Nutshell Physicochemical Characteristics of Different Hazel Cultivars and Their Defensive Activity toward Curculio nucum (Coleoptera: Curculionidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Stand
2.2. Experimental Design
Determination of Insect-Resistant Compounds in the Hazelnut Shells
2.3. Determination of Defense Enzymes in the Hazelnut Shells
2.4. Determination of Phytohormones in the Hazelnut Shells
2.5. Data Analysis and Statistics
3. Results
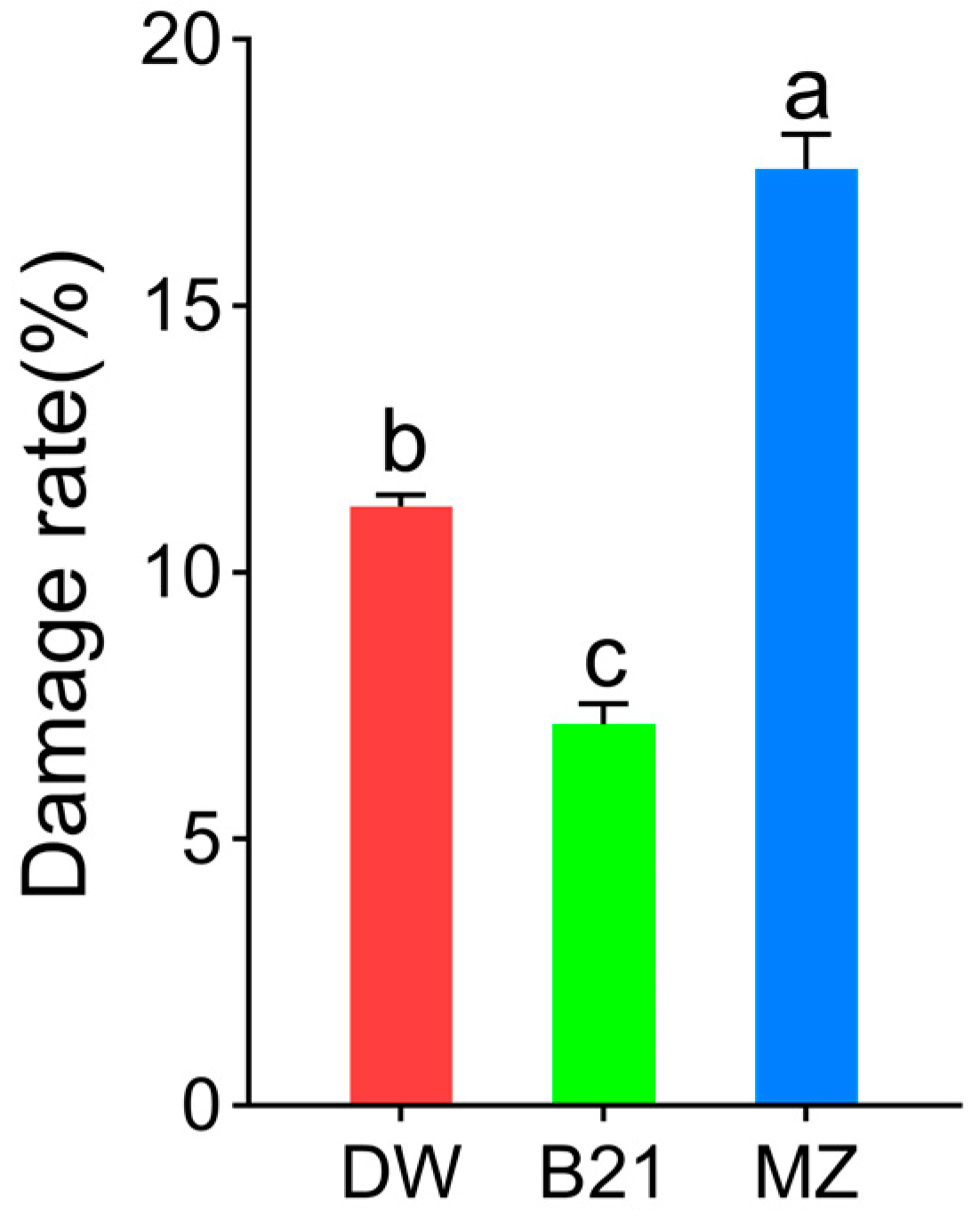
3.1. Resistance of Different Hazel Cultivars to the C. nucum
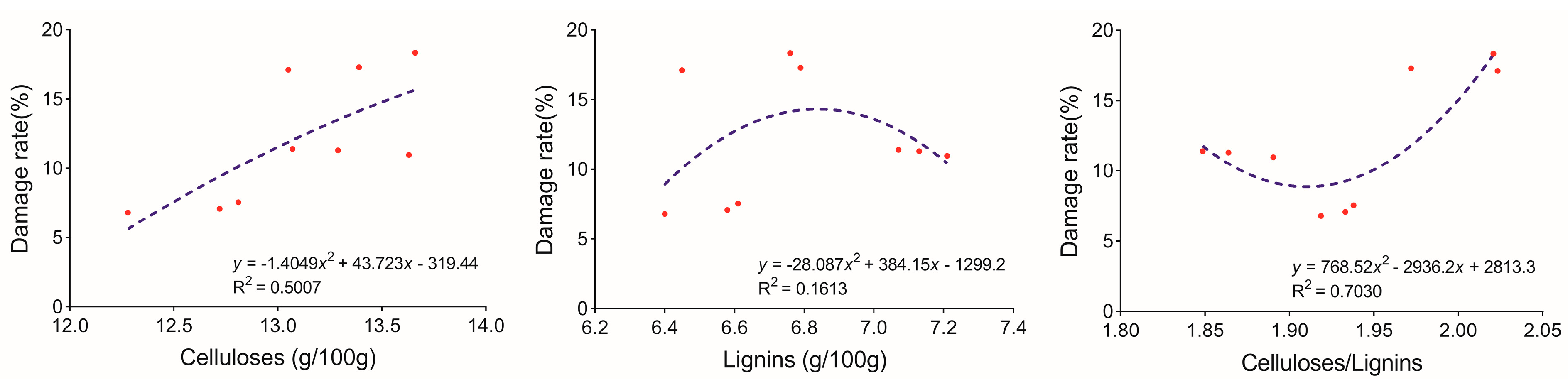
3.2. Insect-Resistant Compounds in Hazelnut Shells
3.3. Effect of Chewing on Insect-Resistant Compounds in Hazelnut Shells
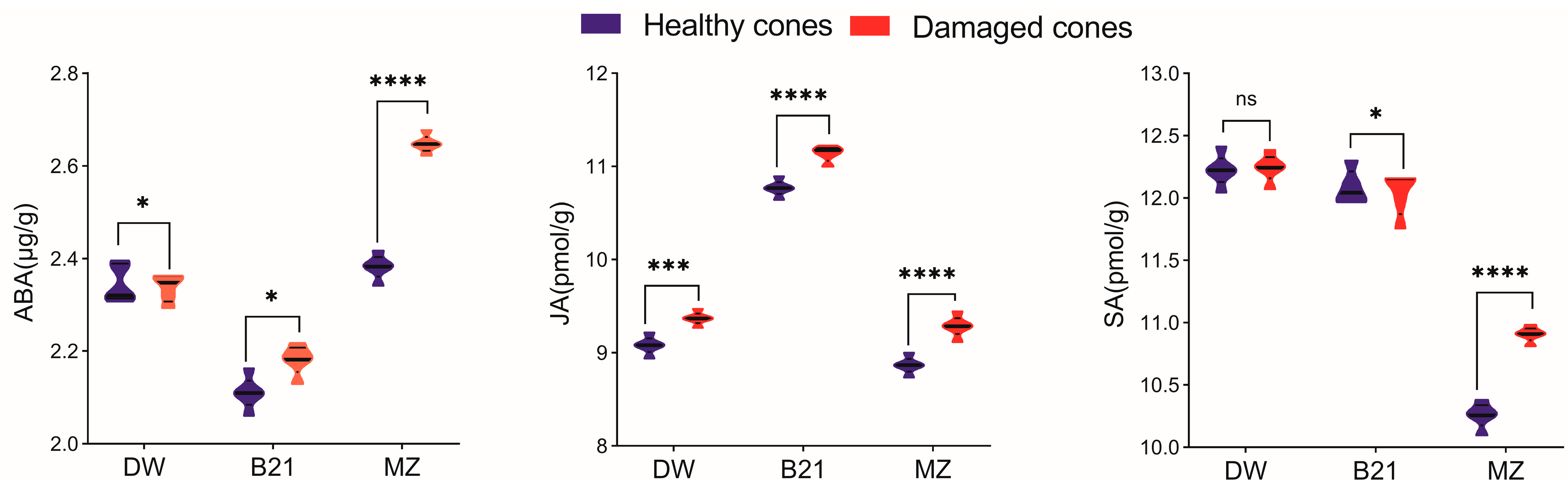
3.4. Effect of Chewing on Defense Enzymes Activity and Phytohormones Contents in Hazelnut Shells
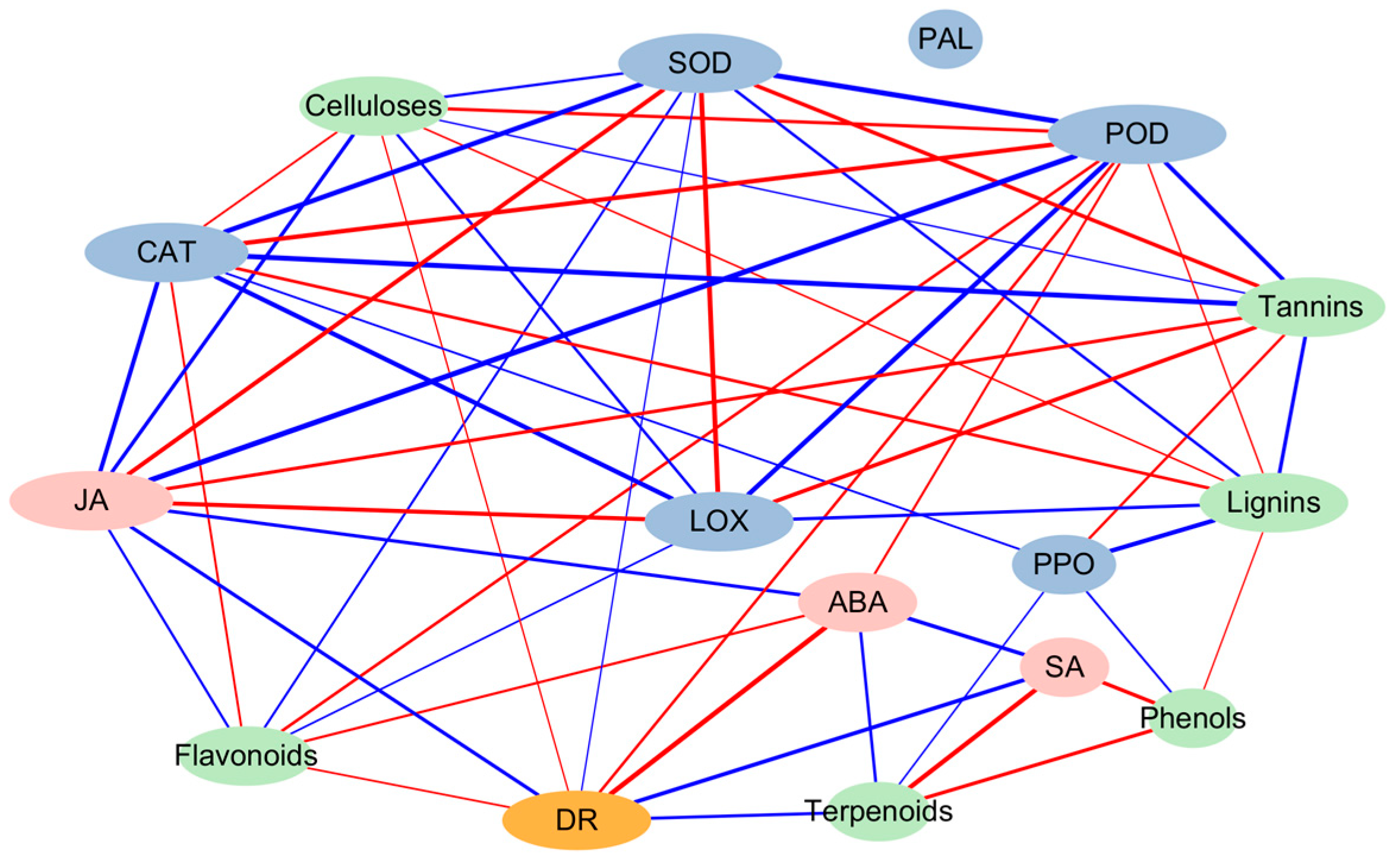
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agrios, G.N. How Plants Defend Themselves against Pathogens. In Plant Pathology; Elsevier: Amsterdam, The Netherlands, 1988; pp. 97–115. [Google Scholar] [CrossRef]
- Ananthakrishnan, T.N.; Gopichandran, R.; Gurusubramanian, G. Influence of Chemical Profiles of Host Plants on the Infestation Diversity of Retithrips syriacus. J. Biosci. 1992, 17, 483–489. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host Plant Quality and Fecundity in Herbivorous Insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Rathjen, J.P. Plant Immunity: Towards an Integrated View of Plant–Pathogen Interactions. Nat. Rev. Genet 2010, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Tosh, C.R.; Powell, G.; Hardie, J. Decision Making by Generalist and Specialist Aphids with the Same Genotype. J. Insect Physiol. 2003, 49, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Woldemariam, M.G.; Baldwin, I.T.; Galis, I. Transcriptional Regulation of Plant Inducible Defenses against Herbivores: A Mini-Review. J. Plant Interact. 2011, 6, 113–119. [Google Scholar] [CrossRef]
