Topography and Soil Organic Carbon in Subtropical Forests of China
Abstract
:1. Introduction
2. Materials and Methods
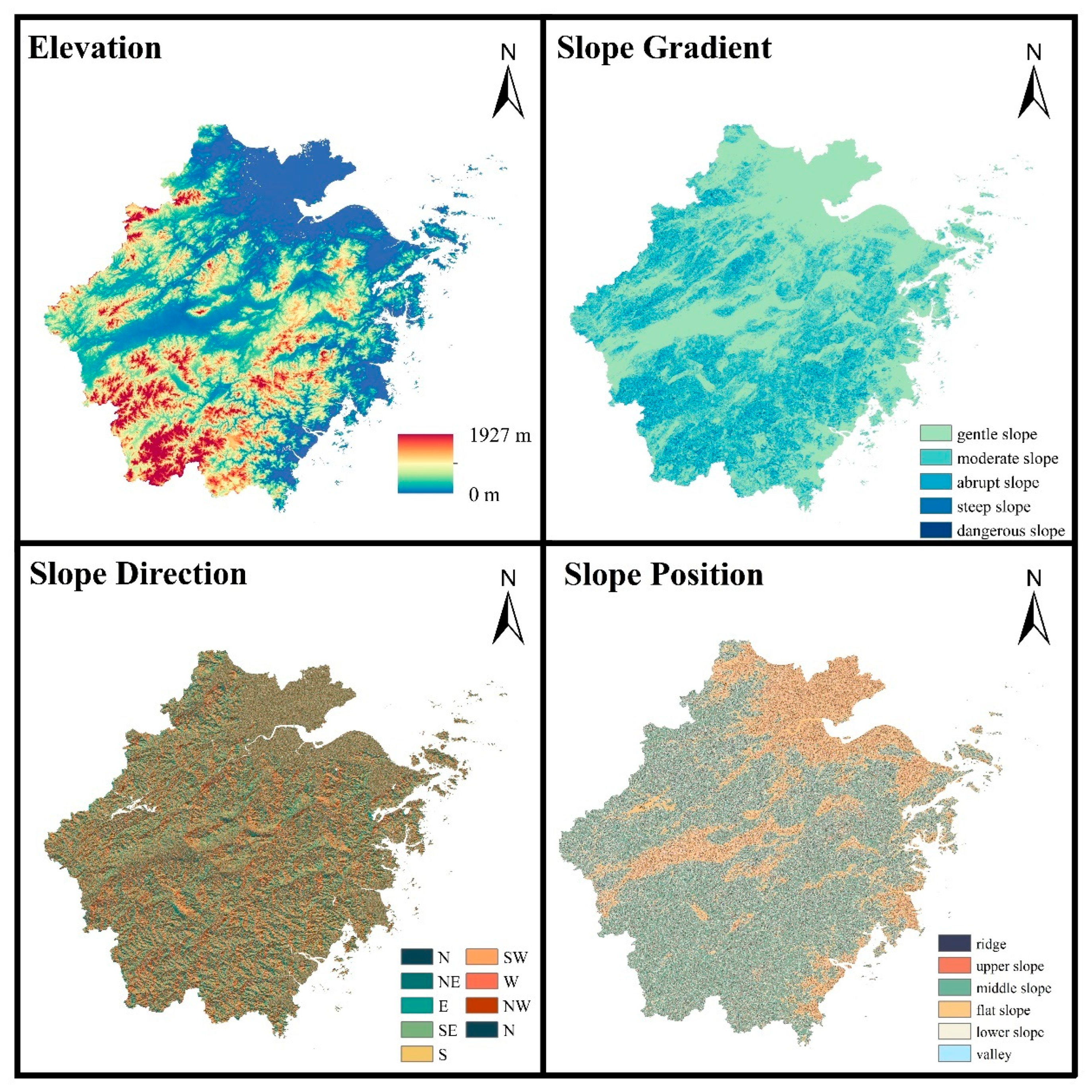
2.1. Study Region
2.2. Data Source
2.3. Sample Treatment and Determination Analysis
2.4. Data Analysis
3. Results
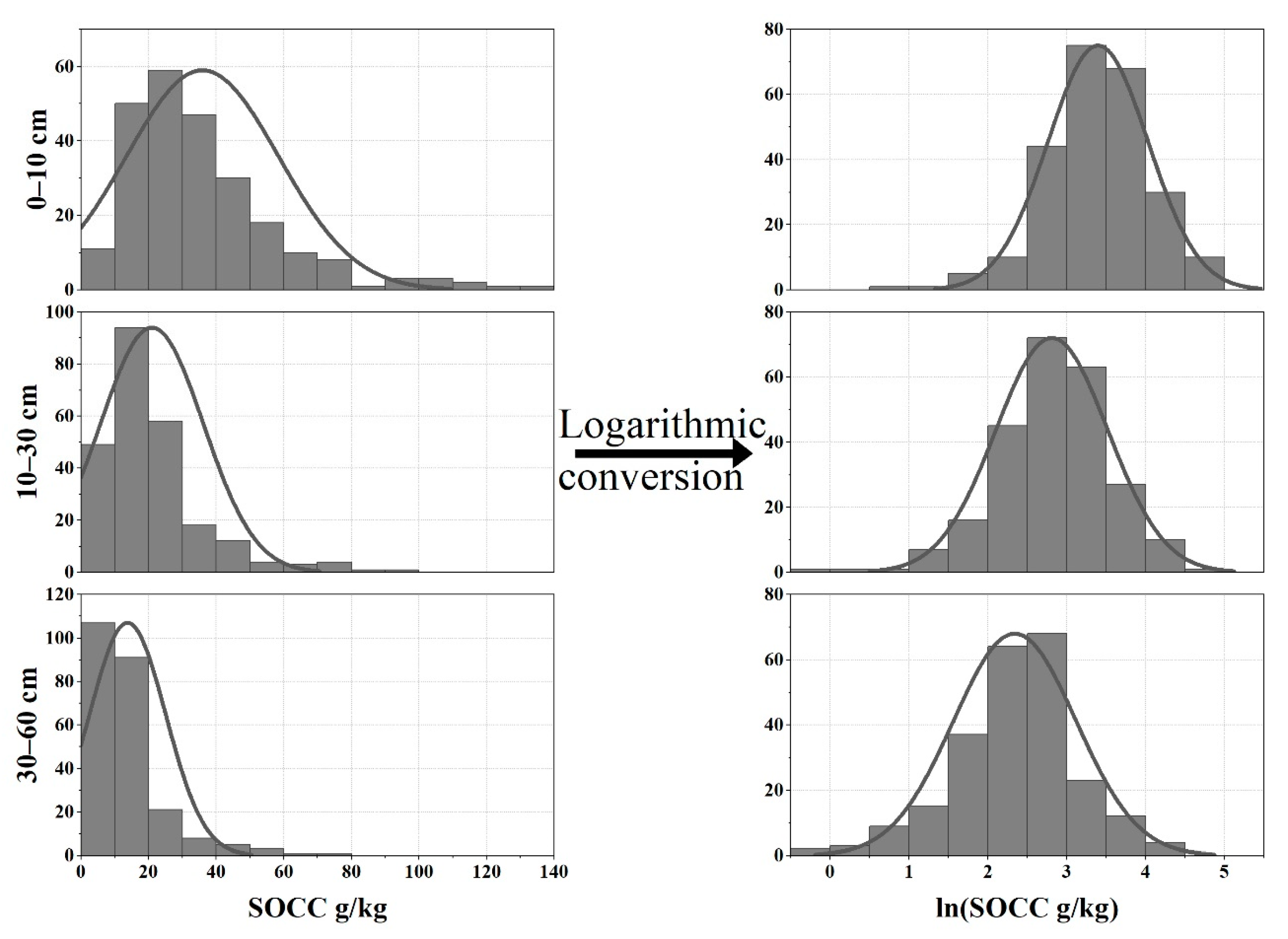
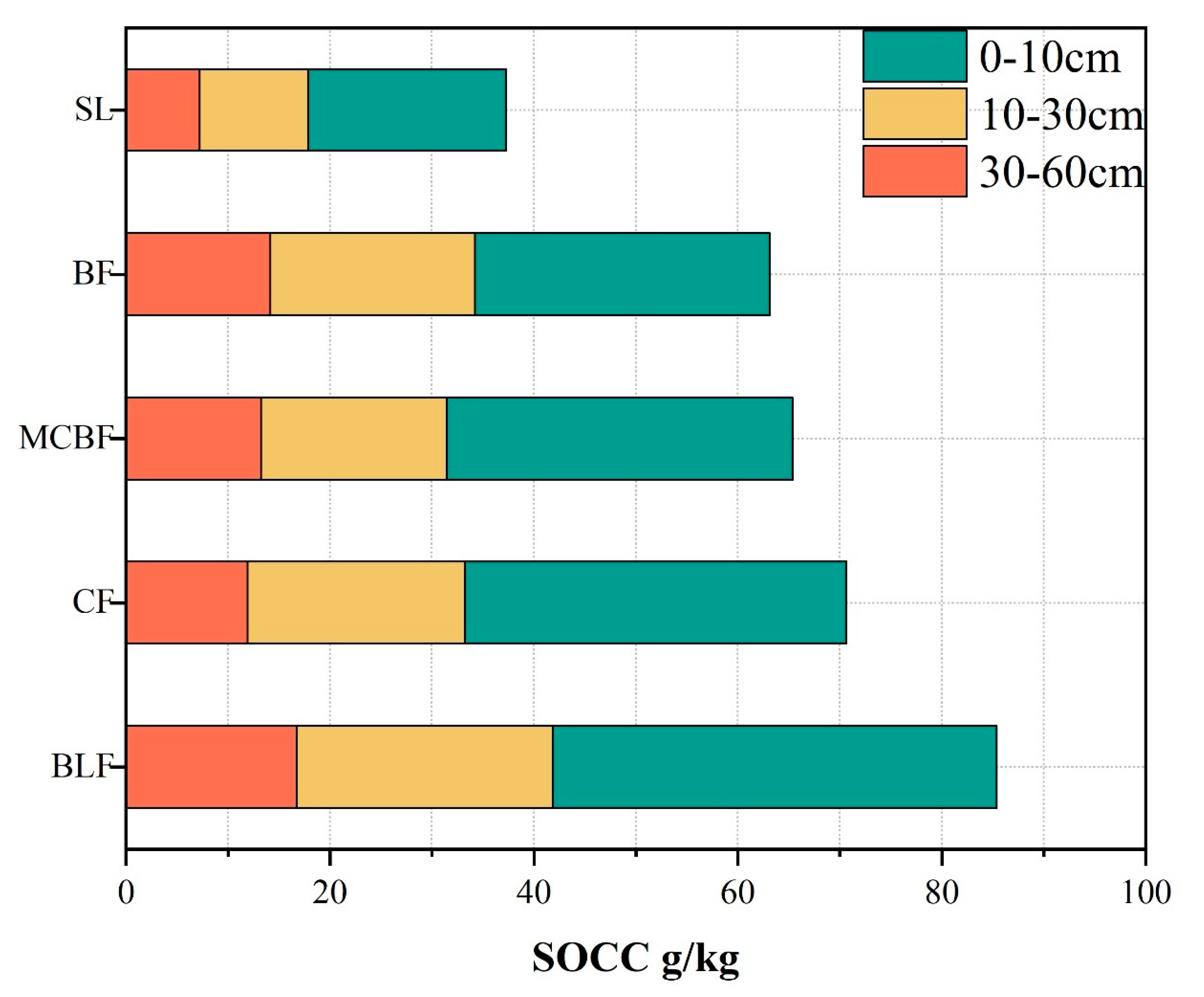
3.1. Characteristics of SOC in Subtropical Forests
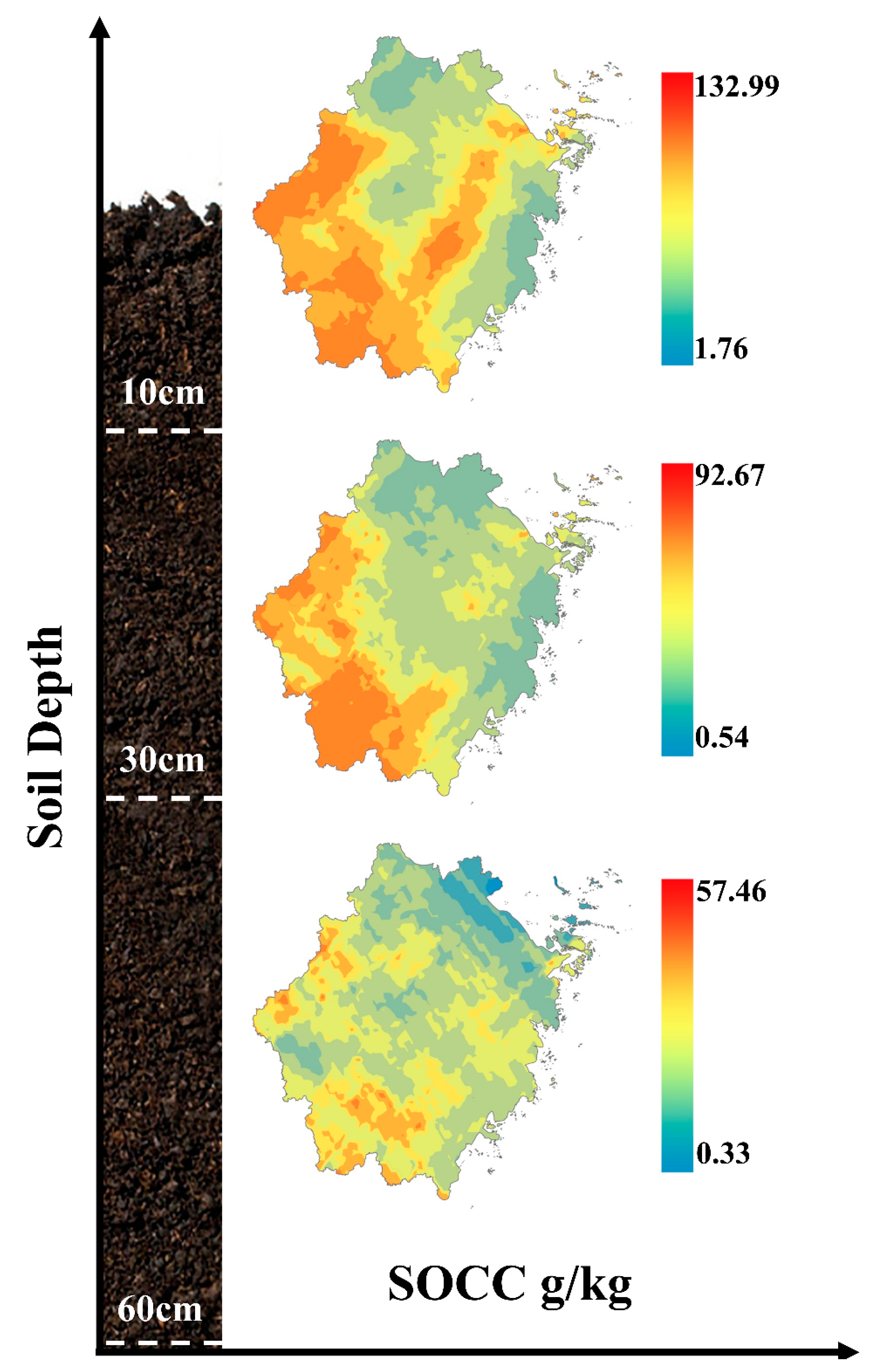
3.2. Spatial Distribution Characteristics of SOC in Subtropical Forests
3.3. Analysis of Topographic Factors of SOC in Subtropical Forests
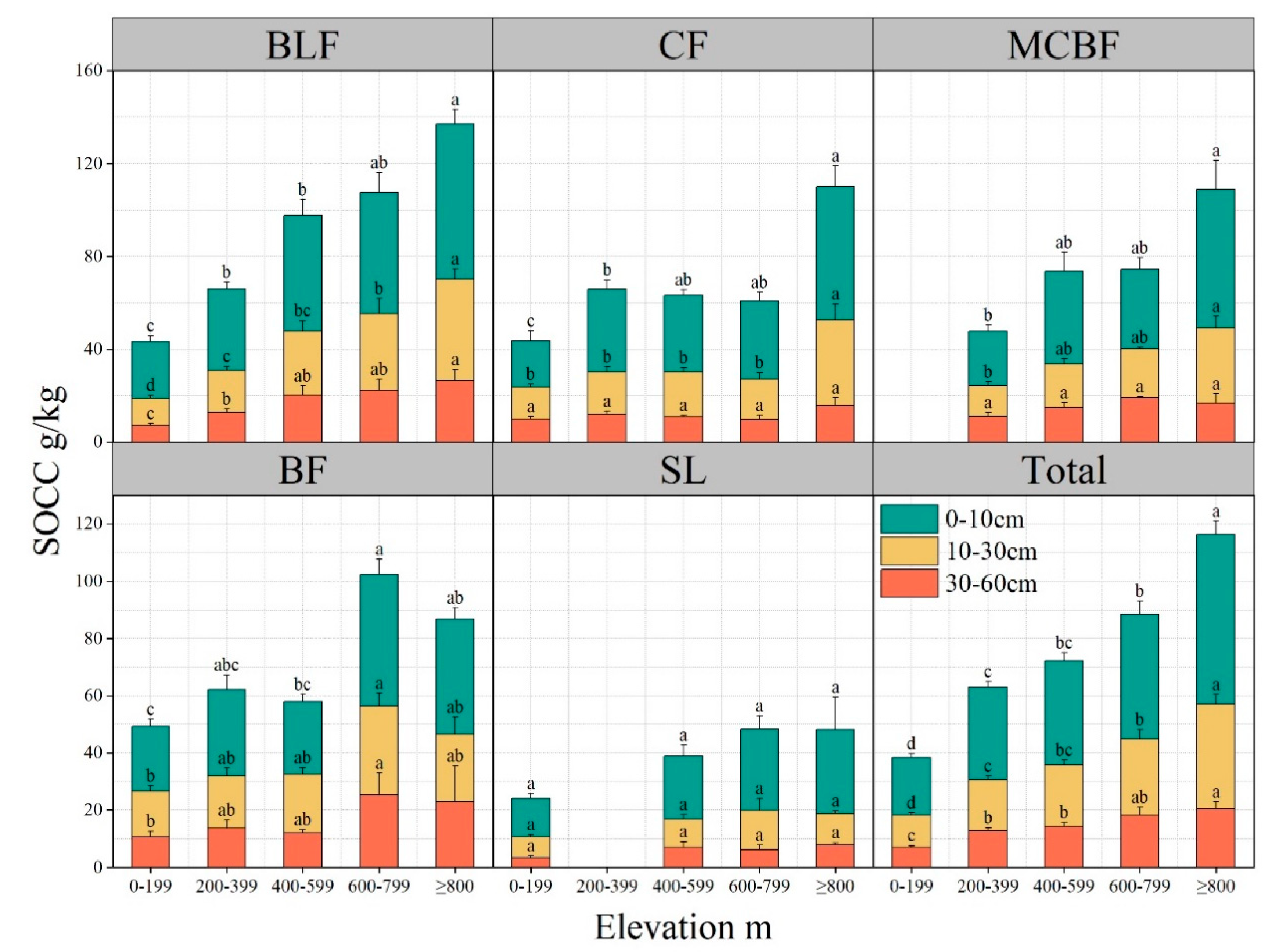
3.3.1. Elevation and Forest Type
3.3.2. Slope Gradient and Forest Type
3.3.3. Slope Position and Forest Type
3.3.4. Slope Direction and Forest Type
3.4. Analysis of Main Control Factors
4. Discussion
4.1. Spatial Distribution of SOC Content in Subtropical Forests
4.2. Topographic Factors Influence SOC Content in Subtropical Forests
4.3. Topographic Factors Influence SOC Spatial Heterogeneity in Subtropical Forests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, Z.; Su, B.; Shangguan, Z. Progress of Research on the Effect of Decomposition of Plant Apoplastic Matter on Soil Organic Carbon Stability. Soil Water Conserv. Res. 2022, 2, 29. [Google Scholar]
- Han, Z.; Zhan, C.; Shi, B.; Wang, L.; Luo, H.; Xu, Q. Effects of Different Treatments of Apoplastic Inputs on Surface Soil Organic Carbon in Warm Temperate Hemlock Forests. Henan For. Sci. Technol. 2022, 2, 42. [Google Scholar]
- Zhou, M.; Xiao, H.; Nie, X.; Li, Z.; Deng, C.; Liu, J.; Zhang, Y. Analysis and Prospect of Soil Organic Carbon Research Process in China and Abroad in the Past 30a. Soil Water Conserv. Res. 2020, 27, 10. [Google Scholar]
- Batjes, N.H. Total Carbon and Nitrogen in the Soils of the World. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Field, C.B. Changes in Ecologically Critical Terrestrial Climate Conditions. Science 2013, 341, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, J.; Meng, M.; Guo, X.; Wu, Y.; Liu, X.; Zhao, K.; Ding, L.; Shao, Y.; Fu, W. Forest-Type Shift and Subsequent Intensive Management Affected Soil Organic Carbon and Microbial Community in Southeastern China. Eur. J. For. Res. 2017, 136, 689–697. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Eisaman, M.D.; Aradó, E.S.P.; Passarini, F.; Wang, T.; Sheehan, S.W. The Role of Carbon Capture, Utilization, and Storage for Economic Pathways That Limit Global Warming to below 1.5 °C. iScience 2022, 25, 104237. [Google Scholar] [CrossRef]
- Chabbi, A.; Rumpel, C.; Grootes, P.M.; González-Pérez, J.A.; Delaune, R.D.; Gonzalez-Vila, F.; Nixdorf, B.; Hüttl, R.F. Lignite Degradation and Mineralization in Lignite-Containing Mine Sediment as Revealed by 14C Activity Measurements and Molecular Analysis. Org. Geochem. 2006, 37, 957–976. [Google Scholar] [CrossRef]
