Extraction of Soluble Salts and Iron Sulfides from the Wood of the “Huaguangjiao I” Shipwreck
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction Procedure
2.3. Monitoring Methods for Extraction Process
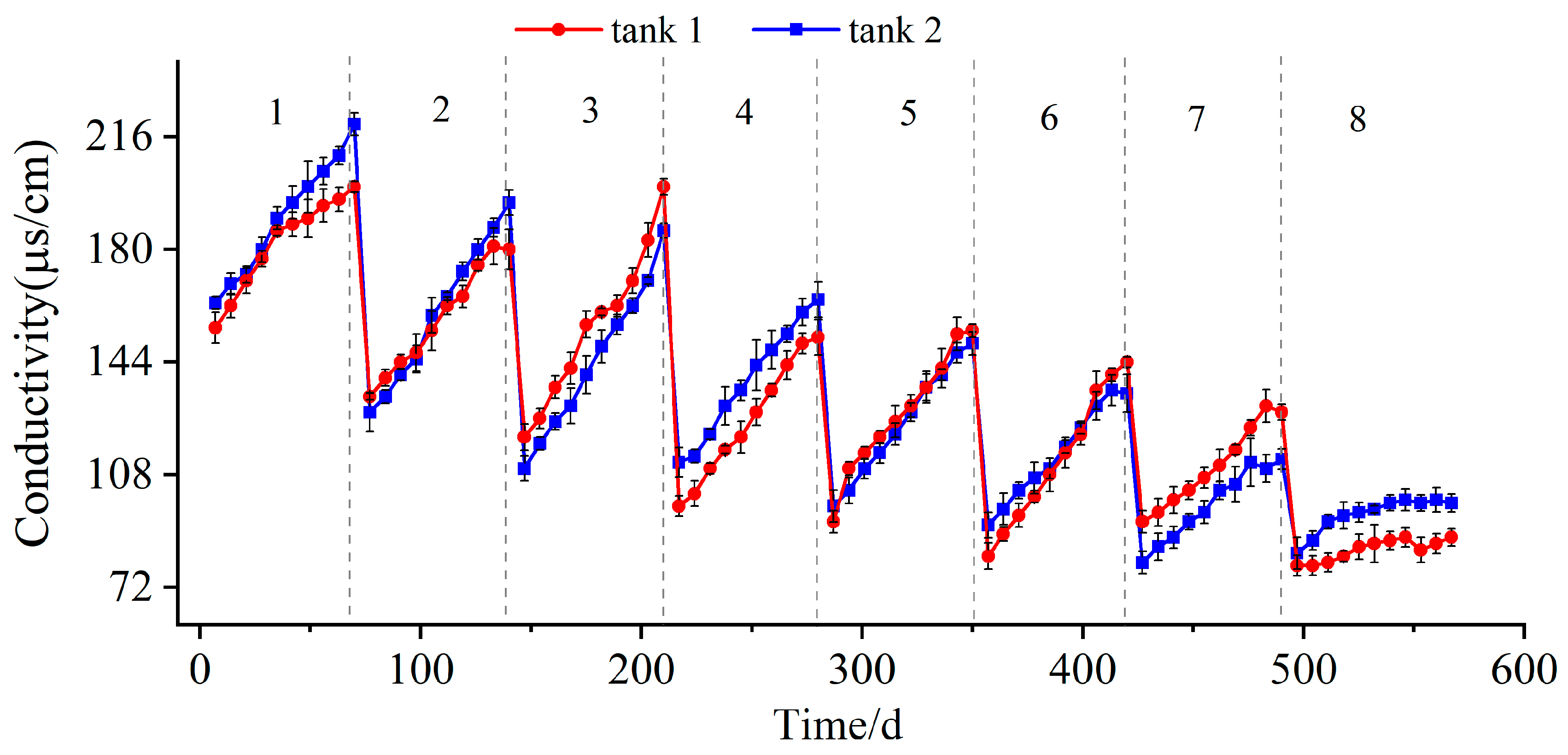
2.3.1. Electrical Conductivity of the Solution
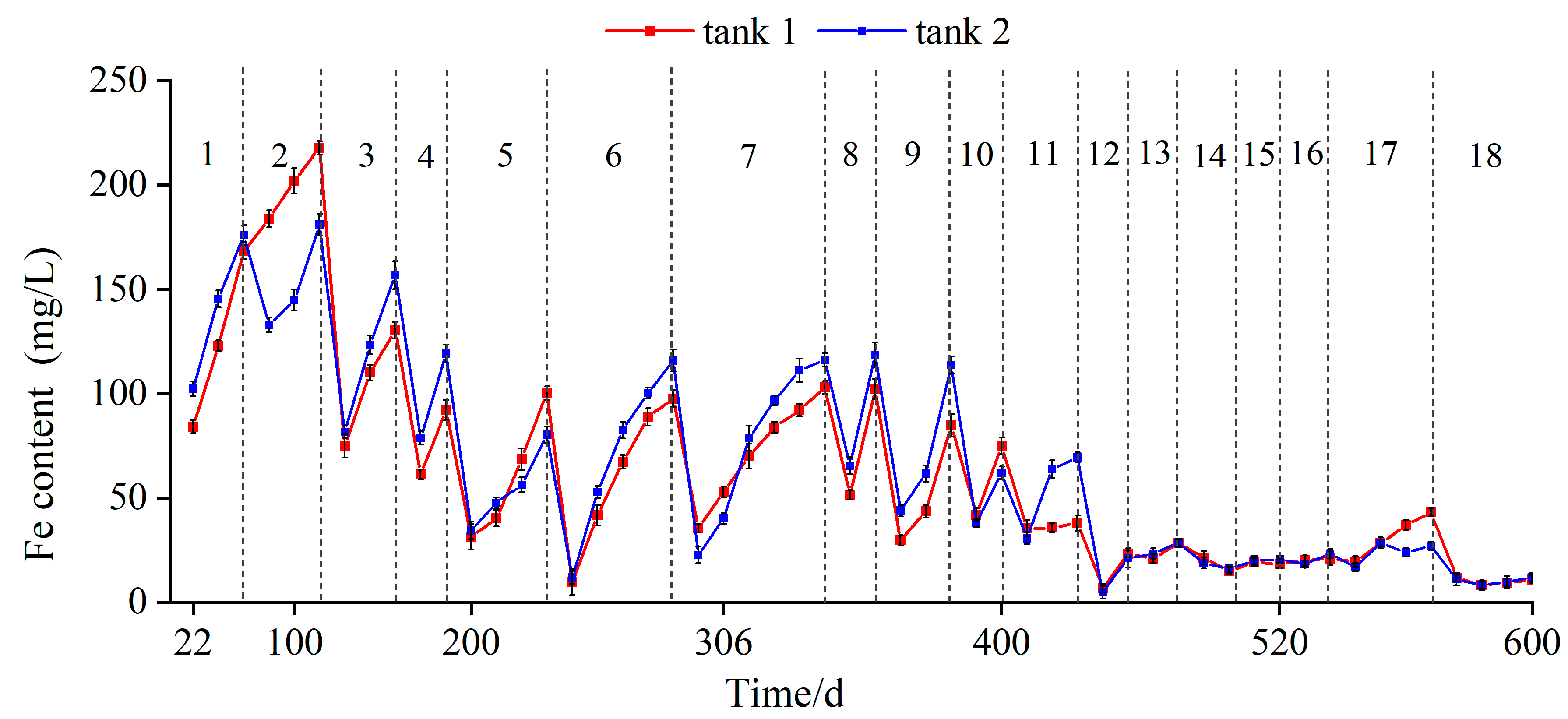
2.3.2. Iron Content of the Solution
2.4. Evaluation Methods for Extraction Effect
2.4.1. Soluble Salt Ions in Wood
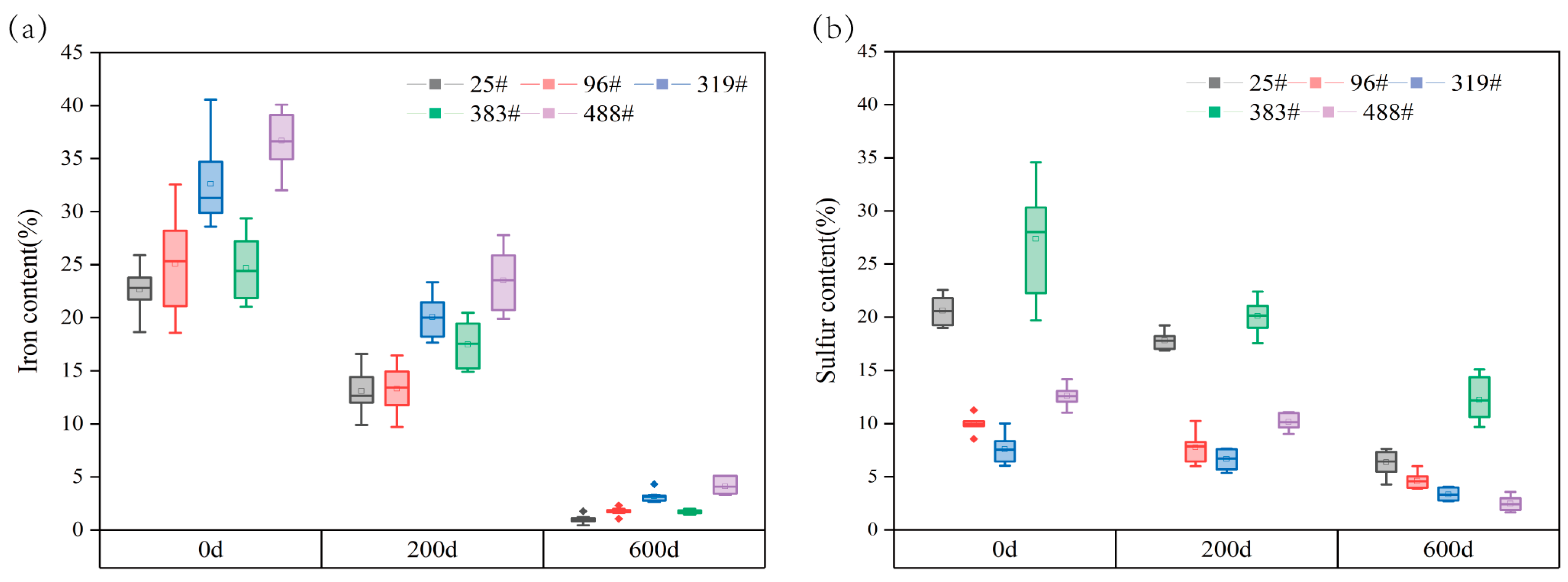
2.4.2. Sulfur and Iron Contents in Wood
2.4.3. Wood Color
2.4.4. Wood Composition
3. Results and Discussion
3.1. Extraction of Soluble Salts
3.2. Extraction of Iron Sulfides
3.3. Effects of Desalination on the Wood
3.3.1. Soluble Salt Content
3.3.2. Iron and Sulfur Contents
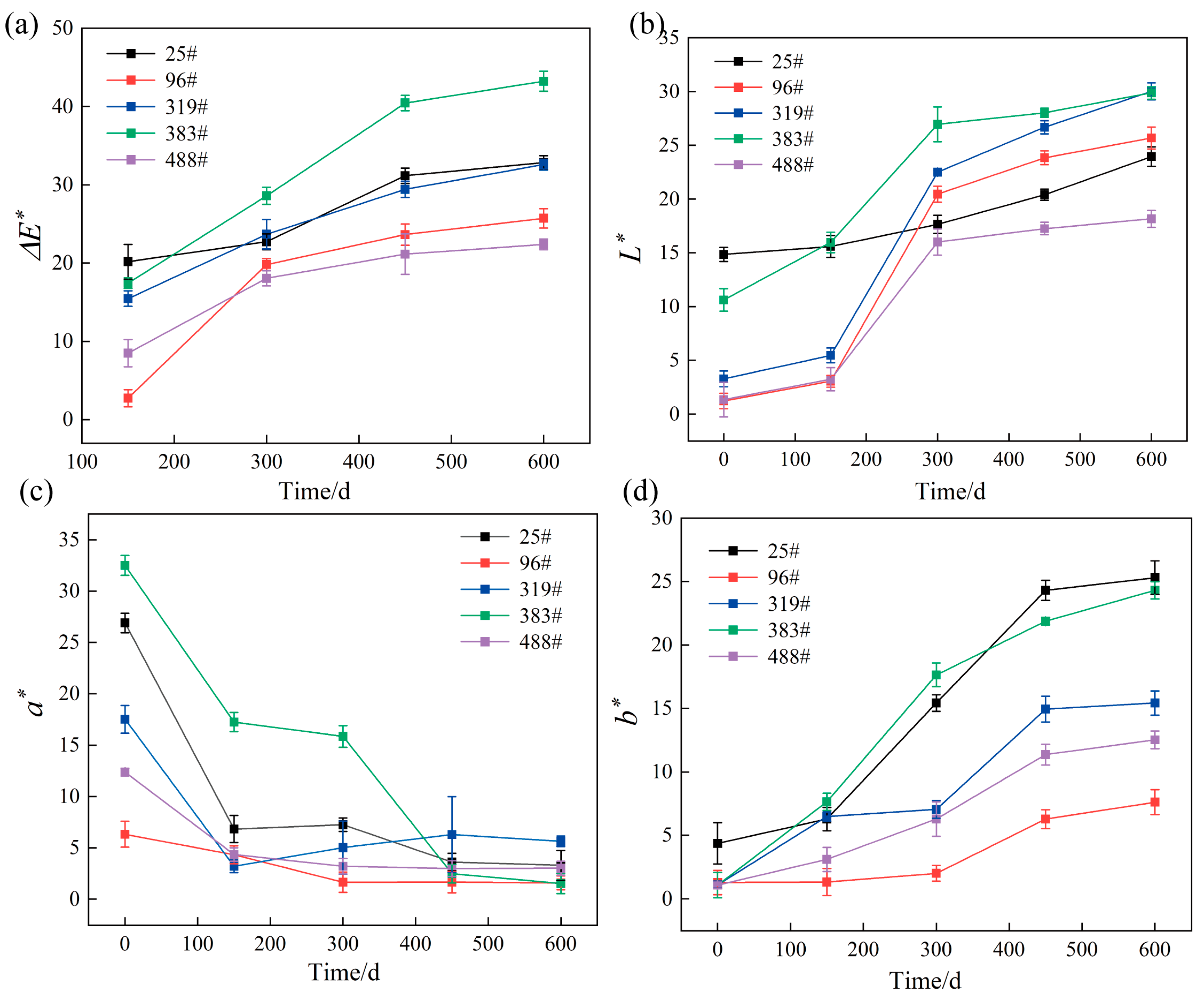
3.3.3. Wood Color
3.3.4. Chemical Composition
4. Conclusions
- (1)
- The extraction process can be monitored using the electric conductivity and iron content of the soaking solution. When these two values are stable in a lower range and do not obviously change with time, the extraction process is considered to be finished. In this study, at the end of extraction, the electrical conductivity of the solution stabilized at 80~100 μs/cm, and the iron content ranged from 8 to 15 mg/L.
- (2)