- Karban, R.; Agrawal, A.A.; Thaler, J.S.; Adler, L.S. Induced Plant Responses and Information Content about Risk of Herbivory. Trends Ecol. Evol. 1999, 14, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Gershenzon, J. Metabolic Costs of Terpenoid Accumulation in Higher Plants. J. Chem. Ecol. 1994, 20, 1281–1328. [Google Scholar] [CrossRef] [PubMed]
- Siemens, D.H.; Keck, A.G.; Ziegenbein, S. Optimal Defense in Plants: Assessment of Resource Allocation Costs. Evol. Ecol. 2010, 24, 1291–1305. [Google Scholar] [CrossRef]
- Lemic, D.; Mikac, K.M.; Genda, M.; Jukić, Ž.; Pajač Živković, I. Durum Wheat Cultivars Express Different Level of Resistance to Granary Weevil, Sitophilus granarius (Coleoptera: Curculionidae) Infestation. Insects 2020, 11, 343. [Google Scholar] [CrossRef]
- Kant, M.R.; Jonckheere, W.; Knegt, B.; Lemos, F.; Liu, J.; Schimmel, B.C.J.; Villarroel, C.A.; Ataide, L.M.S.; Dermauw, W.; Glas, J.J.; et al. Mechanisms and Ecological Consequences of Plant Defence Induction and Suppression in Herbivore Communities. Ann. Bot 2015, 115, 1015–1051. [Google Scholar] [CrossRef]
- Chaudhary, B. Plant Domestication and Resistance to Herbivory. Int. J. Plant Genom. 2013, 2013, 572784. [Google Scholar] [CrossRef]
- Guidone, L.; Valentini, N.; Rolle, L.; Me, G.; Tavella, L. Early Nut Development as a Resistance Factor to the Attacks of Curculio nucum (Coleoptera: Curculionidae). Ann Appl. Biol. 2007, 150, 323–329. [Google Scholar] [CrossRef]
- Dixit, G.; Srivastava, A.; Rai, K.M.; Dubey, R.S.; Srivastava, R.; Verma, P.C. Distinct Defensive Activity of Phenolics and Phenylpropanoid Pathway Genes in Different Cotton Varieties toward Chewing Pests. Plant Signal. Behav. 2020, 15, 1747689. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for Enhanced Defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Vancanneyt, G.; Sanz, C.; Farmaki, T.; Paneque, M.; Ortego, F.; Castañera, P.; Sánchez-Serrano, J.J. Hydroperoxide Lyase Depletion in Transgenic Potato Plants Leads to an Increase in Aphid Performance. Proc. Natl. Acad. Sci. USA 2001, 98, 8139–8144. [Google Scholar] [CrossRef]