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global Soil Carbon: Understanding and Managing the Largest Terrestrial Carbon Pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Martin, M.P.; Wattenbach, M.; Smith, P.; Meersmans, J.; Jolivet, C.; Boulonne, L.; Arrouays, D. Spatial Distribution of Soil Organic Carbon Stocks in France. Biogeosciences 2011, 8, 1053–1065. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, B.; Hua, K.; Luo, Y.; Zhang, J.; Zhang, A. Assessment of Soil Organic Carbon Stock in the Upper Yangtze River Basin. J. Mt. Sci. 2013, 10, 866–872. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. Will Changes in Soil Organic Carbon Act as a Positive or Negative Feedback on Global Warming? Biogeochemistry 2000, 48, 21–51. [Google Scholar] [CrossRef]
- Ozlu, E.; Arriaga, F.J.; Bilen, S.; Gozukara, G.; Babur, E. Carbon Footprint Management by Agricultural Practices. Biology 2022, 11, 1453. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.H.; Xing, H.X.; Scatena, F.N. Controls on Soil Carbon Stocks in El Yunque National Forest, Puerto Rico. Soil Sci. Soc. Am. J. 2015, 79, 294–304. [Google Scholar] [CrossRef]
- Post, W.M.; Emanuel, W.R.; Zinke, P.J.; Stangenberger, A.G. Soil Carbon Pools and World Life Zones. Nature 1982, 298, 156–159. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil Carbon Stocks and Land Use Change: A Meta Analysis. Glob. Change Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Meentemeyer, V. Macroclimate and Lignin Control of Litter Decomposition Rates. Ecology 1978, 59, 465–472. [Google Scholar] [CrossRef]
- Melillo, J.M.; Aber, J.D.; Muratore, J.F. Nitrogen and Lignin Control of Hardwood Leaf Litter Decomposition Dynamics. Ecology 1982, 63, 621–626. [Google Scholar] [CrossRef]
- Mitra, A.; Banerjee, K.; Sett, S. Spatial Variation in Organic Carbon Density of Mangrove Soil in Indian Sundarbans. Natl. Acad. Sci. Lett. 2012, 35, 147–154. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-Scale Similarities in Nitrogen Release Patterns during Long-Term Decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Doetterl, S.; Berhe, A.A.; Nadeu, E.; Wang, Z.; Sommer, M.; Fiener, P. Erosion, Deposition and Soil Carbon: A Review of Process-Level Controls, Experimental Tools and Models to Address C Cycling in Dynamic Landscapes. Earth-Sci. Rev. 2016, 154, 102–122. [Google Scholar] [CrossRef]
- Seibert, J.; Stendahl, J.; Sørensen, R. Topographical Influences on Soil Properties in Boreal Forests. Geoderma 2007, 141, 139–148. [Google Scholar] [CrossRef]
- Mayes, M.; Marin-Spiotta, E.; Szymanski, L.; Akif Erdoǧan, M.; Ozdoǧan, M.; Clayton, M. Soil Type Mediates Effects of Land Use on Soil Carbon and Nitrogen in the Konya Basin, Turkey. Geoderma 2014, 232–234, 517–527. [Google Scholar] [CrossRef]
- Vasques, G.M.; Grunwald, S.; Comerford, N.B.; Sickman, J.O. Regional Modelling of Soil Carbon at Multiple Depths within a Subtropical Watershed. Geoderma 2010, 156, 326–336. [Google Scholar] [CrossRef]
- Gray, J.M.; Bishop, T.F.A.; Wilson, B.R. Factors Controlling Soil Organic Carbon Stocks with Depth in Eastern Australia. Soil Sci. Soc. Am. J. 2015, 79, 1741–1751. [Google Scholar] [CrossRef]
