Arbuscular Mycorrhizal Fungi Adjusts Root Architecture to Promote Leaf Nitrogen Accumulation and Reduce Leaf Carbon–Nitrogen Ratio of Mulberry Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Experimental Design
2.2.2. Sampling and Analysis
2.2.3. Statistical Analysis
3. Results
3.1. Effects of Mycorrhization on the Growth and Development of Mulberry Seedlings
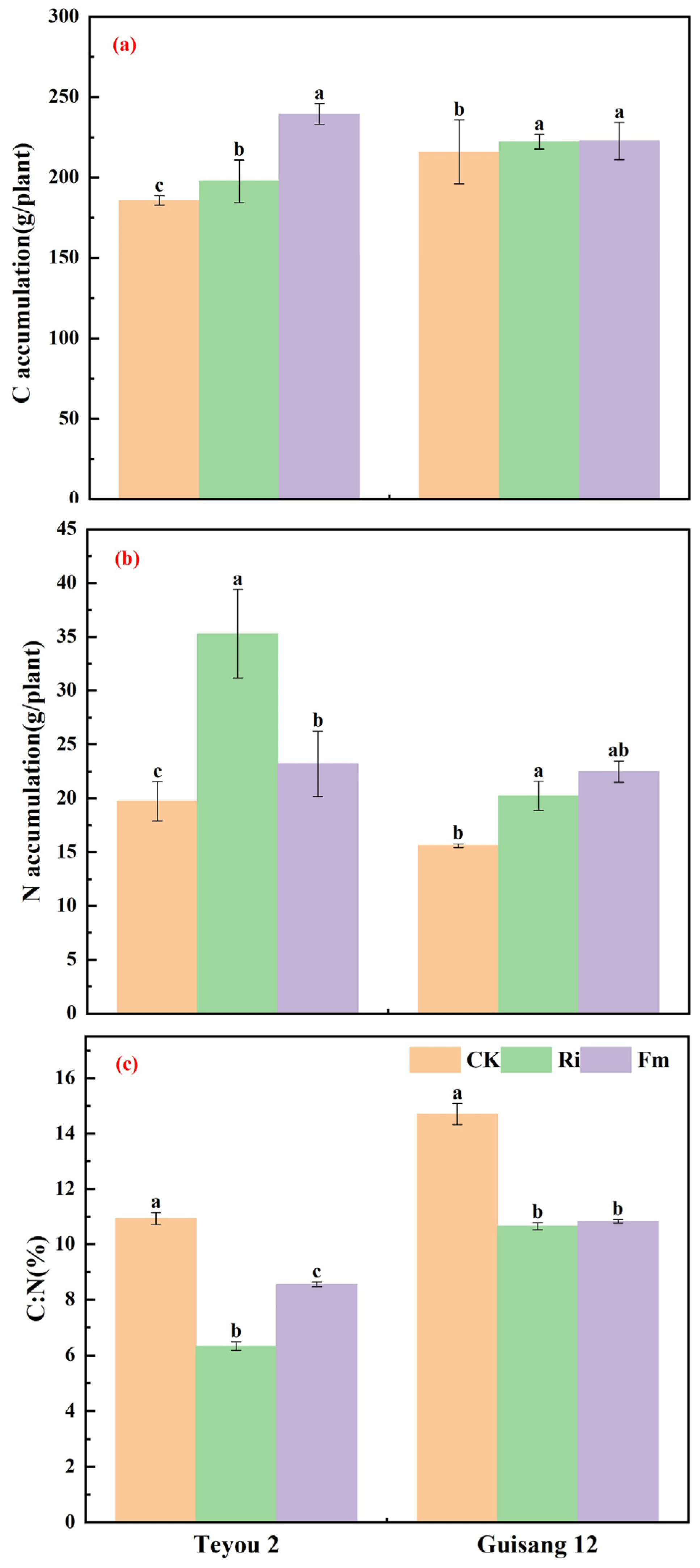
3.2. Changes in Carbon and Nitrogen Accumulation and Stoichiometric Ratio of Mycorrhized Mulberry Seedling Leaves
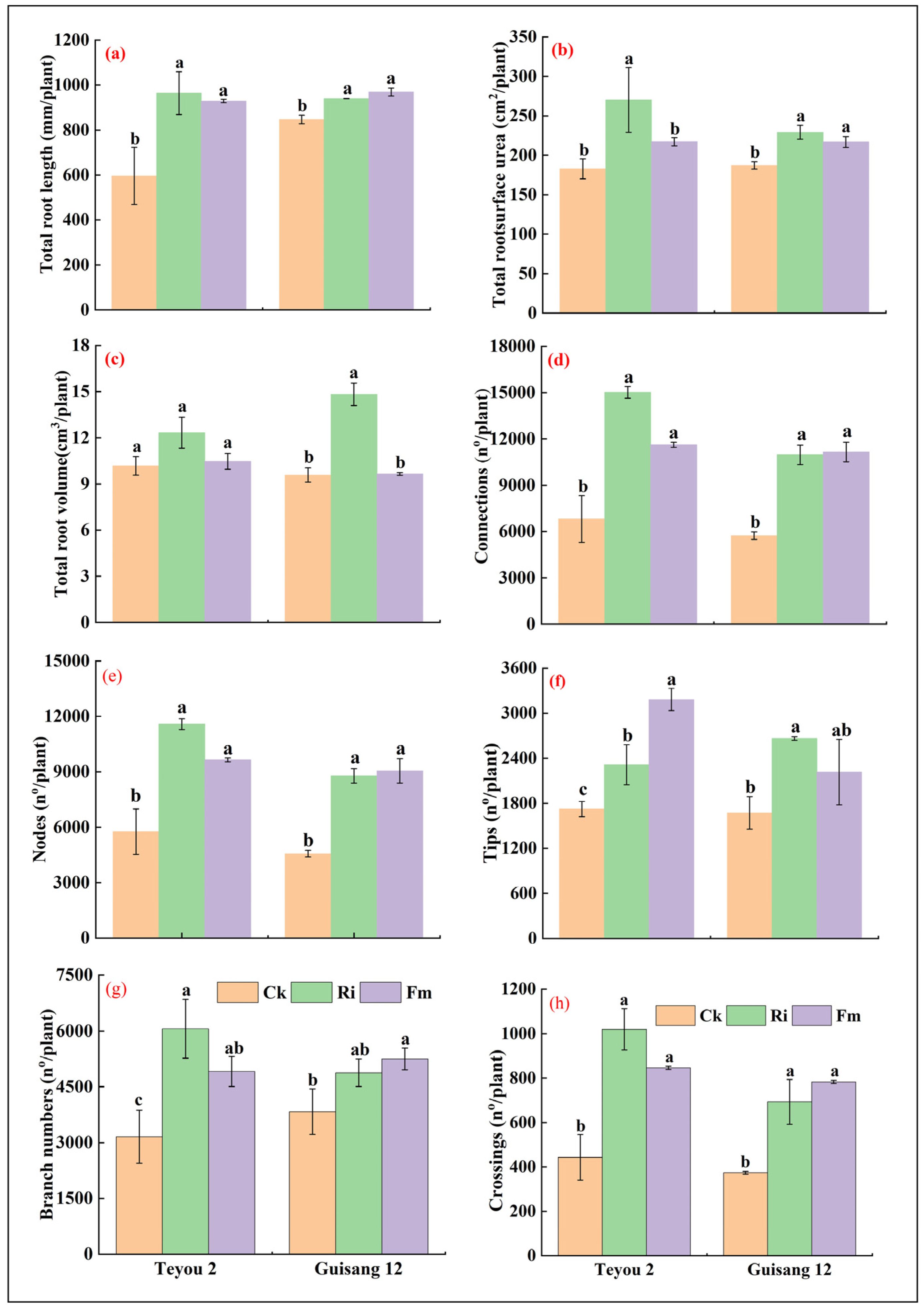
3.3. Morphological Changes in Mycorrhized Mulberry Seedling Root
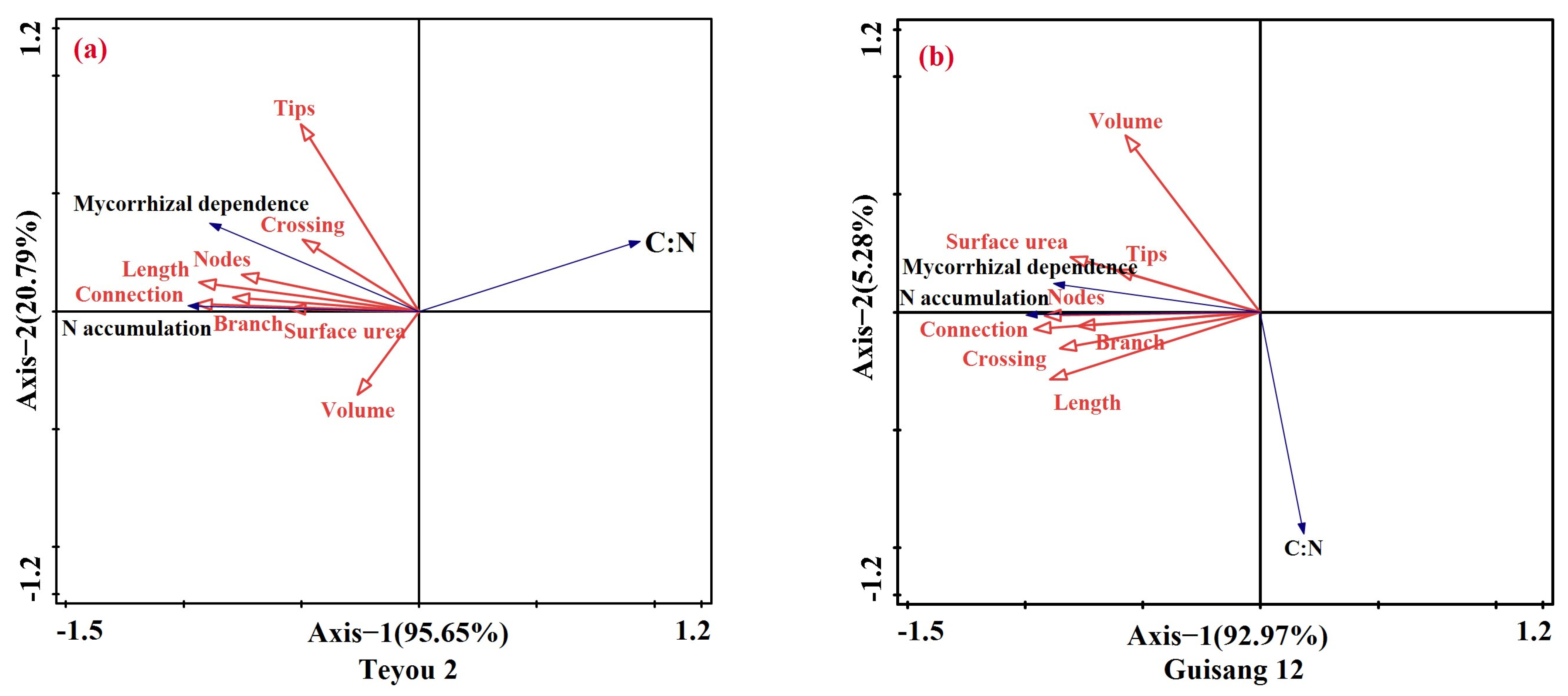
3.4. Coupling Relationship between Root Morphological Characteristics and Leaf Carbon to the Nitrogen Ratio of Mulberry Seedling
4. Discussion
4.1. Mycorrhization of Mulberry Seedlings Can Change Its Absorption and Utilization of Nitrogen
4.2. Mycorrhization of Mulberry Seedlings Could Promote a Positive Change in Root Morphology
4.3. Effects of Root Morphological Changes in Mycorrhized Mulberry Seedlings on Leaf Carbon to Nitrogen Ratio
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Li, D.; Xiao, K.; Tang, H.; Li, C.; Xiao, X. Dynamics of aggregate-associated organic carbon after long-term cropland conversion in a karst region, southwest China. Sci. Rep. 2023, 13, 1773. [Google Scholar]
- Zhu, Y. Molecular regulation of plant developmental transitions and plant architecture via PEPB family proteins: An update on mechanism of action. J. Exp. Bot. 2021, 72, 2301–2311. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Alonso, R.; Carnicer, J.; Fernandez-Martinez, M.; Vivanco, M.G.; Penuelas, J. Factors influencing the foliar elemental composition and stoichiometry in forest trees in Spain. Perspect. Plant Ecol. Evol. Syst. 2016, 18, 52–69. [Google Scholar] [CrossRef]