- Guo, H.; Sun, Y.; Yan, H.; Li, C.; Ge, F. O3-Induced Priming Defense Associated with the Abscisic Acid Signaling Pathway Enhances Plant Resistance to Bemisia tabaci. Front. Plant Sci. 2020, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense Priming: An Adaptive Part of Induced Resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.; Malvar, R.A.; Baamonde, M.D.; Revilla, P.; Souto, X.C. Free Phenols in Maize Pith and Their Relationship with Resistance to Sesamia nonagrioides (Lepidoptera: Noctuidae) Attack. J. Econ. Entomol. 2005, 98, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Koramutla, M.K.; Kaur, A.; Negi, M.; Venkatachalam, P.; Bhattacharya, R. Elicitation of Jasmonate-Mediated Host Defense in Brassica juncea (L.) Attenuates Population Growth of Mustard Aphid Lipaphis erysimi (Kalt.). Planta 2014, 240, 177–194. [Google Scholar] [CrossRef]
- Wojciechowicz-Żytko, E. Infestation of Hazel Nuts by Hazelnut Weevil (Curculio nucum L., Coleoptera, Curculionidae) in Poland. J. Plant Prot. Res. 2005, 45, 59–61. [Google Scholar]
- Paparatti, B.; Speranza, S. Biological Control of Hazelnut Weevil (Curculio nucum L., Coleoptera, Curculionidae) Using the Entomopathogenic Fungus Beauveria bassiana (Balsamo) Vuill. (Deuteromycotina, Hyphomycetes). Acta Hortic. 2005, 686, 407–412. [Google Scholar] [CrossRef]
- Ghorai, N.; Ghorai, N.; Chakraborty, S.; Gucchait, S.; Saha, S.K.; Biswas, S. Estimation of Total Terpenoids Concentration in Plant Tissues Using a Monoterpene, Linalool as Standard Reagent. Protoc. Exch. 2012. [Google Scholar] [CrossRef]
- Walling, L.L. The Myriad Plant Responses to Herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef]
- Kunkel, B.N.; Brooks, D.M. Cross Talk between Signaling Pathways in Pathogen Defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Westfall, C.S.; Sherp, A.M.; Zubieta, C.; Alvarez, S.; Schraft, E.; Marcellin, R.; Ramirez, L.; Jez, J.M. Arabidopsis thaliana GH3.5 Acyl Acid Amido Synthetase Mediates Metabolic Crosstalk in Auxin and Salicylic Acid Homeostasis. Proc. Natl. Acad. Sci. USA 2016, 113, 13917–13922. [Google Scholar] [CrossRef]