- The extraction effect of shipwreck wood can be evaluated by observing the change of soluble salt ion content, iron content, color, and composition of the wood before and after extraction. In this study, after the extraction treatment, the various kinds of soluble salt ion content in the shipwreck wood were all below 5 μg/g, the iron content was below 5%, and the wood returned to its original color with a certain degree of degradation.
- (3)
- The two-step hydrostatic immersion method using deionized water and EDTA-2Na as the extraction solution has a good effect on removing soluble salts and iron sulfides from large-scale shipwreck wood. But this method requires a long time, and EDTA-2Na will cause a certain degree of degradation of wood. It is recommended to seek safer and more effective desalination methods in the future.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, D.; Li, N.; Fu, Y.; Macchioni, N.; Sozzi, L.; Tian, X.; Liu, J. Study on wood preservation state of Chinese ancient shipwreck Huaguangjiao I. J. Cult. Herit. 2018, 32, 53–59. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, H.; Huang, X. Preliminary Analysis of Crystallization of Na2SO4 Solution in Silicate Cultural Relics. Stud. Conserv. 2019, 65, 321–332. [Google Scholar] [CrossRef]
- Almkvist, G.; Persson, I. Extraction of iron compounds from wood from the Vasa. Holzforschung 2006, 60, 678–684. [Google Scholar] [CrossRef]