- Hobley, E.; Wilson, B.; Wilkie, A.; Gray, J.; Koen, T. Drivers of Soil Organic Carbon Storage and Vertical Distribution in Eastern Australia. Plant Soil 2015, 390, 111–127. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil Organic Carbon Storage as a Key Function of Soils—A Review of Drivers and Indicators at Various Scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Chaplot, V.; Bouahom, B.; Valentin, C. Soil Organic Carbon Stocks in Laos: Spatial Variations and Controlling Factors. Glob. Change Biol. 2010, 16, 1380–1393. [Google Scholar] [CrossRef]
- Rossel, R.A.V.; Lee, J.; Behrens, T.; Luo, Z.; Baldock, J.; Richards, A. Continental-Scale Soil Carbon Composition and Vulnerability Modulated by Regional Environmental Controls. Nat. Geosci. 2019, 12, 547–552. [Google Scholar] [CrossRef]
- Sedjo, R.A.; Wisniewski, J.; Sample, A.V.; Kinsman, J.D. The Economics of Managing Carbon via Forestry: Assessment of Existing Studies. Environ. Resour. Econ. 1995, 6, 139–165. [Google Scholar] [CrossRef]
- Dixon, R.K.; Brown, S.; Houghton, R.A.; Solomon, A.M.; Trexler, M.C.; Wisniewski, J. Carbon Pools and Flux of Global Forest Ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, G.; Shi, Y.; Zhou, Y.; Xu, L.; Fan, Y.; Shen, Z.; Li, S.; Lv, Y. Effects of Different Management Measures on the Net Carbon Sink Capacity of Moso Bamboo Forest Ecosystems. For. Sci. 2017, 53, 9. [Google Scholar]
- Yan, L.; Yang, W.; Lin, G.; Dong, P. Impacts of Climate Warming on Forest Ecosystems. Trop. Geogr. 2013, 33, 621–627. [Google Scholar]
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Z.; Piao, S.; Peng, C.; Ciais, P.; Wang, Q.; Lia, X.; Zhu, X. High Carbon Dioxide Uptake by Subtropical Forest Ecosystems in the East Asian Monsoon Region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W. Soil Organic Matter in Major Pedogenic Soil Groups. Geoderma 2021, 384, 114785. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, K.; Jiang, P.; Wu, J.; Fu, W. Revealing Horizontal and Vertical Variation of Soil Organic Carbon, Soil Total Nitrogen and C:N Ratio in Subtropical Forests of Southeastern China. J. Environ. Manag. 2021, 289, 112483. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Xu, W.; Bongers, F.J.; Bao, W.; Chen, B.; Chen, G.; Guo, K.; Lai, J.; Lin, D.; et al. Effects of Diversity, Climate and Litter on Soil Organic Carbon Storage in Subtropical Forests. For. Ecol. Manag. 2020, 476, 118479. [Google Scholar] [CrossRef]
- Chiti, T.; Díaz-Pinés, E.; Rubio, A. Soil Organic Carbon Stocks of Conifers, Broadleaf and Evergreen Broadleaf Forests of Spain. Biol. Fertil. Soils 2012, 48, 817–826. [Google Scholar] [CrossRef]
- Santonja, M.; Pereira, S.; Gauquelin, T.; Quer, E.; Simioni, G.; Limousin, J.M.; Ourcival, J.M.; Reiter, I.M.; Fernandez, C.; Baldy, V. Experimental Precipitation Reduction Slows Down Litter Decomposition but Exhibits Weak to No Effect on Soil Organic Carbon and Nitrogen Stocks in Three Mediterranean Forests of Southern France. Forests 2022, 13, 1485. [Google Scholar] [CrossRef]
- Zhang, F.; Du, Q.; Ge, H.; Liu, A.; Fu, W.; Ji, B. Spatial Distribution of Forest Carbon in Zhejiang Province with Geostatistics Based on CFI Sample Plots. Acta Ecol. Sin. 2012, 32, 5275–5286. [Google Scholar] [CrossRef]