- Urano, K.; Maruyama, K.; Jikumaru, Y.; Kamiya, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Analysis of Plant Hormone Profiles in Response to Moderate Dehydration Stress. Plant J. 2017, 90, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.Y.; Huang, Y.P. The signaling pathways of plant defense response and their interaction. J. Plant Physiol. Mol. Biol. 2005, 31, 347–353. [Google Scholar]
- Wu, J.; Hettenhausen, C.; Meldau, S.; Baldwin, I.T. Herbivory Rapidly Activates MAPK Signaling in Attacked and Unattacked Leaf Regions but Not between Leaves of Nicotiana attenuata. Plant Cell 2007, 19, 1096–1122. [Google Scholar] [CrossRef] [PubMed]
- Skibbe, M.; Qu, N.; Galis, I.; Baldwin, I.T. Induced Plant Defenses in the Natural Environment: Nicotiana attenuata WRKY3 and WRKY6 Coordinate Responses to Herbivory. Plant Cell 2008, 20, 1984–2000. [Google Scholar] [CrossRef] [PubMed]
- Paschold, A.; Halitschke, R.; Baldwin, I.T. Co(i)-Ordinating Defenses: NaCOI1 Mediates Herbivore- Induced Resistance in Nicotiana attenuata and Reveals the Role of Herbivore Movement in Avoiding Defenses: COI1 Mediates Induced Defenses in Nicotiana attenuata. Plant J. 2007, 51, 79–91. [Google Scholar] [CrossRef]
- Liu, J.; Du, H.; Ding, X.; Zhou, Y.; Xie, P.; Wu, J. Mechanisms of Callose Deposition in Rice Regulated by Exogenous Abscisic Acid and Its Involvement in Rice Resistance to Nilaparvata lugens Stål (Hemiptera: Delphacidae): Callose Regulated by ABA and Its Involvement in Rice Resistance to BPH. Pest. Manag. Sci. 2017, 73, 2559–2568. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, H.; Yuan, L.; Wei, J.; Zhang, W.; Ge, F. Plant Stomatal Closure Improves Aphid Feeding under Elevated CO2. Glob. Chang. Biol. 2015, 21, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Narożna, D.; Nowak, W.; Samardakiewicz, S.; Remlein-Starosta, D. Cross-Talk Interactions of Sucrose and Fusarium oxysporum in the Phenylpropanoid Pathway and the Accumulation and Localization of Flavonoids in Embryo Axes of Yellow Lupine. J. Plant Physiol. 