Multi-Century Reconstruction of Pandora Moth Outbreaks at the Warmest/Driest Edge of a Wide-Ranging Pinus Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Site Selection
| Site (Code) [Citation] | Species | Coordinates | Slope (%) | No. of Trees (Dated/Total) | Dates | Mean Series Length | r-Bar | Sensitivity |
|---|---|---|---|---|---|---|---|---|
| Crazy Jug Canyon (CJ) | PIPO | 36.462°, −112.406° | 17.5 | 16/20 | 1646–2018 | 254 | 0.66 | 0.40 |
| Dog Canyon (DG) | PIPO | 36.437°, −112.092° | 5.9 | 17/20 | 1663–2018 | 266 | 0.48 | 0.26 |
| Dry Park (DP) | PIPO | 36.439°, −112.226° | 12.5 | 16/20 | 1666–2018 | 226 | 0.40 | 0.21 |
| Fire Point (FP) [30] | PIPO | 36.358°, −112.347° | 9.9 | 20/20 | 1783–1997 | 181 | 0.66 | 0.36 |
| Fracas Canyon (FR) | PIPO | 36.684°, −112.285° | 29.1 | 19/20 | 1601–2018 | 272 | 0.51 | 0.32 |
| Little Park (LP) [26] | PIPO | 36.331°, −112.127° | 11.4 | 13/20 | 1645–2000 | 207 | 0.29 | 0.25 |
| Orderville Canyon (OR) | PIPO | 36.768°, −112.182° | 9.6 | 19/20 | 1650–2018 | 281 | 0.69 | 0.43 |
| Telephone Hill (TH) | PIPO | 36.603°, −112.156° | 7.4 | 16/20 | 1695–2018 | 215 | 0.54 | 0.26 |
| South Canyon (SC) | PSME | 36.352°, −112.101° | 22.5 | 8/10 | 1850–2018 | 157 | 0.54 | 0.21 |