- Dai, W.; Zhao, K.; Fu, W.; Jiang, P.; Li, Y.; Zhang, C.; Gielen, G.; Gong, X.; Li, Y.; Wang, H.; et al. Spatial Variation of Organic Carbon Density in Topsoils of a Typical Subtropical Forest, Southeastern China. Catena 2018, 167, 181–189. [Google Scholar] [CrossRef]
- Fang, H.; Ji, B.; Deng, X.; Ying, J.; Zhou, G.; Shi, Y.; Xu, L.; Tao, J.; Zhou, Y.; Li, C.; et al. Effects of Topographic Factors and Aboveground Vegetation Carbon Stocks on Soil Organic Carbon in Moso Bamboo Forests. Plant Soil 2018, 433, 363–376. [Google Scholar] [CrossRef]
- Fu, W.; Fu, Z.; Ge, H.; Ji, B.; Jiang, P.; Li, Y.; Wu, J.; Zhao, K. Spatial Variation of Biomass Carbon Density in a Subtropical Region of Southeastern China. Forests 2015, 6, 1966–1981. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, Y.; Chang, J.; Jiang, B.; Jiang, H.; Peng, C.; Zhu, J.; Yuan, W.; Qi, L.; Yu, S. Carbon Storage by Ecological Service Forests in Zhejiang Province, Subtropical China. For. Ecol. Manage. 2007, 245, 64–75. [Google Scholar] [CrossRef]
- Wang, T.; Wang, G.; Innes, J.L.; Seely, B.; Chen, B. ClimateAP: An Application for Dynamic Local Downscaling of Historical and Future Climate Data in Asia Pacific. Front. Agric. Sci. Eng. 2017, 4, 448–458. [Google Scholar] [CrossRef]
- Bao, S. Agricultural Chemistry Committee of China Determination of Soil Organic Matter. In Soil and Agricultural Chemistry Analysis; Agricultural Publishing House Agriculture Press: Beijing, China, 2000; pp. 30–34. [Google Scholar]
- Bell, M.J.; Worrall, F. Estimating a Region’s Soil Organic Carbon Baseline: The Undervalued Role of Land-Management. Geoderma 2009, 152, 74–84. [Google Scholar] [CrossRef]
- Johnson, K.D.; Harden, J.; McGuire, A.D.; Bliss, N.B.; Bockheim, J.G.; Clark, M.; Nettleton-Hollingsworth, T.; Jorgenson, M.T.; Kane, E.S.; Mack, M.; et al. Soil Carbon Distribution in Alaska in Relation to Soil-Forming Factors. Geoderma 2011, 167–168, 71–84. [Google Scholar] [CrossRef]
- Blanchet, G.; Libohova, Z.; Joost, S.; Rossier, N.; Schneider, A.; Jeangros, B.; Sinaj, S. Spatial Variability of Potassium in Agricultural Soils of the Canton of Fribourg, Switzerland. Geoderma 2017, 290, 107–121. [Google Scholar] [CrossRef]
- Goovaerts, P. Geostatistics in Soil Science: State-of-the-Art and Perspectives. Geoderma 1999, 89, 1–45. [Google Scholar] [CrossRef]
- Wang, D.D.; Shi, X.Z.; Lu, X.X.; Wang, H.J.; Yu, D.S.; Sun, W.X.; Zhao, Y.C. Response of Soil Organic Carbon Spatial Variability to the Expansion of Scale in the Uplands of Northeast China. Geoderma 2010, 154, 302–310. [Google Scholar] [CrossRef]
- Beillouin, D.; Cardinael, R.; Berre, D.; Boyer, A.; Corbeels, M.; Fallot, A.; Feder, F.; Demenois, J. A Global Overview of Studies about Land Management, Land-Use Change, and Climate Change Effects on Soil Organic Carbon. Glob. Change Biol. 2022, 28, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, S.; Liu, L.; Liu, J.; Ding, X. Organic Fertilization Increased Soil Organic Carbon Stability and Sequestration by Improving Aggregate Stability and Iron Oxide Transformation in Saline-Alkaline Soil. Plant Soil 2022, 474, 233–249. [Google Scholar] [CrossRef]