2011, 168, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Porta, H.; Rocha-Sosa, M. Plant Lipoxygenases. Physiological and Molecular Features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Mehari, Z.H.; Elad, Y.; Rav-David, D.; Graber, E.R.; Meller Harel, Y. Induced Systemic Resistance in Tomato (Solanum lycopersicum) against Botrytis cinerea by Biochar Amendment Involves Jasmonic Acid Signaling. Plant Soil 2015, 395, 31–44. [Google Scholar] [CrossRef]
- Palma, F.; López-Gómez, M.; Tejera, N.A.; Lluch, C. Salicylic Acid Improves the Salinity Tolerance of Medicago sativa in Symbiosis with Sinorhizobium meliloti by Preventing Nitrogen Fixation Inhibition. Plant Sci. 2013, 208, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Hettenhausen, C.; Heinrich, M.; Baldwin, I.T.; Wu, J. Fatty Acid-Amino Acid Conjugates Are Essential for Systemic Activation of Salicylic Acid-Induced Protein Kinase and Accumulation of Jasmonic Acid in Nicotiana attenuata. BMC Plant Biol. 2014, 14, 326. [Google Scholar] [CrossRef]
- Zhu, F.; Xi, D.-H.; Yuan, S.; Xu, F.; Zhang, D.-W.; Lin, H.-H. Salicylic Acid and Jasmonic Acid Are Essential for Systemic Resistance Against Tobacco mosaic virus in Nicotiana benthamiana. MPMI 2014, 27, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of Secondary Metabolites in Plant Defense against Pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Stotz, H.U.; Pittendrigh, B.R.; Kroymann, J.; Weniger, K.; Fritsche, J.; Bauke, A.; Mitchell-Olds, T. Induced Plant Defense Responses against Chewing Insects. Ethylene Signaling Reduces Resistance of Arabidopsis against Egyptian Cotton Worm But Not Diamondback Moth. Plant Physiol. 2000, 124, 1007–1018. [Google Scholar] [CrossRef]