- Pan, R.; Wang, X.; Li, N. Phytophysiology; Higher Education Press: Beijin, China, 2001. [Google Scholar]
- Xiao, N. Habitat heterogeneity drives arbuscular mycorrhizal fungi and shrub communities in karst ecosystems. Catena 2023, 233, 107513. [Google Scholar] [CrossRef]
- Debatosh, D.; Michael, P.; Karen, H.; Michael, G.; Corinna, D.; Ming, L.H.; Jianhua, Z.; Moxian, C.; Caroline, G. PHOSPHATE STARVATION RESPONSE transcription factors enable arbuscular mycorrhiza symbiosis. Nat. Commun. 2022, 13, 477. [Google Scholar]
- Li, S.-L.; Liu, C.-Q.; Li, J.; Xue, Z.; Guan, J.; Lang, Y.; Ding, H.; Li, L. Evaluation of nitrate source in surface water of southwestern China based on stable isotopes. Environ. Earth Sci. 2013, 68, 219–228. [Google Scholar] [CrossRef]
- Gómez-Leyva, J.F.; Segura-Castruita, M.A.; Hernández-Cuevas, L.V.; Íñiguez-Rivas, M. Arbuscular Mycorrhizal Fungi Associated with Maize (Zea mays L.) in the Formation and Stability of Aggregates in Two Types of Soil. Microorganisms 2023, 11, 2615. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, X.; Feng, G.; Li, Z.; Zhang, Y.; Cao, N. Arbuscular mycorrhizal enhancement of phosphorus uptake and yields of maize under high planting density in the black soil region of China. Sci. Rep. 2021, 11, 1100. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; Zhang, J.; Bai, H.; Shen, Y. How arbuscular mycorrhizal fungi drives herbaceous plants’ C: N: P stoichiometry? A meta-analysis. Sci. Total Environ. 2023, 862, 160807. [Google Scholar] [CrossRef]