- Han, J.; Zhang, A.; Kang, Y.; Han, J.; Yang, B.; Hussain, Q.; Wang, X.; Zhang, M.; Khan, M.A. Biochar Promotes Soil Organic Carbon Sequestration and Reduces Net Global Warming Potential in Apple Orchard: A Two-Year Study in the Loess Plateau of China. Sci. Total Environ. 2022, 803, 150035. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Z.; Zhou, Y.; Wang, X.; Zhang, J.; Zhou, X. Characteristics of Soil Organic Carbon under Different Karst Landforms. Carbonates Evaporites 2021, 36, 40. [Google Scholar] [CrossRef]
- Tsozue, D.; Noubissie, N.M.M.; Mamdem, E.L.T.; Basga, S.D.; Oyono, D.L.B. Effects of Environmental Factors and Soil Properties on Soil Organic Carbon Stock in a Natural Dry Tropical Area of Cameroon. SOIL 2021, 7, 677–691. [Google Scholar] [CrossRef]
- Fu, W.J.; Jiang, P.K.; Zhou, G.M.; Zhao, K.L. Using Moran’s i and GIS to Study the Spatial Pattern of Forest Litter Carbon Density in a Subtropical Region of Southeastern China. Biogeosciences 2014, 11, 2401–2409. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, J.; Zhu, L.; Qin, J.; Liu, M.; Shi, J.; Li, G. Revealing the Scale- and Location-Specific Relationship between Soil Organic Carbon and Environmental Factors in China’s North-South Transition Zone. Geoderma 2022, 409, 115600. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Moorman, T.B.; Novak, J.M.; Parkin, T.B.; Karlen, D.L.; Turco, R.F.; Konopka, A.E. Field-Scale Variability of Soil Properties in Central Iowa Soils. Soil Sci. Soc. Am. J. 1994, 58, 1501–1511. [Google Scholar] [CrossRef]
- Li, Y.; Gong, J.; Liu, J.; Hou, W.; Moroenyane, I.; Liu, Y.; Jin, J.; Liu, J.; Xiong, H.; Cheng, C.; et al. Effects of Different Land Use Types and Soil Depth on Soil Nutrients and Soil Bacterial Communities in a Karst Area, Southwest China. Soil Syst. 2022, 6, 20. [Google Scholar] [CrossRef]
- Yu, H.; Zha, T.; Zhang, X.; Ma, L. Vertical Distribution and Influencing Factors of Soil Organic Carbon in the Loess Plateau, China. Sci. Total Environ. 2019, 693, 133632. [Google Scholar] [CrossRef] [PubMed]
- Dad, J.M.; Shafiq, M. ul Spatial Distribution of Soil Organic Carbon in Apple Orchard Soils of Kashmir Himalaya, India. Carbon Manag. 2021, 12, 485–498. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Guo, S.L. Contribution of Root Biomass to Soil Organic Carbon Under Complex Landforms Conditions. Huanjing Kexue/Environ. Sci. 2019, 40, 961–969. [Google Scholar]
- Du, Y.X.; Wu, C.J.; Zhou, S.X.; Huang, L.; Han, S.M.; Xu, X.F.; Ding, Y. Forest Soil Organic Carbon Density and Its Distribution Characteristics along an Altitudinal Gradient in Lushan Mountains of China. Chin. J. Appl. Ecol. 2011, 22, 1675–1681. [Google Scholar]
- Jakšić, S.; Ninkov, J.; Milić, S.; Vasin, J.; Živanov, M.; Jakšić, D.; Komlen, V. Influence of Slope Gradient and Aspect on Soil Organic Carbon Content in the Region of Niš, Serbia. Sustainability 2021, 13, 8332. [Google Scholar] [CrossRef]