- Handrick, V.; Robert, C.A.M.; Ahern, K.R.; Zhou, S.; Machado, R.A.R.; Maag, D.; Glauser, G.; Fernandez-Penny, F.E.; Chandran, J.N.; Rodgers-Melnick, E.; et al. Biosynthesis of 8-O-Methylated Benzoxazinoid Defense Compounds in Maize. Plant Cell 2016, 28, 1682–1700. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Wang, X.; Durachko, D.M.; Zhang, S.; Cosgrove, D.J. Molecular Insights into the Complex Mechanics of Plant Epidermal Cell Walls. Science 2021, 372, 706–711. [Google Scholar] [CrossRef]
- Maag, D.; Dalvit, C.; Thevenet, D.; Köhler, A.; Wouters, F.C.; Vassão, D.G.; Gershenzon, J.; Wolfender, J.-L.; Turlings, T.C.J.; Erb, M.; et al. 3-β-d-Glucopyranosyl-6-Methoxy-2-Benzoxazolinone (MBOA-N-Glc) Is an Insect Detoxification Product of Maize 1,4-Benzoxazin-3-Ones. Phytochemistry 2014, 102, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Silva-Brandão, K.L.; Murad, N.F.; Peruchi, A.; Martins, C.H.Z.; Omoto, C.; Figueira, A.; Brandão, M.M.; Trigo, J.R. Transcriptome Differential Co-expression Reveals Distinct Molecular Response of Fall-armyworm Strains to DIMBOA. Pest Manag. Sci. 2021, 77, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Meihls, L.N.; Handrick, V.; Glauser, G.; Barbier, H.; Kaur, H.; Haribal, M.M.; Lipka, A.E.; Gershenzon, J.; Buckler, E.S.; Erb, M.; et al. Natural Variation in Maize Aphid Resistance Is Associated with 2,4-Dihydroxy-7-Methoxy-1,4-Benzoxazin-3-One Glucoside Methyltransferase Activity. Plant Cell 2013, 25, 2341–2355. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of Flavonoids in Plant Resistance: A Review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Thomas, A.M.; Williams, R.S.; Swarthout, R.F. Distribution of the Specialist Aphid Uroleucon nigrotuberculatum (Homoptera: Aphididae) in Response to Host Plant Semiochemical Induction by the Gall Fly Eurosta solidaginis (Diptera: Tephritidae). Environ. Entomol. 2019, 48, 1138–1148. [Google Scholar] [CrossRef]
- Huang, X.-Z.; Xiao, Y.-T.; Köllner, T.G.; Jing, W.-X.; Kou, J.-F.; Chen, J.-Y.; Liu, D.-F.; Gu, S.-H.; Wu, J.-X.; Zhang, Y.-J.; et al. The Terpene Synthase Gene Family in Gossypium hirsutum Harbors a Linalool Synthase GhTPS12 Implicated in Direct Defence Responses against Herbivores. Plant Cell Environ. 