| Saddle Mtn. (AZ505) [32] | PSME | 36.42°, −112.00° | NR | 21/21 | 1708–1975 | 133 | 0.60 | 0.20 |
| Yovimpa Point (UT515) [33] | PSME | 37.47°, −112.25° | NR | 17/17 | 1436–1998 | 405 | 0.71 | 0.25 |
2.3. Study Area
2.4. Lab Procedures
2.5. Non-Host Chronology Modifications
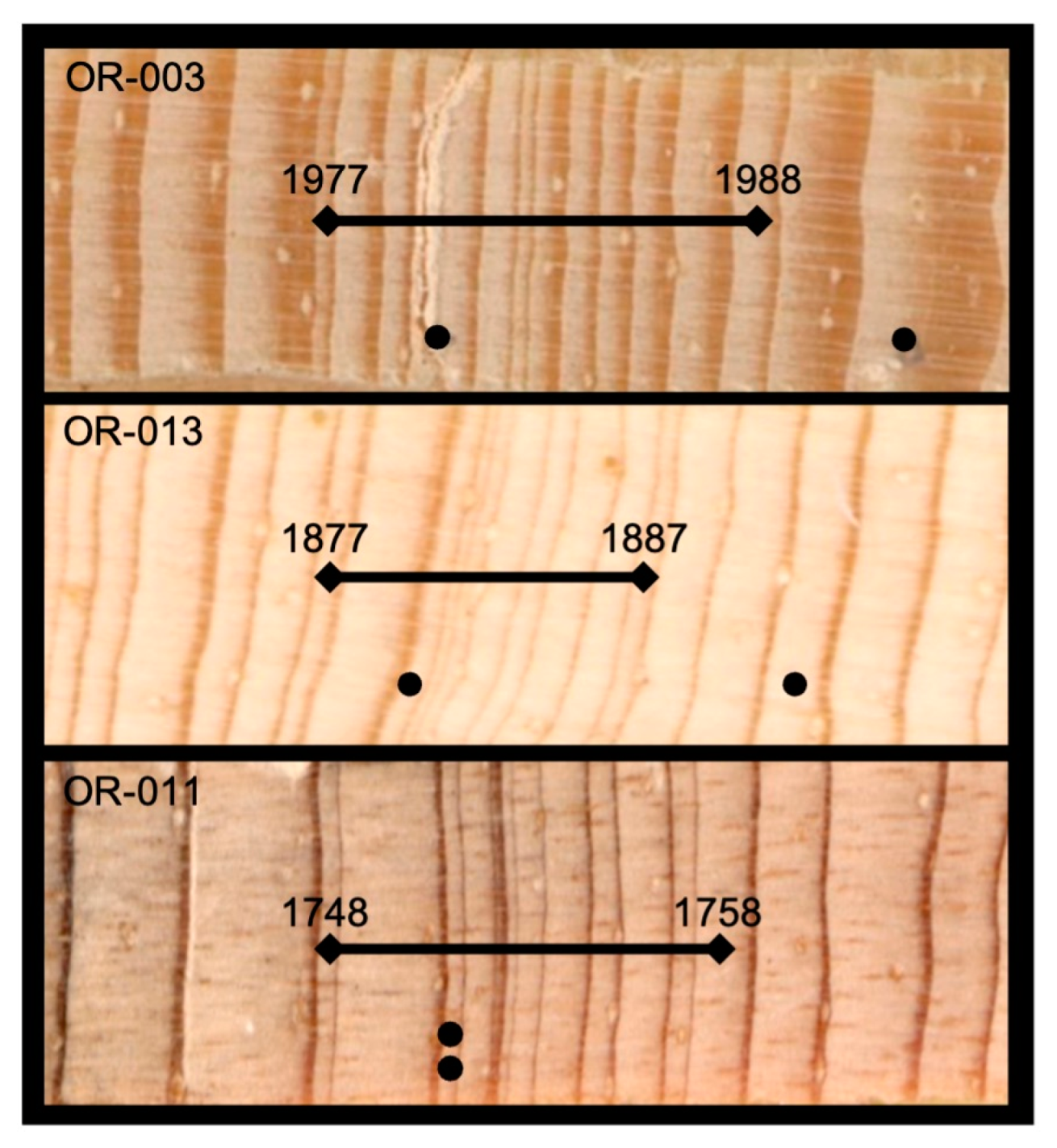
2.6. Outbreak Analyses
3. Results
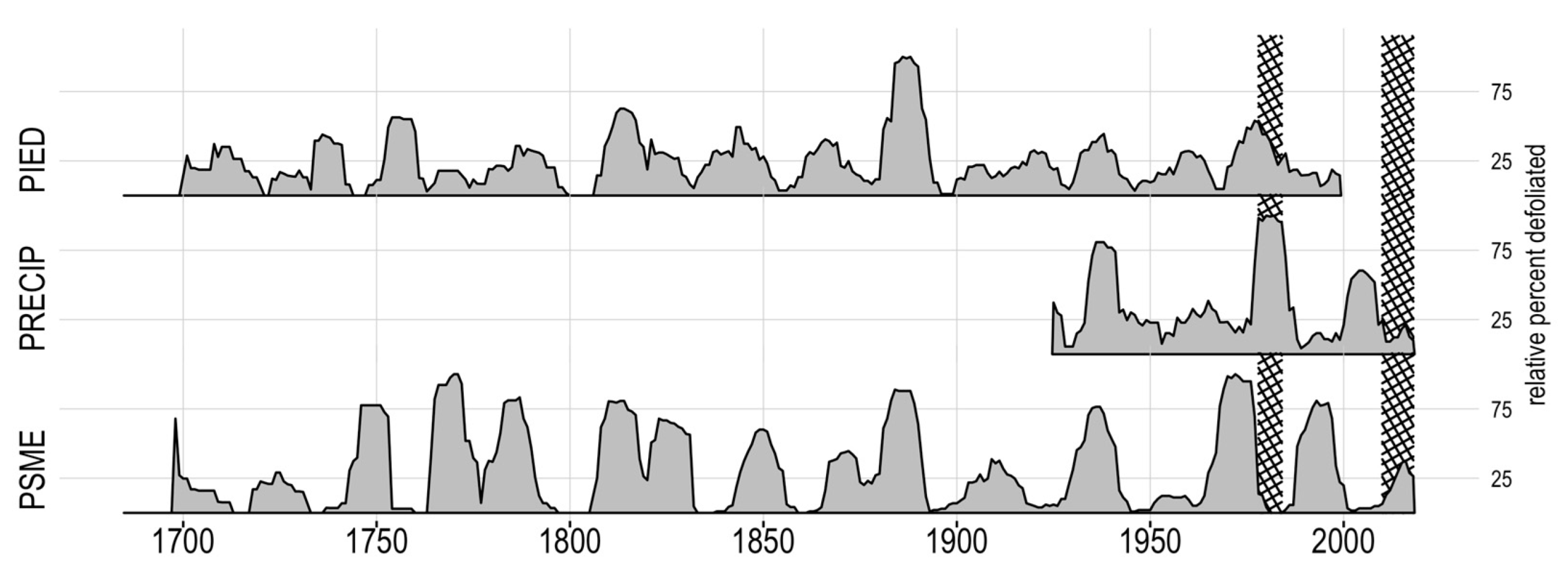
3.1. Kaibab Pandora Moth Signature
3.2. Scale
3.3. Fire Exclusion
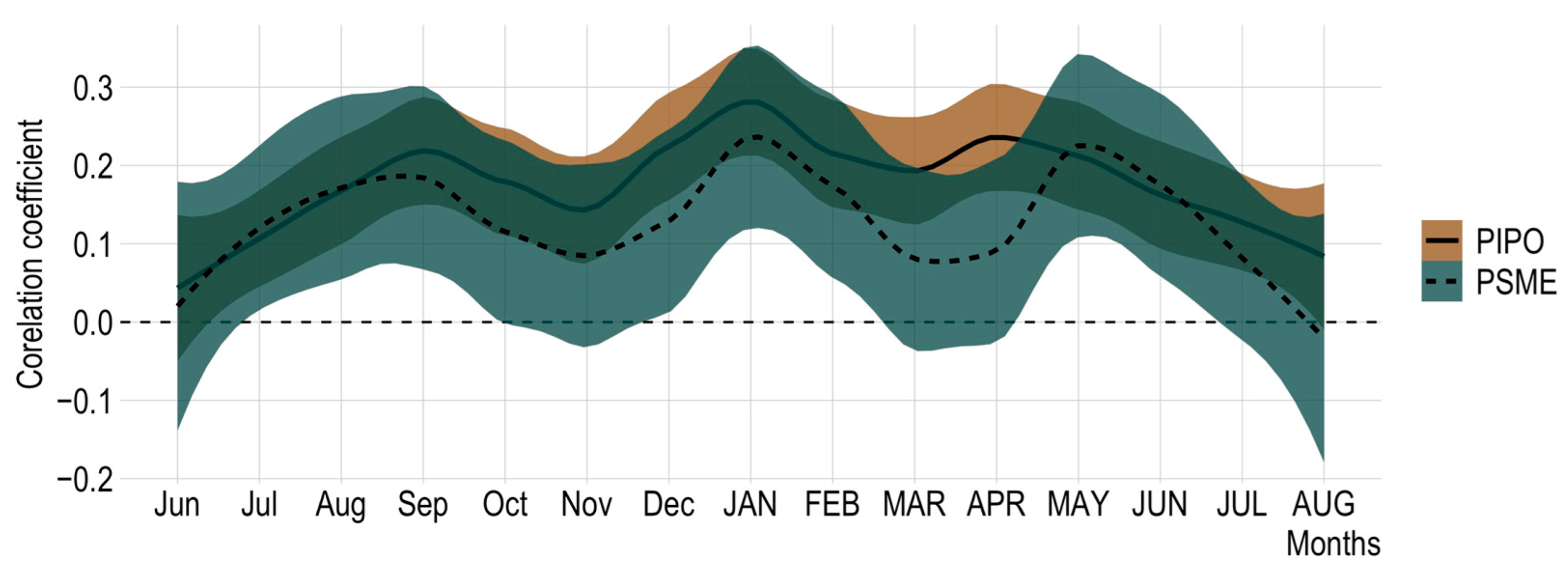
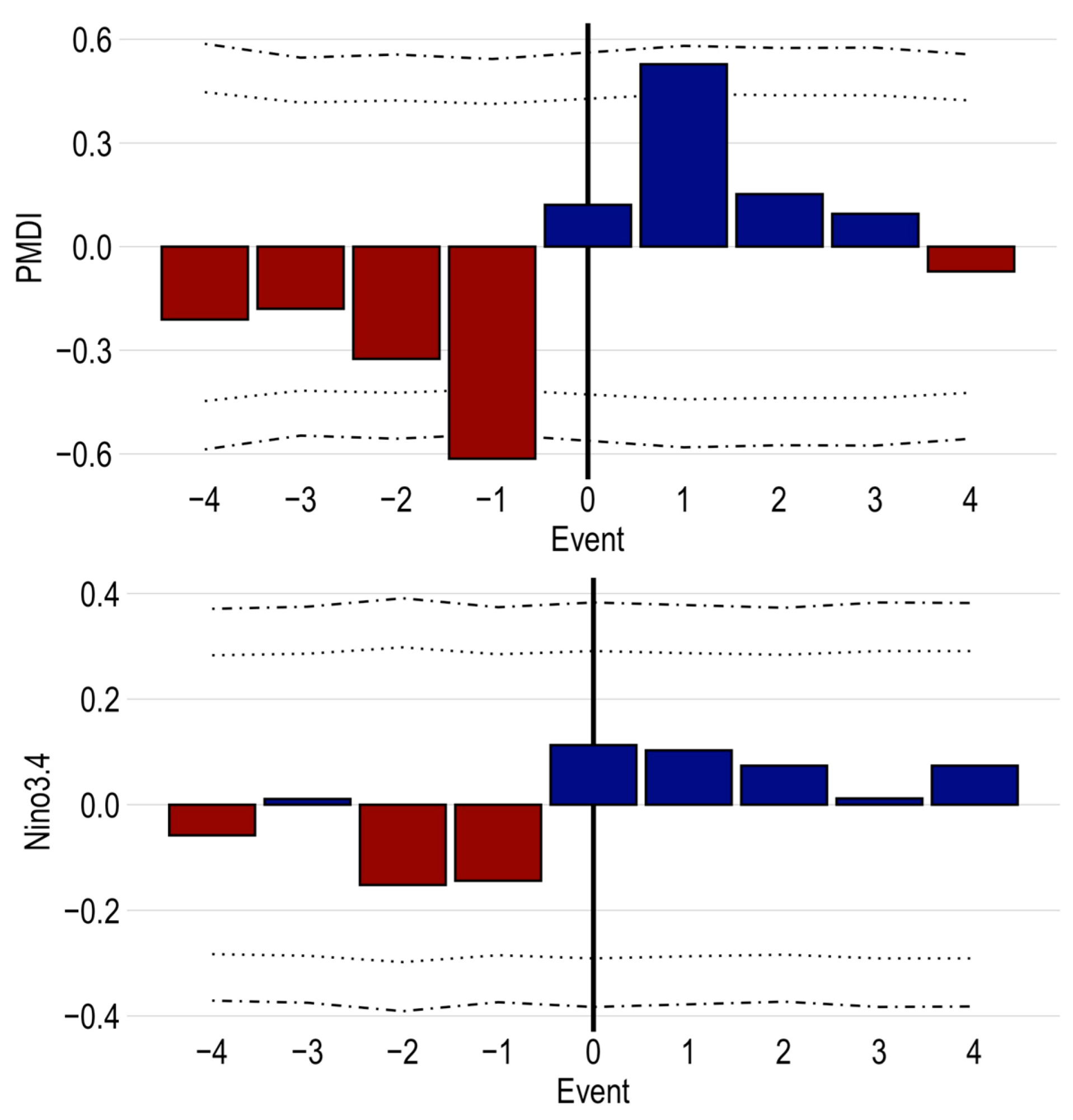
3.4. Climate
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive Shifts in Forest Dynamics in a Changing World. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest Disturbances under Climate Change. Nat. Clim. Change 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eschtruth, A.K.; Battles, J.J. Ephemeral Disturbances Have Long-Lasting Impacts on Forest Invasion Dynamics. Ecology 2014, 95, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.R.; Seager, R.; Heim, R.R.; Vose, R.S.; Herweijer, C.; Woodhouse, C. Megadroughts in North America: Placing IPCC Projections of Hydroclimatic Change in a Long-Term Palaeoclimate Context. J. Quat. Sci. 2010, 25, 48–61. [Google Scholar] [CrossRef] [Green Version]