- Mei, L.; Yang, X.; Cao, H.; Zhang, T.; Guo, J. Arbuscular Mycorrhizal Fungi Alter Plant and Soil C:N:P Stoichiometries Under Warming and Nitrogen Input in a Semiarid Meadow of China. Int. J. Environ. Res. Public Health 2019, 16, 397. [Google Scholar] [CrossRef]
- Henneron, L.; Kardol, P.; Wardle, D.A.; Cros, C.; Fontaine, S. Rhizosphere control of soil nitrogen cycling: A key component of plant economic strategies. New Phytol. 2020, 228, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Erratum: Evolutionary history resolves global organization of root functional traits. Nature 2018, 556, 135. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Xin, M.; Xu, C.C.; Zhu, W.Y.; Mao, C.Z.; Chen, X.; Cheng, L. Effects of arbuscular mycorrhizal fungi and nitrogen addition on nitrogen uptake in rice with different root morphology genotypes. J. Plant Ecol. 2021, 45, 728–737. [Google Scholar] [CrossRef]
- Yang, X.; Shen, K.; Xia, T.; He, Y.; Guo, Y.; Wu, B.; Han, X.; Yan, J.; Jiao, M. Invasive and Native Plants Differentially Respond to Exogenous Phosphorus Addition in Root Growth and Nutrition Regulated by Arbuscular Mycorrhizal Fungi. Plants 2023, 12, 2195. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-G.; Tang, M. Effect of arbuscular mycorrhizal fungi inoculation on root traits and root volatile organic compound emissions of Sorghum bicolor. S. Afr. J. Bot. 2013, 88, 373–379. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Cao, M.-Q.; Zou, Y.-N.; Wu, C.; He, X.-H. Mycorrhizal colonization represents functional equilibrium on root morphology and carbon distribution of trifoliate orange grown in a split-root system. Sci. Hortic. 2016, 199, 95–102. [Google Scholar]