- Tu, C.; He, T.; Lu, X.; Luo, Y.; Smith, P. Extent to Which PH and Topographic Factors Control Soil Organic Carbon Level in Dry Farming Cropland Soils of the Mountainous Region of Southwest China. Catena 2018, 163, 204–209. [Google Scholar] [CrossRef]
- Yu, H.; Zha, T.; Zhang, X.; Nie, L.; Ma, L.; Pan, Y. Spatial Distribution of Soil Organic Carbon May Be Predominantly Regulated by Topography in a Small Revegetated Watershed. Catena 2020, 188, 104459. [Google Scholar] [CrossRef]
- Yuan, Z.Q.; Fang, C.; Zhang, R.; Li, F.M.; Javaid, M.M.; Janssens, I.A. Topographic Influences on Soil Properties and Aboveground Biomass in Lucerne-Rich Vegetation in a Semi-Arid Environment. Geoderma 2019, 344, 137–143. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, L.; Su, Z. Changes in Soil Organic Carbon along an Altitudinal Gradient in Subtropical Mountain Forests of Northern Guangdong. J. Ecol. Rural Environ. 2012, 28, 6. [Google Scholar]
- Njeru, C.M.; Ekesi, S.; Mohamed, S.A.; Kinyamario, J.I.; Kiboi, S.; Maeda, E.E. Assessing Stock and Thresholds Detection of Soil Organic Carbon and Nitrogen along an Altitude Gradient in an East Africa Mountain Ecosystem. Geoderma Reg. 2017, 10, 29–38. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Z.; Chen, L.; Wang, J.; Shen, Z. Spatial Distribution Characteristics of Soil Organic Carbon in Subtropical Forests of Mountain Lushan, China. Environ. Monit. Assess. 2018, 190, 545. [Google Scholar] [CrossRef] [PubMed]
- Yüksek, T.; Yüksek, F. Effects of Altitude, Aspect, and Soil Depth on Carbon Stocks and Properties of Soils in a Tea Plantation in the Humid Black Sea Region. L. Degrad. Dev. 2021, 32, 4267–4276. [Google Scholar] [CrossRef]
- Sauer, T.J.; Cambardella, C.A.; Brandle, J.R. Soil Carbon and Tree Litter Dynamics in a Red Cedar-Scotch Pine Shelterbelt. Agrofor. Syst. 2007, 71, 163–174. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Y.; Xu, X.; Ruan, H.; Wang, J. Temperature Sensitivity of Soil Organic Carbon Mineralization along an Elevation Gradient in the Wuyi Mountains, China. PLoS ONE 2013, 8, e53914. [Google Scholar] [CrossRef]
- Tang, X.; Xia, M.; Pérez-Cruzado, C.; Guan, F.; Fan, S. Spatial Distribution of Soil Organic Carbon Stock in Moso Bamboo Forests in Subtropical China. Sci. Rep. 2017, 7, srep42640. [Google Scholar] [CrossRef]
- Ni, H.; Su, W.; Fan, S.; Chu, H. Effects of Intensive Management Practices on Rhizosphere Soil Properties, Root Growth, and Nutrient Uptake in Moso Bamboo Plantations in Subtropical China. For. Ecol. Manag. 2021, 493, 119083. [Google Scholar] [CrossRef]
- Yang, C.; Wang, A.; Zhu, Z.; Lin, S.; Bi, Y.; Du, X. Impact of Extensive Management System on Soil Properties and Carbon Sequestration under an Age Chronosequence of Moso Bamboo Plantations in Subtropical China. For. Ecol. Manag. 2021, 497, 119535. [Google Scholar] [CrossRef]
- Deng, X.; Yin, J.; Xu, L.; Shi, Y.; Zhou, G.; Li, Y.; Chen, G.; Ye, Y.; Zhang, F.; Zhou, Y.; et al. Effects of Abandonment Management on Soil C and N Pools in Moso Bamboo Forests. Sci. Total Environ. 2020, 729, 138949. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Ji, X.; Zhang, Z.; Zhang, H.; Zha, T.; Jiang, L. Elevation and Total Nitrogen Are the Critical Factors That Control the Spatial Distribution of Soil Organic Carbon Content in the Shrubland on the Bashang Plateau, China. Catena 2021, 204, 105415. [Google Scholar] [CrossRef]