- Hocker, E. From the Micro- to the Macro-: Managing the Conservation of the Warship, Vasa. Macromol. Symp. 2010, 238, 16–21. [Google Scholar] [CrossRef]
- Sandström, M.; Jalilehvand, F.; Damian, E.; Fors, Y.; Gelius, U.; Jones, M.; Salomé, M. Sulfur accumulation in the timbers of King Henry VIII’s warship Mary Rose: A pathway in the sulfur cycle of conservation concern. Proc. Natl. Acad. Sci. USA 2005, 102, 14165–14170. [Google Scholar] [CrossRef] [PubMed]
- Fors, Y.; Jalilehvand, F.; Risberg, E.D.; Björdal, C.; Phillips, E.; Sandström, M. Sulfur and iron analyses of marine archaeological wood in shipwrecks from the Baltic Sea and Scandinavian waters. J. Archaeol. Sci. 2012, 39, 2521–2532. [Google Scholar] [CrossRef]
- Fors, Y.; Sandström, M. Sulfur and iron in shipwrecks cause conservation concerns. Chem. Soc. Rev. 2006, 35, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Almkvist, G.; Persson, I. Distribution of iron and sulfur and their speciation in relation to degradation processes in wood from the Swedish warship Vasa. New J. Chem. 2011, 35, 1491–1502. [Google Scholar] [CrossRef]
- Gunnar, A.; Dal, L.; Persson, I. Extraction of iron compounds. In Proceedings of the 9th ICOM Group on Wet Organic Archaeological Materials Conference, Copenhagen, Denmark, 7–11 June 2004; pp. 203–212. [Google Scholar]