- Yuan, J.; Shi, K.; Zhou, X.; Wang, L.; Xu, C.; Zhang, H.; Zhu, G.; Si, C.; Wang, J.; Zhang, Y. Interactive impact of potassium and arbuscular mycorrhizal fungi on the root morphology and nutrient uptake of sweet potato (Ipomoea batatas L.). Front. Microbiol. 2023, 13, 1075957. [Google Scholar] [CrossRef]
- Yan, M.; Bu, C.; Huang, G.; Liu, G.; Dong, T.; Xu, X. Positive effects of arbuscular mycorrhizal fungi on aboveground parts of Morus alba. J. Plant Physiol. 2020, 56, 2647–2654. [Google Scholar] [CrossRef]
- Qu, M.H.; Yu, Y.C.; Wang, J.; Xue, L.; Wang, Z.F.; Li, S. Effects of arbuscular mycorrhizal fungui on biomass distribution and root architecture characters of Zenia insigins seedlings in karst soil. J. Ecol. 2021, 40, 766–776. [Google Scholar] [CrossRef]
- Fan, W.; Luo, Y. Growth status, root morphology and physiological characteristics of four citrus rootstocks under different phosphorus levels. Sci. Agric. Sin. 2015, 48, 534–545. [Google Scholar]
- Ju, C.; Buresh, R.J.; Wang, Z.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Root and shoot traits for rice varieties with higher grain yield and higher nitrogen use efficiency at lower nitrogen rates application. Field Crop. Res. 2015, 175, 47–55. [Google Scholar] [CrossRef]
- Chen, M.; Chen, G.; Di, D.; Kronzucker, H.J.; Shi, W. Higher nitrogen use efficiency (NUE) in hybrid super rice links to improved morphological and physiological traits in seedling roots. J. Plant Physiol. 2020, 251, 153191. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Hu, L.; Zhu, Y.; Xu, D.; Zheng, L.; Chen, Z.; Hu, Y.; Cui, P.; Guo, B.; Dai, Q.; et al. Different characteristics of nutrient absorption and utilization between inbred japonica super rice and inter-sub-specific hybrid super rice. Field Crop. Res. 2018, 218, 88–96. [Google Scholar] [CrossRef]