2018, 41, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Growth of the Plant Cell Wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, J.W.; Krekling, T.; Franceschi, V.R. Distribution of Calcium Oxalate Crystals in the Secondary Phloem of Conifers: A Constitutive Defense Mechanism? New Phytol. 2003, 159, 677–690. [Google Scholar] [CrossRef]

| Test Indexes | Sum of Squares | Df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Total terpenoids | 0.001 | 4 | <0.000 | 39.753 | <0.001 |
| Tannins | 0.161 | 4 | 0.081 | 111.800 | <0.001 |
| Total phenols | 0.009 | 4 | 0.004 | 7.463 | 0.024 |
| Flavonoids | 0.003 | 4 | 0.001 | 4.433 | 0.066 |
| Celluloses | 0.060 | 4 | 0.030 | 1.000 | 0.422 |
| Lignins | 0.136 | 4 | 0.068 | 1.694 | 0.261 |
| Cultivar | Test Indexes (mg/g) | |||||
|---|---|---|---|---|---|---|
| Celluloses | Lignins | Total Terpenoids | Tannins | Total Phenols | Flavonoids | |
| DW | 126.33 ± 1.20 a | 66.67 ± 0.88 a | 0.84 ± 0.02 a | 3.17 ± 0.19 c | 1.63 ± 0.09 b | 0.47 ± 0.09 b |
| B21 | 125.33 ± 0.88 a | 63.67 ± 1.76 a | 0.87 ± 0.02 a | 6.40 ± 0.17 a | 2.27 ± 0.18 a | 0.87 ± 0.07 a |
| MZ | 124.33 ± 0.88 a | 65.33 ± 0.33 a | 0.65 ± 0.02 b | 5.27 ± 0.09 b | 1.57 ± 0.15 b | 0.83 ± 0.15 a |
| Test Indexes | Sum of Squares | Df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Total terpenoids | 0.017 | 4 | 0.008 | 1719.023 | <0.001 |
| Tannins | 0.257 | 4 | 0.129 | 131.602 | <0.001 |
| Total phenols | 0.009 | 4 | 0.004 | 11.400 | 0.009 |
| Flavonoids | 0.003 | 4 | 0.002 | 5.148 | 0.050 |
| Celluloses | 1.127 | 4 | 0.563 | 9.054 | 0.015 |
| Lignins | 0.527 | 4 | 0.263 | 6.237 | 0.034 |
| Cultivar | Test Indexes (mg/g) | |||||
|---|---|---|---|---|---|---|
| Celluloses | Lignins | Total Terpenoids | Tannins | Total Phenols | Flavonoids | |
| DW | 133.30 ± 1.63 a | 71.37 ± 0.41 a | 1.66 ± 0.01 a | 3.67 ± 0.12 c | 3.00 ± 0.15 a | 1.17 ± 0.12 ab |
| B21 | 126.03 ± 1.64 b | 65.30 ± 0.66 b | 1.37 ± 0.01 b | 7.80 ± 0.21 a | 2.67 ± 0.09 a | 0.83 ± 0.09 b |
| MZ | 133.67 ± 1.76 a | 66.67 ± 1.09 b | 0.63 ± 0.01 c | 5.50 ± 0.20 b | 2.23 ± 0.09 b | 1.27 ± 0.09 a |