- Dad, J.M.; Abdollahi, L. Changes in Soil Organic Carbon, Nitrogen and Sulphur along a Slope Gradient in Apple Orchard Soils of Kashmir Himalaya. J. Mt. Sci. 2021, 18, 2377–2387. [Google Scholar] [CrossRef]
- Hu, W.; Zhai, X.; Du, S.; Zhang, X. Impacts of Slope and Longitudinal Ridge on Soil Organic Carbon Dynamics in the Typical Mollisols Sloping Farmland (China). Eurasian Soil Sci. 2021, 54, 951–963. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, H.; Guo, S. Soil Organic Carbon as a Function of Land Use and Topography on the Loess Plateau of China. Ecol. Eng. 2015, 83, 249–257. [Google Scholar] [CrossRef]
- Zhang, X.; Adamowski, J.F.; Liu, C.; Zhou, J.; Zhu, G.; Dong, X.; Cao, J.; Feng, Q. Which Slope Aspect and Gradient Provides the Best Afforestation-Driven Soil Carbon Sequestration on the China’s Loess Plateau? Ecol. Eng. 2020, 147, 105782. [Google Scholar] [CrossRef]
- Jin, C. A Theoretical Study on Critical Erosion Slope Gradient. Acta Geograohica Sinca 1995, 50, 234–239. [Google Scholar]
- Lin, W.; Cui, X. The Influences of Topographic Factors on Soil Organic Carbon Storage in Cool Conifer Forest in the North of Great Xing ’ an Mountain. Fore. Eng. 2017, 33, 1–6. [Google Scholar] [CrossRef]
- Xue, L.; Xue, Y.; Lie, G.; Ye, L.; Huang, X. Soil Organic Carbon Storage on Different Slope Positions in Cunninghamia Lanceolata Stands. Bull. Soil Water Conserv. 2012, 32, 43–46. [Google Scholar] [CrossRef]
- Jakšić, S.; Ninkov, J.; Milić, S.; Vasin, J.; Živanov, M.; Perović, V.; Banjac, B.; Vučković, S.; Dozet, G.; Komlen, V. Topographic Position, Land Use and Soil Management Effects on Soil Organic Carbon (Vineyard Region of Niš, Serbia). Agronomy 2021, 11, 1438. [Google Scholar] [CrossRef]
- Abebe, G.; Tsunekawa, A.; Haregeweyn, N.; Takeshi, T.; Wondie, M.; Adgo, E.; Masunaga, T.; Tsubo, M.; Ebabu, K.; Berihun, M.L.; et al. Effects of Land Use and Topographic Position on Soil Organic Carbon and Total Nitrogen Stocks in Different Agro-Ecosystems of the Upper Blue Nile Basin. Sustainability 2020, 12, 2425. [Google Scholar] [CrossRef]
- Schöning, I.; Totsche, K.U.; Kögel-Knabner, I. Small Scale Spatial Variability of Organic Carbon Stocks in Litter and Solum of a Forested Luvisol. Geoderma 2006, 136, 631–642. [Google Scholar] [CrossRef]
- Román-Sánchez, A.; Vanwalleghem, T.; Peña, A.; Laguna, A.; Giráldez, J.V. Controls on Soil Carbon Storage from Topography and Vegetation in a Rocky, Semi-Arid Landscapes. Geoderma 2018, 311, 159–166. [Google Scholar] [CrossRef]
- Oueslati, I.; Allamano, P.; Bonifacio, E.; Claps, P. Vegetation and Topographic Control on Spatial Variability of Soil Organic Carbon. Pedosphere 2013, 23, 48–58. [Google Scholar] [CrossRef]


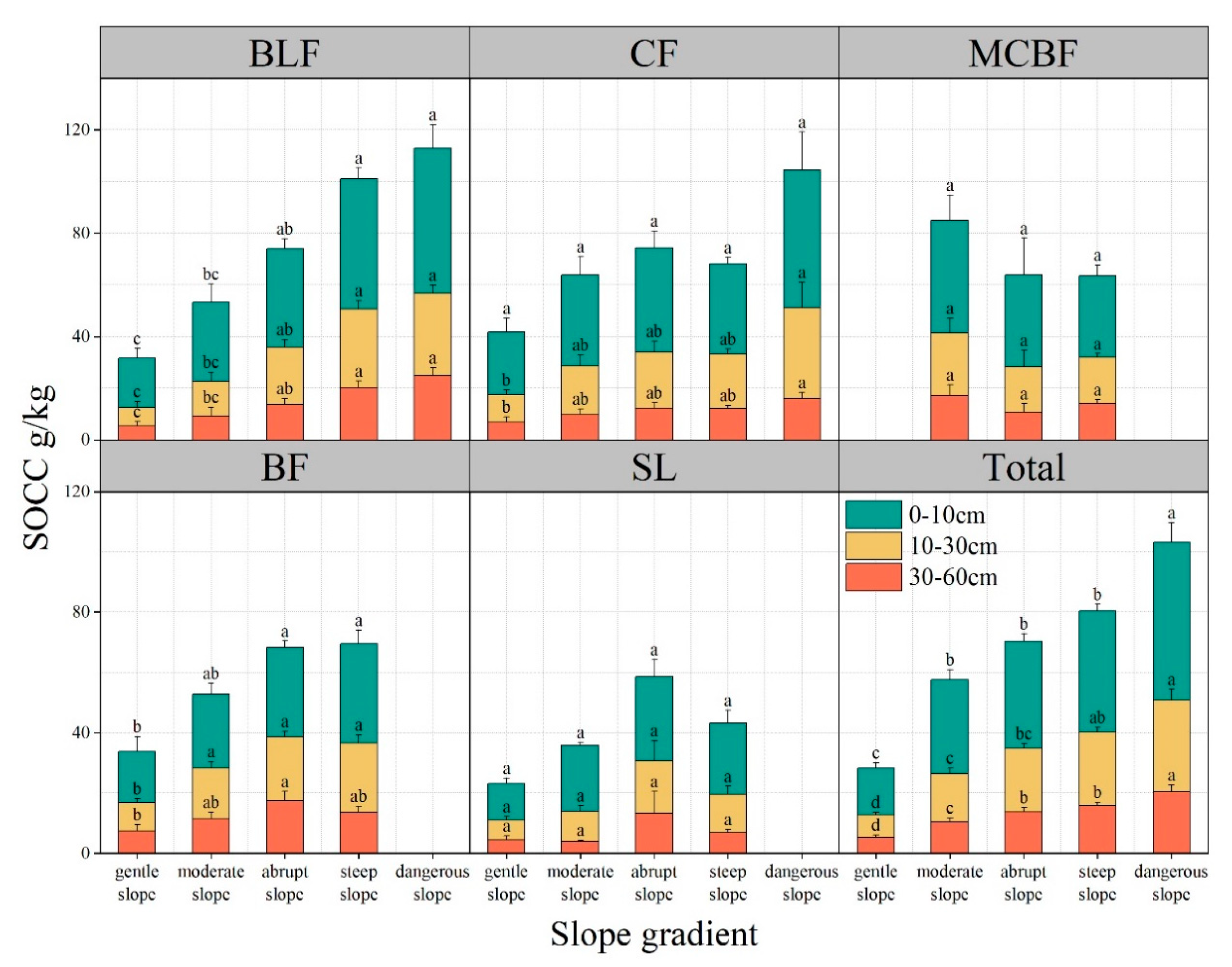
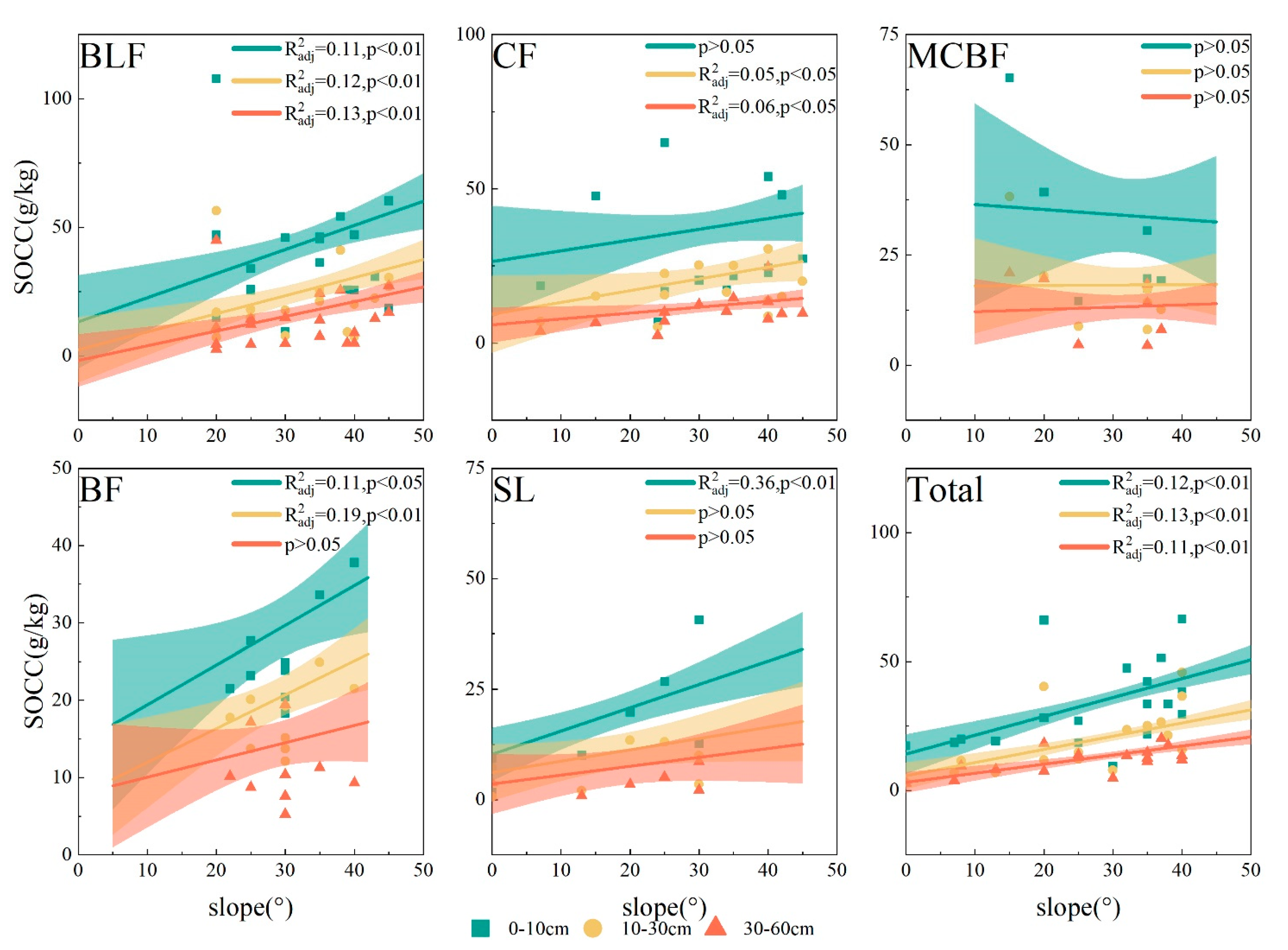
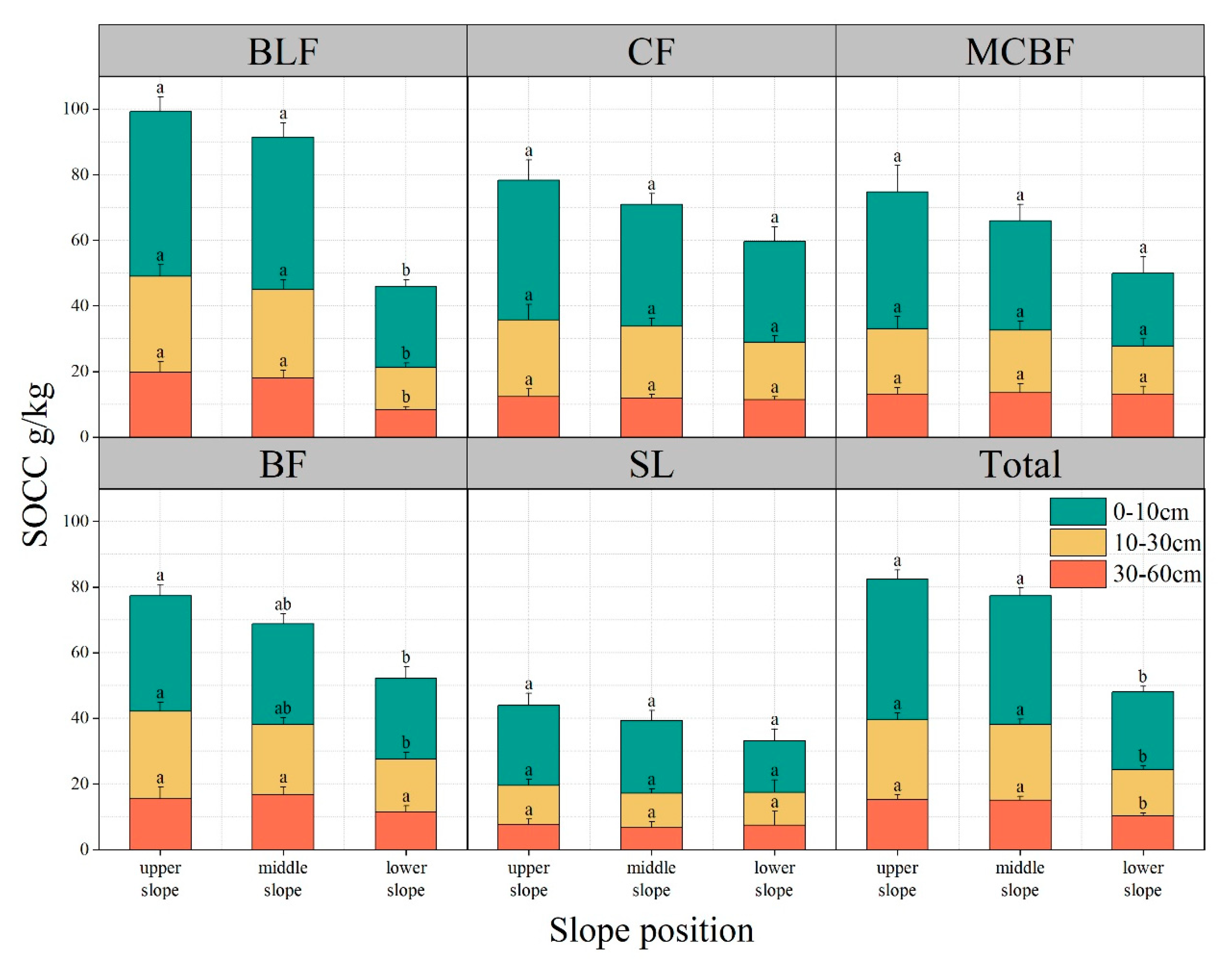