- de Graauw, K. Tree-Ring Analysis of Outbreak Dynamics across an Insect’s Entire Range: The Pandora Moth. Master’s Thesis, Indiana State University, Terre Haute, IN, USA, 2012. [Google Scholar]
- Pohl, K.A.; Hadley, K.S.; Arabas, K.B. Decoupling Tree-Ring Signatures of Climate Variation, Fire, and Insect Outbreaks in Central Oregon. Tree-Ring Res. 2006, 62, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Speer, J.H.; Swetnam, T.W.; Wickman, B.E.; Youngblood, A. Changes in Pandora Moth Outbreak Dynamics during the Past 622 Years. Ecology 2001, 82, 679–697. [Google Scholar] [CrossRef]
- Clark, P.W.; Speer, J.H.; Winship, L.J. Identifying and Separating Pandora Moth Outbreaks and Climate from A 1500-Year Ponderosa Pine Chronology from Central Oregon. Tree Ring Res. 2017, 73, 113–125. [Google Scholar] [CrossRef]
- Ryerson, D.E.; Swetnam, T.W.; Lynch, A.M. A Tree-Ring Reconstruction of Western Spruce Budworm Outbreaks in the San Juan Mountains, Colorado, U.S.A. Can. J. For. Res. 2003, 33, 1010–1028. [Google Scholar] [CrossRef]
- Flower, A.; Gavin, D.G.; Heyerdahl, E.K.; Parsons, R.A.; Cohn, G.M. Drought-Triggered Western Spruce Budworm Outbreaks in the Interior Pacific Northwest: A Multi-Century Dendrochronological Record. For. Ecol. Manag. 2014, 324, 16–27. [Google Scholar] [CrossRef]
