleHe, Jianing, Caiwen Ning, Wentao Zhang, Ümüt Halik, and Zehao Shen. 2023. "The Effect of Elevation on the Population Structure, Spatial Patterning and Intraspecific Interactions of Picea schrenkiana in the Eastern Tianshan Mountains: A Test of the Stress Gradient Hypothesis" Forests 14, no. 10: 2092. https://doi.org/10.3390/f14102092