- Sandstro, M.; Fors, Y.; Jalilehvand, F.; Damian, E.; Gelius, U. Analyses of Sulfur and Iron in Marine Archaeological Wood. In Proceedings of the 9th ICOM Group Wet Organic Archaeological Materials Conference, Copenhagen, Denmark, 7–11 June 2004; pp. 181–202. [Google Scholar]
- Chelazzi, D.; Giorgi, R.; Baglioni, P. Nanotechnology for Vasa Wood De-Acidification. Macromol. Symp. 2006, 238, 30–36. [Google Scholar] [CrossRef]
- Schofield, E.J.; Sarangi, R.; Mehta, A.; Jones, A.M.; Mosselmans, F.J.; Chadwick, A.V. Nanoparticle de-acidification of the Mary Rose. Mater. Today 2011, 14, 354–358. [Google Scholar] [CrossRef]
- Longo, S.; Fazio, E.; Pizzo, B.; Riminesi, C. Real-time detection of salts content in waterlogged archaeological wood by evanescent field dielectrometry (EFD): Preliminary results. J. Phys. Conf. Ser. 2021, 2204, 012058. [Google Scholar] [CrossRef]
- Berenguer, M.A.; Monachon, M.; Jacquet, C.; Junier, P.; Rémazeilles, C.; Schofield, E.J.; Joseph, E. Biological oxidation of sulfur compounds in artificially degraded wood. Int. Biodeterior. Biodegrad. 2019, 141, 62–70. [Google Scholar] [CrossRef]
- Pelé, C.; Guilminot, E.; Labroche, S.; Lemoine, G.; Baron, G. Iron removal from waterlogged wood: Extraction by electrophoresis and chemical treatments. Stud. Conserv. 2015, 60, 155–171. [Google Scholar] [CrossRef]