- Carolin, V.M.; Knopf, J.A.E. The Pandora Moth; Department of Agriculture, Forest Service: Washington, DC, USA, 1968; Volume 114.
- Jacquet, J.-S.; Orazio, C.; Jactel, H. Defoliation by Processionary Moth Significantly Reduces Tree Growth: A Quantitative Review. Ann. For. Sci. 2012, 69, 857–866. [Google Scholar] [CrossRef] [Green Version]
- Swank, W.T.; Waide, J.B.; Crossley, D.A.; Todd, R.L. Insect Defoliation Enhances Nitrate Export from Forest Ecosystems. Oecologia 1981, 51, 297–299. [Google Scholar] [CrossRef]
- Patterson, J.E. The Pandora Moth, a Periodic Pest of Western Pine Forests. USDA Tech. Bull. 1929, 137, 20. [Google Scholar] [CrossRef]
- Slaton, M.R.; Holmquist, J.G.; Meyer, M.; Andrews, R.; Beidl, J. Traditional Ecological Knowledge Used in Forest Restoration Benefits Natural and Cultural Resources: The Intersection between Pandora Moths, Jeffrey Pine, People, and Fire. Nat. Areas J. 2019, 39, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Wagner, M.R.; Mathiasen, R.L. Dwarf Mistletoe-Pandora Moth Interaction and Its Contribution to Ponderosa Pine Mortality in Arizona. Great Basin Naturalist 1984, 45, 423–426. [Google Scholar]