- Xing, D.; Wang, Z.; Zhang, A. Research of Ecological Restoration of Mycorrhizal Mulberry in Karst Rocky Desertification Area. Agric. Sci. Technol. 2014, 15, 1998–2002. [Google Scholar] [CrossRef]
- Xing, D.; Han, S.-Y.; Luo, C.; Yang, S.; Zhang, F.; Chen, T. Effects of Arbuscular Mycorrhizal Fungi on Mulberry Growth and Water Use Efficiency Under Drought Condition. Silkworm Sci. 2019, 45, 475–483. [Google Scholar] [CrossRef]
- Phillips, J.; Hayman, D.S. Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Elmostapha, O.; Hanane, D.; Faissal, A.; Adnane, B.; Robin, D.; Lahcen, O. The first use of morphologically isolated arbuscular mycorrhizal fungi single-species from Moroccan ecosystems to improve growth, nutrients uptake and photosynthesis in Ceratonia siliqua seedlings under nursery conditions. Saudi J. Biol. Sci. 2022, 29, 2121–2130. [Google Scholar]
- Chen, W.; Mou, X.; Meng, P.; Chen, J.; Tang, X.; Meng, G.; Xin, K.; Zhang, Y.; Wang, C. Effects of arbuscular mycorrhizal fungus inoculation on the growth and nitrogen metabolism of Catalpa bungei C.A.Mey. under different nitrogen levels. Front. Plant Sci. 2023, 14, 1138184. [Google Scholar] [CrossRef]
- Virginia, M.; Vitousek, P.M. N:P stoichiometry and protein:RNA ratios in vascular plants: An evaluation of the growth-rate hypothesis. Ecol. Lett. 2009, 12, 765–771. [Google Scholar]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF Inoculation Enhances Growth and Improves the Nutrient Uptake Rates of Transplanted, Salt-Stressed Tomato Seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef]