- Berko, A.; Smith, A.D.; Jones, A.M.; Schofield, E.J.; Mosselmans, J.F.W.; Chadwick, A.V. XAS studies of the effectiveness of iron chelating treatments of Mary Rose timbers. J. Phys. Conf. Ser. 2009, 190, 12147. [Google Scholar] [CrossRef]
- Pecoraro, E.; Pelé-Meziani, C.; Macchioni, N.; Lemoine, G.; Guilminot, E.; Shen, D.; Pizzo, B. The removal of iron from waterlogged archaeological wood: Efficacy and effects on the room temperature wood properties. Wood Mater. Sci. Eng. 2023, 18, 672–689. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Li, N.S.; Tian, X.L.; Liu, J.; Shen, D.W. Research on the removal of the iron sulfides in the Qing Dynasty marine shipwreck, Ningbo Xiaobaijiao No. 1. Sci. Conserv. Archaeol. 2014, 26, 30–38. [Google Scholar] [CrossRef]
- Luo, M.R.; Lo, M.C.; Kuo, W.G. The LLAB (l:c) colour model. Color Res. Appl. 1996, 21, 412–429. [Google Scholar] [CrossRef]
- Dzurenda, L. Natural Variability of the Color of Beech Wood in the Color Space CIE L*a*b*. Forests 2023, 14, 1103. [Google Scholar] [CrossRef]
- GB/T 2677.3-93; Fibrous Raw Material-Determination of Ash. Standardization Administration of China: Beijing, China, 1993.
- GB/T 2677.10-1995; Fibrous Raw Material-Determination of Hollocellulose. Standardization Administration of China: Beijing, China, 1996.
- GB/T 2677.8-94; Fibrous Raw Material-Determination of Lignin. Standardization Administration of China: Beijing, China, 1995.