- García-González, I.; Baisan, C.; Swetnam, T.; Lynch, A. Fire and Pandora Moth History in Northern Arizona; Department of Agriculture, Rocky Mountain Research Station and University of Arizona: Tucson, AZ, USA, 2005. [Google Scholar]
- Speer, A. A Dendrochronological Record of Pandora Moth (Coloradia Pandora, Blake) Outbreaks in Central Oregon. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1997. [Google Scholar]
- Speer, J.H.; Kulakowski, D. Creating a Buzz: Insect Outbreaks and Disturbance Interactions. In Dendroecology: Tree-Ring Analyses Applied to Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2017; Volume 231, pp. 231–255. [Google Scholar]
- Speer, J. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010. [Google Scholar]
- Guiterman, C.H.; Lynch, A.M.; Axelson, J.N. DfoliatR: An R Package for Detection and Analysis of Insect Defoliation Signals in Tree Rings. Dendrochronologia 2020, 63, 125750. [Google Scholar] [CrossRef]
- Lynch, A.; Anhold, J.; McMillan, J.; Dudley, S.; Fitzgibbon, R.; Fairweather, M. Forest Insect and Disease Activity on the Kaibab National Forest and Grand Canyon National Park, 1918-2006; Department of Agriculture, Rocky Mountain Research Station: Tucson, AZ, USA, 2008.