- Zai, X.M.; Hao, Z.P.; Wang, H.; Ji, Y.F.; Li, Y.P. Arbuscular Mycorrhizal Fungi (AMF) on Growth and Nutrient Uptake of Beach Plum (Prunus maritima) under Salt Stress. Appl. Mech. Mater. 2014, 618, 268–272. [Google Scholar] [CrossRef]
- Moreira, H.; Pereira, S.I.; Vega, A.; Castro, P.M.; Marques, A.P. Synergistic effects of arbuscular mycorrhizal fungi and plant growth-promoting bacteria benefit maize growth under increasing soil salinity. J. Environ. Manag. 2020, 257, 109982. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, A.; Xie, K.; Yang, X.; Luo, Z.; Chen, J.; Zeng, D.; Ren, Y.; Yang, C.; Wang, L.; et al. Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 16649–16659. [Google Scholar] [CrossRef] [PubMed]
- Mariano, A.B.; Busso, M. Arbuscular mycorrhizal fungi and common mycorrhizal networks benefit plants through morphological, physiological and productive traits and soil quality. Lilloa 2022, 59, 301–331. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, X.X.; Zhang, D.; Dong, Z.; Ji, H.; Li, H. Localized nutrient supply promotes maize growth and nutrient acquisition by shaping root morphology and physiology and mycorrhizal symbiosis. Soil Tillage Res. 2023, 225, 105550. [Google Scholar] [CrossRef]
- Sakha, M.A.; Jefwa, J.; Gweyi-Onyango, J.P. Effects of Arbuscular Mycorrhizal Fungal Inoculation on Growth and Yield of Two Sweet Potato Varieties. J. Agric. Ecol. Res. Int. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, D.; Hu, X.; Wu, Q.-S.; Jiang, C.; Ting-Jun, X.; Gao, X.-B.; Kuca, K. Mycorrhiza-induced changes in root growth and nutrient absorption of tea plants. Plant Soil Environ. 