- Li, R.; Guo, J.; Macchioni, N.; Pizzo, B.; Xi, G.; Tian, X.; Chen, J.; Sun, J.; Jiang, X.; Cao, J.; et al. Characterisation of waterlogged archaeological wood from Nanhai No. 1 shipwreck by multidisciplinary diagnostic methods. J. Cult. Herit. 2022, 56, 25–35. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Tran, K.; Guilminot, E.; Conforto, E.; Refait, P. Study of Fe(II) sulphides in waterlogged archaeological wood. Stud. Conserv. 2013, 58, 297–307. [Google Scholar] [CrossRef]
- Lin, H.F.; Liu, J.B.; Yan, M.; Bai, Y.; Liu, B. Molecular dynamics simulation on diffusion rules of CO2/CH4 in coal reservior. J. Saf. Technol. 2017, 1, 6. [Google Scholar] [CrossRef]
- Romualdas, M.; Andrejus, J.; Donatas, L. Research on the electrical capacitance and electrical conductivity of char resulting from natural and treated wood. J. Civ. Eng. Manag. 2015, 21, 11–20. [Google Scholar] [CrossRef]
- Simatupang, M.H.; Lange, H.; Kasim, A. Semimicro Electrical Conductivity Method to Trace the Hydration of β-Gypsum Hemihydrate in the Presence of Wood Extractives of some Tropical Wood Species. Holzforschung 1992, 46, 357–359. [Google Scholar] [CrossRef]
- Fors, Y.; Nilsson, T.; Risberg, E.D.; Sandström, M.; Torssander, P. Sulfur accumulation in pinewood (Pinus sylvestris) induced by bacteria in a simulated seabed environment: Implications for marine archaeological wood and fossil fuels. Int. Biodeterior. Biodegrad. 2008, 62, 336–347. [Google Scholar] [CrossRef]
- Bytner, O.; Drożdżek, M.; Laskowska, A.; Zawadzki, J. Temperature, Time, and Interactions between Them in Relation to Colour Parameters of Black Poplar (Populus nigra L.) Thermally Modified in Nitrogen Atmosphere. Materials 2022, 15, 824. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.M.; Hale, M.D. Colour in thermally modified wood of beech, Norway spruce and Scots pine. Part 2: Property predictions from colour changes. Holzforschung 2009, 63, 394–401. [Google Scholar] [CrossRef]
- Cogulet, A.; Blanchet, P.; Landry, V. Wood degradation under UV irradiation: A lignin characterization. J. Photochem. Photobiol. B Biol. 2016, 158, 184–191. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, C.; Yao, Y.; He, S.; Wang, X.; Li, J.; Gao, J.; Gan, W.; Jiang, B.; Cui, M.; et al. In Situ Lignin Modification toward Photonic Wood. Adv. Mater. 2021, 33, 2001588. [Google Scholar] [CrossRef]
- Chen, Y.; Tshabalala, M.A.; Gao, J.; Stark, N.M.; Fan, Y. Color and Surface Chemistry Changes of Pine Wood Flour after Extraction and Delignification. BioResources 2014, 9, 2937–2948. [Google Scholar] [CrossRef]
- Preklet, E.; Tolvaj, L.; Banadics, E.A.; Alpar, T.; Varga, D. Colour modification and homogenisation of larch wood by steaming. Wood Res. 2019, 64, 811–820. [Google Scholar]
- Fors, Y.; Grudd, H.; Rindby, A.; Jalilehvand, F.; Sandström, M.; Cato, I.; Bornmalm, L. Sulfur and iron accumulation in three marine-archaeological shipwrecks in the Baltic Sea: The Ghost, the Crown and the Sword. Sci. Rep. 2014, 4, 4222. [Google Scholar] [CrossRef] [PubMed]
- Krutul, D.; Radomski, A.; Zawadzki, J. Comparison of the Chemical Composition of the Fossil and Recent Oak Wood. Wood Res. 2010, 55, 113–120. Available online: http://www.woodresearch.sk/articles/5-17-143435_WR201003_11krutul.pdf (accessed on 1 November 2023).