- Schmid, J.M.; Bennett, D.D. The North Kaibab Pandora Moth Outbreak, 1978–1984; Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1988.
- Hofstetter, R. Personal Communication, 2019.
- Miller, K.; Wagner, M. Effect of Pandora Moth (Lepidoptera: Saturniidae) Defoliation on Growth of Ponderosa Pine in Arizona. Econ. Entomol. 1989, 82, 1682–1686. [Google Scholar] [CrossRef]
- Fulé, P.Z.; Crouse, J.E.; Heinlein, T.A.; Moore, M.M.; Covington, W.W.; Verkamp, G. Mixed-Severity Fire Regime in a High-Elevation Forest of Grand Canyon, Arizona, USA. Landsc. Ecol. 2003, 18, 465–485. [Google Scholar] [CrossRef]
- Flatley, W.T.; Fulé, P.Z. Are Historical Fire Regimes Compatible with Future Climate? Implications for Forest Restoration. Ecosphere 2016, 7, e01471. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, I. Biotic Communities of Kaibab Plateau, Arizona. Eclological Monogr. 1941, 11, 229–275. [Google Scholar] [CrossRef]
- Cooperative Climatological Data Summaries. Available online: http://wrcc.dri.edu/climatedata/climsum/ (accessed on 4 June 2022).
- Fulé, P.Z.; Covington, W.W.; Moore, M.M.; Heinlein, T.A.; Waltz, A.E.M. Natural Variability in Forests of the Grand Canyon, USA. J. Biogeogr. 2002, 29, 31–47. [Google Scholar] [CrossRef]
- Young, C.E. Tree Rings and Kaibab North Deer Hunting Success, 1925–1975. J. Ariz.-Nev. Acad. Sci. 1979, 14, 61–65. [Google Scholar]
- Young, C.E. NOAA/WDS Paleoclimatology—Young—Saddle Mountain Road—PSME—ITRDB AZ505. NOAA Natl. Cent. Environ. Inf. 1996. [Google Scholar] [CrossRef]
- Grow, D. NOAA/WDS Paleoclimatology—Grow—Yovimpa Point—PSME—ITRDB UT515. NOAA Natl. Cent. Environ. Inf. 2003. [Google Scholar] [CrossRef]
- White, A.S. Presettlement Regeneration Patterns in a Southwestern Ponderosa Pine Stand. Ecology 1985, 66, 589–594. [Google Scholar] [CrossRef]
- Larsson, L.; Larsson, P. CDendro, CooRecorder, Cybis Elektronik & Data AB, Saltsjöbaden, Sweden. 2006. Available online: https://www.cybis.se (accessed on 4 June 2022).
- Holmes, R.L. Computer-Assisted Quality Control in Tree- Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Bunn, A.G. Statistical and Visual Crossdating in R Using the DplR Library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020.
- Vose, R.S.; Applequist, S.; Squires, M.; Durre, I.; Menne, M.J.; Williams, C.N., Jr.; Fenimore, C.; Gleason, K.; Arndt, D. NOAA Monthly U.S. Climate Divisional Database (NClimDiv). NOAA Natl. Clim. Data Cent. 2014. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Treeclim: An R Package for the Numerical Calibration of Proxy-Climate Relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Stokes, M.A. NOAA/WDS Paleoclimatology—Stokes—Kaibab Plateau—PIED—ITRDB AZ129. NOAA Natl. Cent. Environ. Inf. 1996. [Google Scholar] [CrossRef]
- Lyubchich, V.; Gel, Y.R. A Local Factor Nonparametric Test for Trend Synchronism in Multiple Time Series. J. Multivar. Anal. 2016, 150, 91–104. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.P.; Cook, E.R.; Morales, M.S.; Christie, D.A.; Johnson, N.C.; Chen, F.; D’Arrigo, R.; Fowler, A.M.; Gou, X.; et al. El Niño Modulations over the Past Seven Centuries. Nat. Clim. Change 2013, 3, 822–826. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.P.; Cook, E.R.; Smerdon, J.E.; Cook, B.I.; Abatzoglou, J.T.; Bolles, K.; Baek, S.H.; Badger, A.M.; Livneh, B. Large Contribution from Anthropogenic Warming to an Emerging North American Megadrought. Science 2020, 368, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.; Andrews, M. Biological Evaluation, Pandora Moth; USDA Forest Service: Washington, DC, USA, 1983.