2018, 64, 283–289. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, J.; Han, Y.; Hua, Y.; Guan, C.; Zhang, Z. Low Nitrogen Enhances Nitrogen Use Efficiency by Triggering NO3− Uptake and Its Long-Distance Translocation. J. Agric. Food Chem. 2019, 67, 6736–6747. [Google Scholar] [CrossRef]
- Liu, B.; Junyu, W.; Shuaiqi, Y.; John, S.; Yinbo, G. Nitrate regulation of lateral root and root hair development in plants. J. Exp. Bot. 2020, 71, 4405–4414. [Google Scholar] [CrossRef]
- Huang, G.M.; Zou, Y.N.; Wu, Q.S.; Xu, Y.J.; Kuca, K. Mycorrhizal roles in plant growth, gas exchange, root morphology, and nutrient uptake of walnuts. Plant Soil Environ. 2020, 66, 295–302. [Google Scholar] [CrossRef]
- Xiu, L.; Zhang, W.; Wu, D.; Sun, Y.; Zhang, H.; Gu, W.; Wang, Y.; Meng, J.; Chen, W. Biochar can improve biological nitrogen fixation by altering the root growth strategy of soybean in Albic soil. Sci. Total Environ. 2021, 773, 144564. [Google Scholar] [CrossRef] [PubMed]
- Dunbabin, V.M.; Postma, J.A.; Schnepf, A.; Pagès, L.; Javaux, M.; Wu, L.; Leitner, D.; Chen, Y.L.; Rengel, Z.; Diggle, A.J. Modelling root-soil interactions using three-dimensional models of root growth, architecture and function. Plant Soil 2013, 372, 93–124. [Google Scholar] [CrossRef]
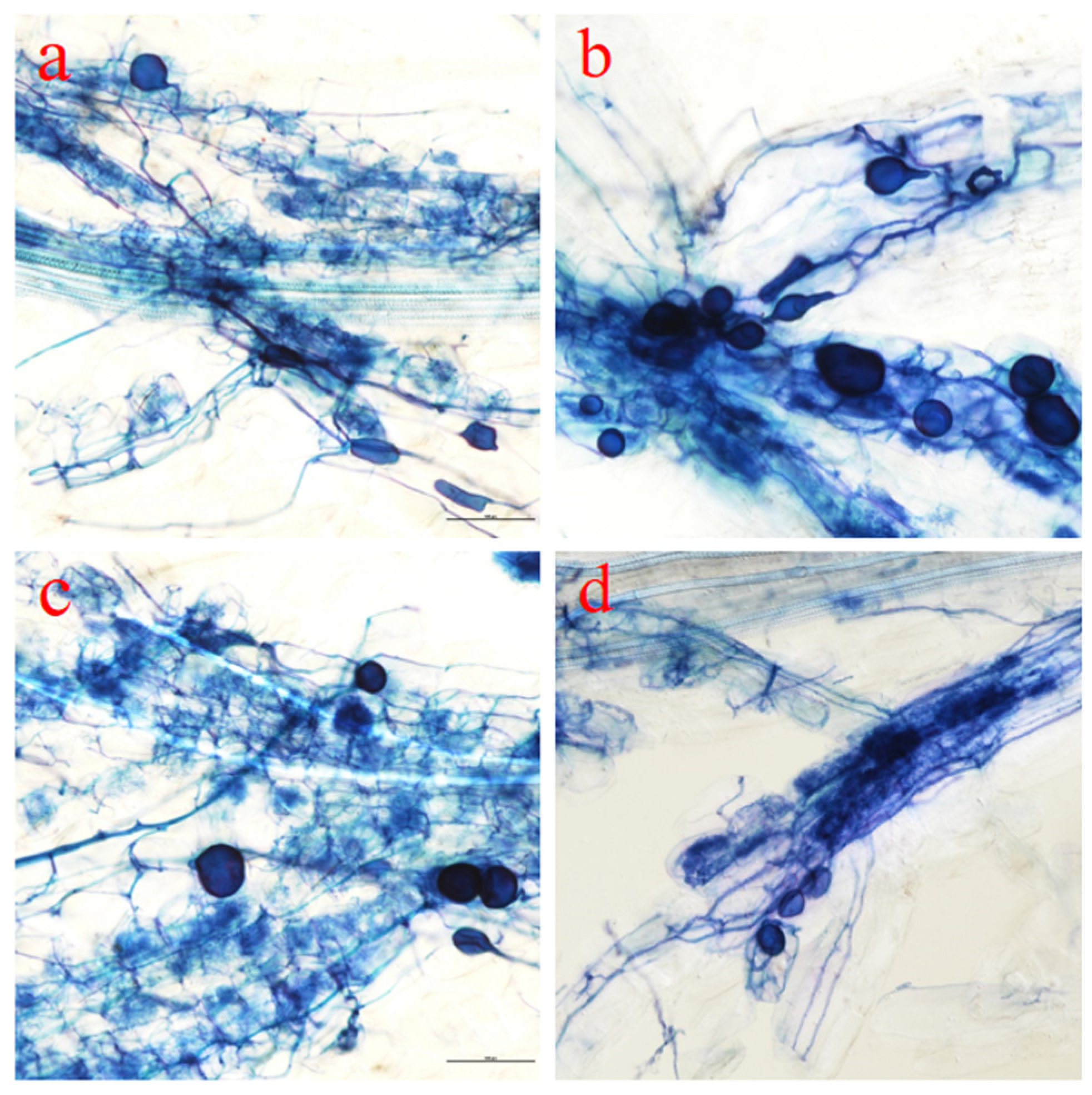
| Parameters | Soil |
|---|---|
| pH | 6.91 |
| Organic matter (g/kg) | 26.9 |
| Total nitrogen (g/kg) | 2.32 |
| Total phosphorus (g/kg) | 0.65 |
| Alkali-hydrolyzed nitrogen (mg/kg) | 110.11 |
| Available phosphorus (mg/kg) | 7.77 |
| Plant Height (mm) | Stem Diameter (mm) | Root Shoot Ratio (mm) | ||||
|---|---|---|---|---|---|---|
| Teyou 2 | Guisang 12 | Teyou 2 | Guisang 12 | Teyou 2 | Guisang 12 | |