- Shmulsky, R.; Jones, P.D. Forest Products and Wood Science an Introduction; Wiley Blackwell: Hoboken, NJ, USA, 2019; ISBN 9781119426431. [Google Scholar]
- Zoia, L.; Salanti, A.; Orlandi, M. Chemical characterization of archaeological wood: Softwood Vasa and hardwood Riksapplet case studies. J. Cult. Herit. 2015, 16, 428–437. [Google Scholar] [CrossRef]
- Liu, X.; Tu, X.; Ma, W.; Zhang, C.; Huang, H.; Varodi, A.M. Consolidation and Dehydration of Waterlogged Archaeological Wood from Site Huaguangjiao No.1. Forests 2022, 13, 1919. [Google Scholar] [CrossRef]

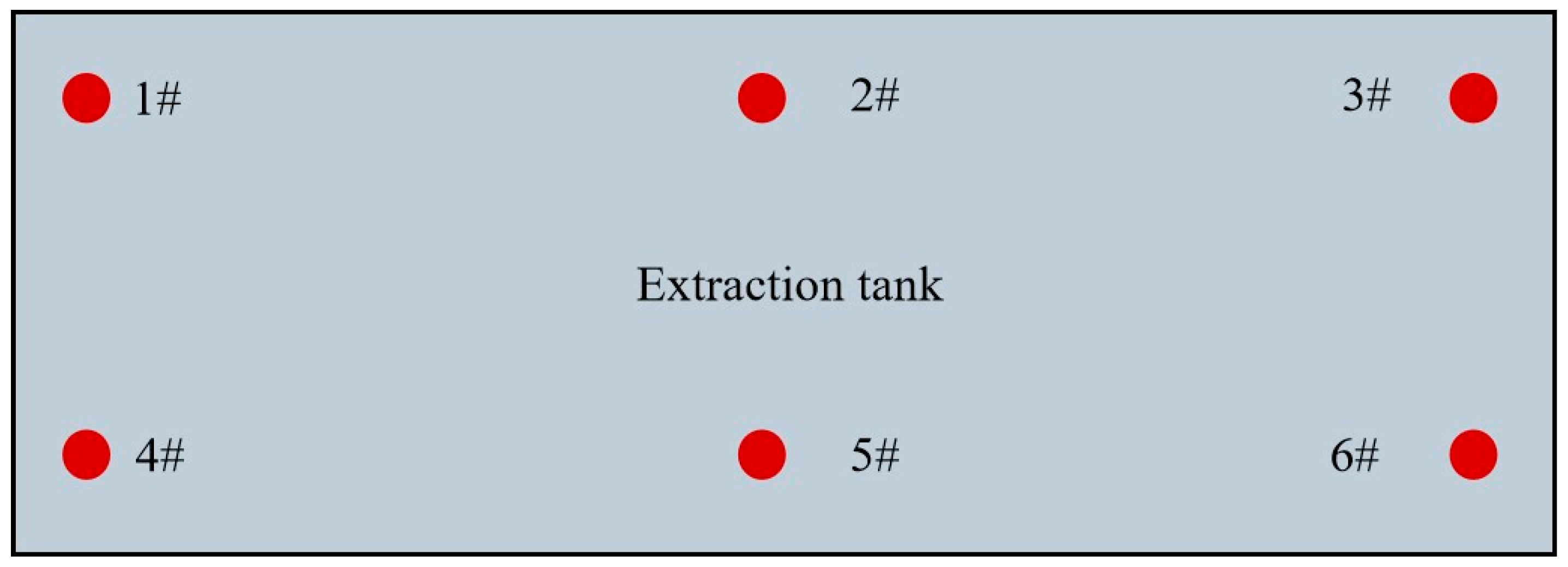
| Samples | Extraction Tank | Shelf Layer | Sample Size (mm) | Wood Species | Sampling Position |
|---|---|---|---|---|---|
| 25# | No. 1 | 2 | 1870 × 65 × 300 | Pinus massoniana Lamb. | Northeast of the hull |
| 96# | No. 1 | 1 | 620 × 350 × 50 | Pinus massoniana Lamb. | The position is not sure |
| 319# | No. 2 | 2 | 4590 × 110 × 45 | Pinus massoniana Lamb. | The third layer of the hull |
| 383# | No. 2 | 1 | 690 × 290 × 35 | Pinus massoniana Lamb. | The fourth layer of the hull |
| 488# | No. 2 | 2 | 2380 × 245 × 45 | Pinus massoniana Lamb. | The fifth layer of the hull |
| Samples | Time | Ca2+ | SO42− | Na+ | Mg2+ | Cl− | K+ |
|---|---|---|---|---|---|---|---|
| 25# | 0 d | 12.59 1 (4.77) | 24.65 (6.30) | 47.33 (2.48) | 1.00 (10.35) | 1.62 (7.68) | 3.91 (3.56) |
| 200 d | 10.65 (7.58) | 6.92 (5.92) | 30.46 (3.05) | 0.83 (8.62) | 1.48 (9.66) | 1.81 (9.56) | |
| 520 d | 0.54 (7.65) | 0.98 (10.62) | 13.70 (4.62) | 0.21 (7.66) | 0.33 (5.68) | 0.09 (11.08) | |
| 96# | 0 d | 20.58 (3.51) | 32.2 (5.03) | 28.95 (5.34) | 2.09 (5.89) | 2.54 (5.90) | 4.05 (3.29) |
| 200 d | 15.25 (9.21) | 19.65 (5.98) | 20.46 (11.28) | 1.65 (6.45) | 1.85 (10.95) | 2.59 (3.99) | |
| 520 d | 1.65 (6.98) | 0.12 (6.59) | 4.42 (8.23) | 0.11 (5.46) | 0.28 (5.68) | 0.04 (10.58) | |
| 319# | 0 d | 35.66 (8.21) | 25.12 (2.34) | 19.58 (6.56) | 2.85 (9.64) | 1.49 (13.24) | 2.89 (3.56) |
| 200 d | 25.16 (9.60) | 20.01 (4.62) | 13.65 (3.95) | 2.09 (8.25) | 1.03 (8.36) | 1.69 (6.29) | |
| 520 d | 1.28 (5.88) | 1.67 (4.88) | 1.93 (5.44) | 0.05 (11.20) | 0.28 (9.24) | 0.07 (5.89) | |