- Miller, K.K.; Wagner, M.R. Factors Influencing Pupal Distribution of the Pandora Moth (Lepidoptera: Saturniidae) and Their Relationship to Prescribed Burning. Environ. Entomol. 1984, 13, 430–431. [Google Scholar] [CrossRef]
- Swetnam, T.W.; Lynch, A.M. A Tree-Ring Reconstruction of Western Spruce Budworm History in the Southern Rocky Mountains. For. Sci. 1989, 35, 962–986. [Google Scholar]
- Fulé, P.Z.; Covington, W.W.; Moore, M.M. Determining Reference Conditions for Ecosystem Management of Southwestern Ponderosa Pine Forests. Ecol. Appl. 1997, 7, 895–908. [Google Scholar] [CrossRef]
- Cochran, P.H. Reduction in Growth of Pole-Sized Ponderosa Pine Related to a Pandora Moth Outbreak in Central Oregon; U.S. Dept. of Agriculture, Forest Service, Pacific Northwest Research Station: Corvallis, OR, USA, 1998.
- Schoennagel, T.; Veblen, T.T.; Romme, W.H.; Sibold, J.S.; Cook, E.R. ENSO and PDO Variability Affect Drought-Induced Fire Occurrence in Rocky Mountain Subalpine Forests. Ecol. Appl. 2005, 15, 2000–2014. [Google Scholar] [CrossRef]
- Swetnam, T.W.; Betancourt, J.L. Mesoscale Disturbance and Ecological Response to Decadal Climatic Variability in the American Southwest. J. Clim. 1998, 11, 3128–3147. [Google Scholar] [CrossRef]
- Peltonen, M.; Liebhold, A.M.; Bjørnstad, O.N.; Williams, D.W. Spatial Synchrony in Forest Insect Outbreaks: Roles of Regional Stochasticity and Dispersal. Ecology 2002, 83, 3120–3129. [Google Scholar] [CrossRef]
- Klemola, T.; Huitu, O.; Klemola, K.R.; Ruohomäki, O.; Klemola, T.; Huitu, O.; Ruohomä, K. Geographically Partitioned Spatial Synchrony among Cyclic Moth Populations. Oikos 2006, 114, 349–359. [Google Scholar] [CrossRef]