| Cultivar | Test Indexes | Test of Homogeneity of Variances | Df | Sig. (Bilateral) | |
|---|---|---|---|---|---|
| F | Sig. | ||||
| DW | POD | 1.984 | 0.197 | 8 | <0.001 |
| CAT | 0.247 | 0.632 | 8 | <0.001 | |
| SOD | 0.034 | 0.858 | 8 | <0.001 | |
| PAL | 1.142 | 0.316 | 8 | 0.010 | |
| LOX | 0.872 | 0.378 | 8 | <0.001 | |
| PPO | 1.043 | 0.337 | 8 | <0.001 | |
| ABA | 0.762 | 0.408 | 8 | 0.045 | |
| JA | 0.163 | 0.697 | 8 | 0.001 | |
| SA | 0.017 | 0.899 | 8 | 0.803 | |
| B21 | POD | 2.811 | 0.132 | 8 | 0.083 |
| CAT | 0.452 | 0.520 | 8 | <0.001 | |
| SOD | 0.075 | 0.791 | 8 | <0.001 | |
| PAL | 0.889 | 0.373 | 8 | 0.008 | |
| LOX | 0.005 | 0.947 | 8 | 0.052 | |
| PPO | 0.130 | 0.728 | 8 | <0.001 | |
| ABA | 0.000 | 0.999 | 8 | 0.012 | |
| JA | 0.093 | 0.768 | 8 | <0.001 | |
| SA | 0.224 | 0.649 | 8 | 0.036 | |
| MZ | POD | 0.013 | 0.913 | 8 | 0.545 |
| CAT | 0.009 | 0.925 | 8 | <0.001 | |
| SOD | 0.062 | 0.810 | 8 | 0.120 | |
| PAL | 0.056 | 0.819 | 8 | <0.001 | |
| LOX | 0.074 | 0.792 | 8 | 0.001 | |
| PPO | 0.044 | 0.839 | 8 | <0.001 | |
| ABA | 0.189 | 0.675 | 8 | <0.001 | |
| JA | 0.038 | 0.850 | 8 | <0.001 | |
| SA | 0.488 | 0.505 | 8 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xiu, D.; Huang, J.; Yu, B.; Jia, S.; Song, L. Nutshell Physicochemical Characteristics of Different Hazel Cultivars and Their Defensive Activity toward Curculio nucum (Coleoptera: Curculionidae). Forests 2023, 14, 319. https://doi.org/10.3390/f14020319
Li X, Xiu D, Huang J, Yu B, Jia S, Song L. Nutshell Physicochemical Characteristics of Different Hazel Cultivars and Their Defensive Activity toward Curculio nucum (Coleoptera: Curculionidae). Forests. 2023; 14(2):319. https://doi.org/10.3390/f14020319
Chicago/Turabian StyleLi, Xingpeng, Dongying Xiu, Jinbin Huang, Bo Yu, Shuxia Jia, and Liwen Song. 2023. "Nutshell Physicochemical Characteristics of Different Hazel Cultivars and Their Defensive Activity toward Curculio nucum (Coleoptera: Curculionidae)" Forests 14, no. 2: 319. https://doi.org/10.3390/f14020319