| 383# | 0 d | 18.95 (4.65) | 19.28 (6.50) | 40.12 (2.38) | 0.98 (8.03) | 3.02 (13.25) | 5.02 (10.28) |
| 200 d | 10.36 (5.02) | 11.28 (5.02) | 25.39 (7.62) | 0.54 (5.98) | 2.18 (15.68) | 3.59 (9.35) | |
| 520 d | 1.64 (9.06) | 0.88 (9.16) | 0.50 (8.06) | 0.08 (9.68) | 0.29 (3.28) | 0.10 (8.66) | |
| 488# | 0 d | 21.08 (7.06) | 32.05 (3.19) | 21.08 (7.30) | 1.06 (10.28) | 2.18 (12.62) | 3.50 (8.63) |
| 200 d | 16.81 (4.67) | 25.28 (4.92) | 16.37 (4.35) | 0.78 (6.89) | 1.85 (11.35) | 2.56 (11.25) | |
| 520 d | 2.01 (3.59) | 1.59 (7.77) | 1.92 (9.21) | 0.05 (9.76) | 0.31 (6.87) | 0.07 (13.64) |
| 25# | 96# | 319# | 383# | 488# | |
|---|---|---|---|---|---|
| Iron | 99.15 1 (2.36) | 99.32 (2.02) | 99.03 (1.84) | 98.91 (1.38) | 99.16 (2.01) |
| Sulfur | 68.66 (8.77) | 52.39 (13.51) | 53.05 (10.19) | 55.55 (7.24) | 79.94 (8.35) |
| Samples | Extraction Status | Holocellulose | Lignin | Ash |
|---|---|---|---|---|
| 25# | before | 52.56 1 (5.67) | 43.42 (10.24) | 55.33 (7.62) |
| after | 36.43 (6.42) | 64.66 (8.99) | 2.11 (3.15) | |
| 96# | before | 49.50 (7.21) | 61.72 (5.68) | 39.44 (6.84) |
| after | 35.36 (6.88) | 53.53 (4.38) | 2.92 (2.45) | |
| 319# | before | 41.95 (8.25) | 49.02 (9.68) | 49.21 (4.77) |
| after | 27.77 (6.11) | 49.09 (6.44) | 7.28 (4.28) | |
| 383# | before | 50.44 (4.28) | 47.83 (5.97) | 53.09 (3.65) |
| after | 38.03 (9.24) | 57.53 (8.23) | 6.01 (6.68) | |
| 488# | before | 48.62 (7.16) | 43.18 (4.59) | 46.67 (9.55) |
| after | 33.07 (5.42) | 41.85 (6.48) | 4.39 (5.34) | |
| Vasa [39] | before | 70.5 | 26.6 | 2.9 |
| Luoyang I [40] | before | 32–38 | 53–57 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, N. Extraction of Soluble Salts and Iron Sulfides from the Wood of the “Huaguangjiao I” Shipwreck. Forests 2023, 14, 2432. https://doi.org/10.3390/f14122432
Wang X, Li N. Extraction of Soluble Salts and Iron Sulfides from the Wood of the “Huaguangjiao I” Shipwreck. Forests. 2023; 14(12):2432. https://doi.org/10.3390/f14122432
Chicago/Turabian StyleWang, Xueyu, and Naisheng Li. 2023. "Extraction of Soluble Salts and Iron Sulfides from the Wood of the “Huaguangjiao I” Shipwreck" Forests 14, no. 12: 2432. https://doi.org/10.3390/f14122432