- Cory, J.S.; Myers, J.H. The Ecology and Evolution of Insect Baculoviruses. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 239–272. [Google Scholar] [CrossRef] [Green Version]
- Speer, J.; Holmes, R. Effects of Pandora Moth Outbreaks on Ponderosa Pine Wood Volume. Tree Ring Res. 2004, 60, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Grüning, M.M.; Simon, J.; Rennenberg, H.; L-M-Arnold, A. Defoliating Insect Mass Outbreak Affects Soil N Fluxes and Tree N Nutrition in Scots Pine Forests. Front. Plant Sci. 2017, 8, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]

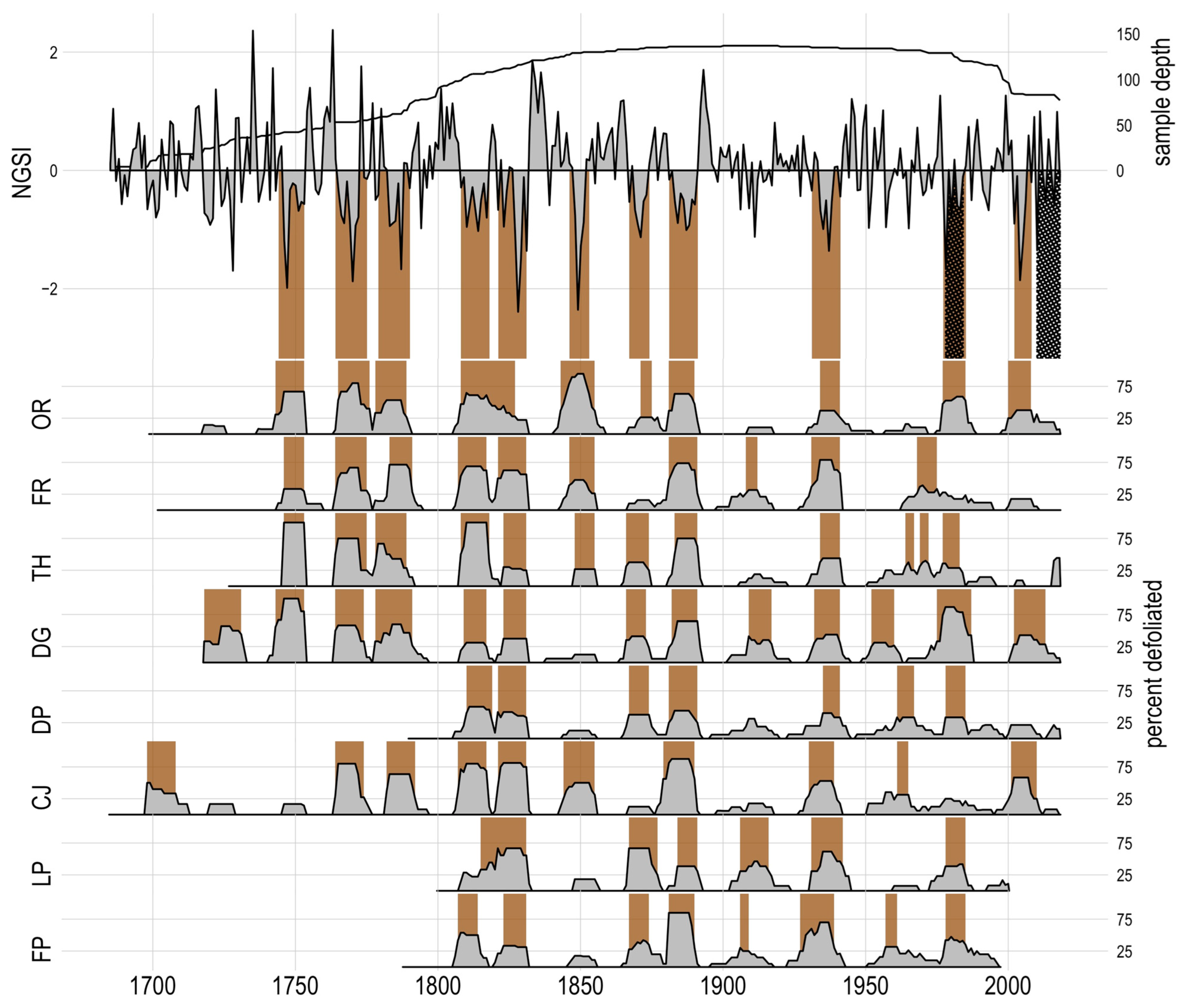
| Dates | Duration | Interval | Min nGSI (Year) |
|---|---|---|---|
| 1744–1753 | 10 | - | −1.986 (1747) |
| 1764–1775 | 12 | 20 | −1.875 (1770) |
| 1779–1790 | 12 | 15 | −1.671 (1787) |
| 1808–1818 | 11 | 29 | −1.025 (1811) |
| 1821–1831 | 11 | 13 | −2.391 (1823) |
| 1846–1853 | 8 | 25 | −2.356 (1849) |
| 1867–1874 | 8 | 21 | −1.130 (1872) |
| 1881–1891 | 11 | 14 | −1.009 (1884) |
| 1931–1941 | 11 | 50 | −1.361 (1936) |
| 1977–1985 | 9 | 46 | −1.669 (1978) |
| 2002–2008 | 7 | 25 | −1.856 (2004) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, L.; Fulé, P.Z.; Hofstetter, R.W. Multi-Century Reconstruction of Pandora Moth Outbreaks at the Warmest/Driest Edge of a Wide-Ranging Pinus Species. Forests 2023, 14, 444. https://doi.org/10.3390/f14030444
O’Neill L, Fulé PZ, Hofstetter RW. Multi-Century Reconstruction of Pandora Moth Outbreaks at the Warmest/Driest Edge of a Wide-Ranging Pinus Species. Forests. 2023; 14(3):444. https://doi.org/10.3390/f14030444
Chicago/Turabian StyleO’Neill, Leo, Peter Z. Fulé, and Richard W. Hofstetter. 2023. "Multi-Century Reconstruction of Pandora Moth Outbreaks at the Warmest/Driest Edge of a Wide-Ranging Pinus Species" Forests 14, no. 3: 444. https://doi.org/10.3390/f14030444