| CK | 34.44 ± 2.57 c | 78.60 ± 3.87 c | 1.34 ± 0.07 b | 1.83 ± 0.04 b | 0.09 ± 0.01 b | 0.16 ± 0.02 b |
| FM | 79.31 ± 4.80 a | 91.11 ± 4.67 b | 2.21 ± 0.12 a | 2.16 ± 0.04 a | 0.12 ± 0.02 b | 0.27 ± 0.04 ab |
| RI | 63.06 ± 1.67 b | 104.09 ± 0.91 a | 1.94 ± 0.13 a | 2.29 ± 0.06 a | 0.31 ± 0.04 a | 0.30 ± 0.06 a |
| Mycorrhizal Infection Rate (%) | Mycorrhizal Dependence (%) | |||
|---|---|---|---|---|
| Teyou 2 | Guisang 12 | Teyou 2 | Guisang 12 | |
| CK | —— | —— | —— | —— |
| FM | 42.55 ± 1.78 b | 70.64 ± 3.46 b | 80.58 ± 0.02 b | 48.35 ± 0.10 b |
| RI | 63.60 ± 5.18 a | 75.97 ± 2.77 a | 78.29 ± 0.03 b | 44.60 ± 0.13 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Cheng, H.; Twagirayezu, G.; Zhang, F.; Shi, Y.; Luo, C.; Yan, F.; Wang, Z.; Xing, D. Arbuscular Mycorrhizal Fungi Adjusts Root Architecture to Promote Leaf Nitrogen Accumulation and Reduce Leaf Carbon–Nitrogen Ratio of Mulberry Seedlings. Forests 2023, 14, 2448. https://doi.org/10.3390/f14122448
Zhang H, Cheng H, Twagirayezu G, Zhang F, Shi Y, Luo C, Yan F, Wang Z, Xing D. Arbuscular Mycorrhizal Fungi Adjusts Root Architecture to Promote Leaf Nitrogen Accumulation and Reduce Leaf Carbon–Nitrogen Ratio of Mulberry Seedlings. Forests. 2023; 14(12):2448. https://doi.org/10.3390/f14122448
Chicago/Turabian StyleZhang, Huirong, Hongguang Cheng, Gratien Twagirayezu, Fang Zhang, Yanjin Shi, Chaobin Luo, Fan Yan, Zhenhong Wang, and Dan Xing. 2023. "Arbuscular Mycorrhizal Fungi Adjusts Root Architecture to Promote Leaf Nitrogen Accumulation and Reduce Leaf Carbon–Nitrogen Ratio of Mulberry Seedlings" Forests 14, no. 12: 2448. https://doi.org/10.3390/f14122448