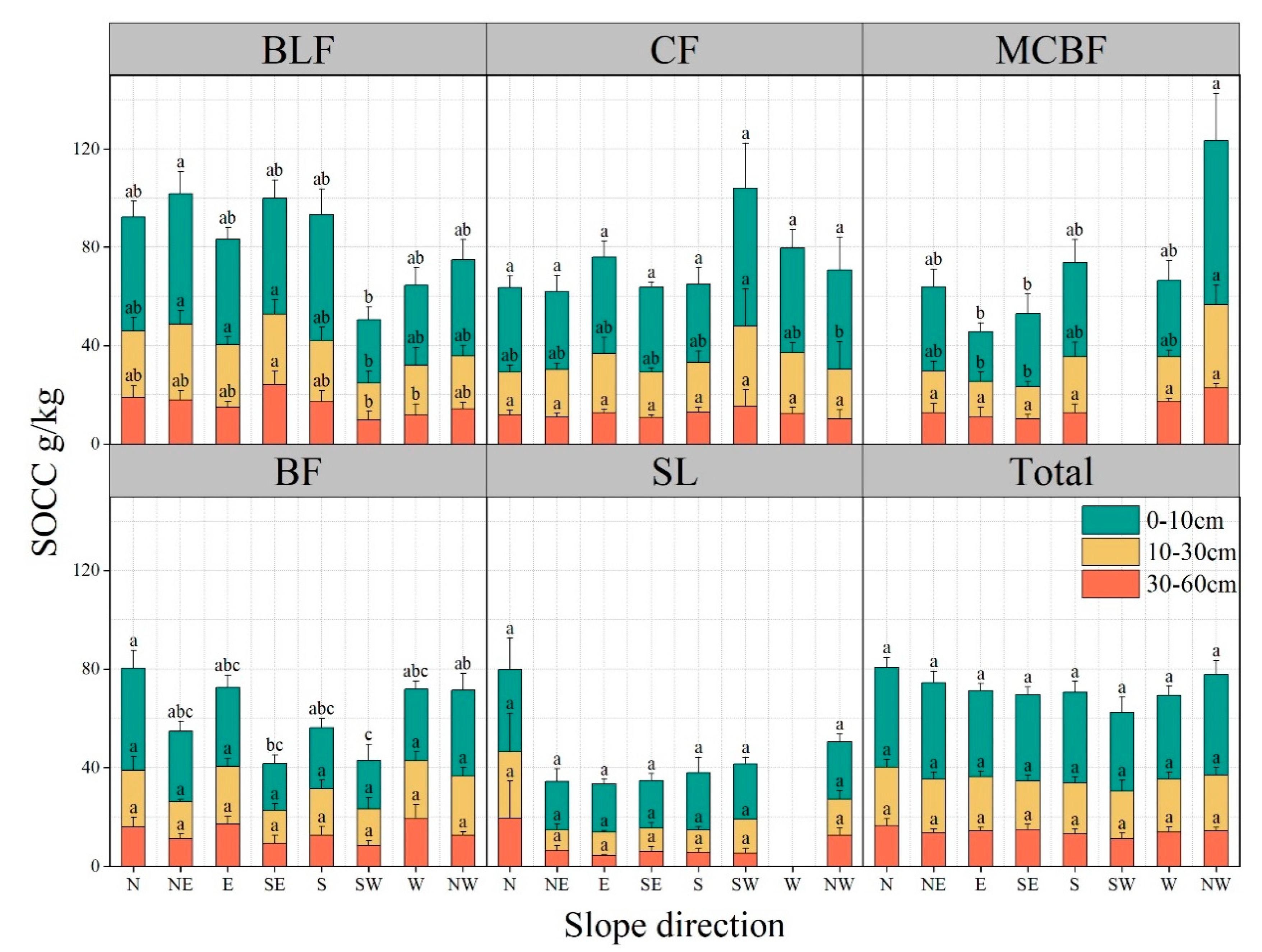

| Forest Type | Elevation (m) | Dominant Species | Soil Type | Soil Moisture (%) |
|---|---|---|---|---|
| BLF | 8–1270 | alder (Alnus cremastogyne Burkill), hackberry (Celtis sinesis Pers.), schima (Schima superba Gardner & Champ.), etc. | red soil, yellow soil, limestone and paddy soil | 7.65–44.10 |
| CF | 2–1550 | masson pine (Pinus massoniana Lamb.), china fir (Cunninghamia lanceolata (Lamb.) Hook.), cypress (Cupressus funebris Endl.), etc. | red soil, yellow soil, and limestone | 12.10–34.96 |
| MCBF | 170–1180 | pine–broadleaf mixed, cypress–broadleaf mixed | red soil and yellow soil | 6.08–36.05 |
| BF | 70–830 | moso bamboo (Phyllostachys edulis (Carrière) J. Houz.), etc. | red soil and yellow soil | 10.93–33.70 |
| SL | 2–980 | pear (Pyrus spp), hickory (Carya cathayensis Sarg.), tea-leaf (Camellia sinensis (L.) Kuntze), etc. | red soil, yellow soil and paddy soil | 15.08–29.68 |
| Depth (cm) | Count | Mean | Median | Maximum | Minimum | SD | CV (%) | K-Sp (p > 0.05) | |
|---|---|---|---|---|---|---|---|---|---|
| SOCC | 0–10 | 244 | 35.95 | 30.40 | 132.99 | 1.76 | 22.58 | 62.81 | 0.200 |
| 10–30 | 244 | 20.98 | 17.44 | 92.67 | 0.67 | 15.26 | 72.74 | 0.099 | |
| 30–60 | 237 | 13.77 | 10.59 | 70.11 | 0.89 | 11.28 | 81.92 | 0.056 | |
| SANC | 0–10 | 244 | 145.42 | 118.86 | 606.23 | 17.39 | 91.32 | 62.79 | 0.201 |
| 10–30 | 244 | 101.61 | 82.17 | 554.20 | 13.94 | 75.16 | 73.97 | 0.086 | |
| 30–60 | 244 | 74.91 | 62.80 | 328.71 | 10.46 | 49.12 | 65.57 | 0.063 |
| Depth (cm) | Model | Nugget Coefficient C0/C0 + C | Range (km) | Radj2 | Residual SS | |
|---|---|---|---|---|---|---|
| SOCC | 0–10 | Exponential | 0.809 | 157.74 | 0.506 | 0.00509 |
| 10–30 | Gaussian | 0.846 | 286.15 | 0.560 | 0.00526 | |
| 30–60 | Gaussian | 0.977 | 11.313 | 0.572 | 0.01810 |
| Topographic Factor | Depth (cm) | p | |
|---|---|---|---|
| SOCC | Elevation | 0–10 | <0.001 |
| 10–30 | <0.001 | ||
| 30–60 | <0.001 | ||
| Slope gradient | 0–10 | <0.001 | |
| 10–30 | <0.001 | ||
| 30–60 | <0.001 | ||
| Slope position | 0–10 | <0.001 | |
| 10–30 | <0.001 | ||
| 30–60 | <0.01 | ||
| Slope direction | 0–10 | >0.05 | |
| 10–30 | >0.05 | ||
| 30–60 | >0.05 |
| Depth (cm) | Topographic Factor | r | Radj2 | p |
|---|---|---|---|---|
| 0–10 | EL | 0.550 | 0.300 | <0.001 |
| SG | 0.462 | 0.210 | <0.001 | |
| SP | 0.372 | 0.134 | <0.001 | |
| SD | 0.070 | 0.001 | 0.282 | |
| FT | 0.372 | 0.135 | <0.001 | |
| EL/FT | 0.582 | 0.333 | <0.001 | |
| EL/FT/SG | 0.611 | 0.366 | <0.001 | |
| 10–30 | EL | 0.534 | 0.282 | <0.001 |
| SG | 0.502 | 0.248 | <0.001 | |
| SP | 0.276 | 0.072 | <0.001 | |
| SD | 0.069 | 0.001 | 0.289 | |
| FT | 0.288 | 0.079 | <0.001 | |
| EL/SG | 0.590 | 0.343 | <0.001 | |
| 30–60 | EL | 0.425 | 0.177 | <0.001 |
| SG | 0.468 | 0.216 | <0.001 | |
| SP | 0.172 | 0.026 | 0.008 | |
| SD | 0.058 | 0.001 | 0.379 | |
| FT | 0.216 | 0.042 | 0.001 | |
| EL/SG | 0.521 | 0.265 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Lv, Y.; Xie, B.; Xu, L.; Zhou, Y.; Mei, T.; Li, Y.; Yuan, N.; Shi, Y. Topography and Soil Organic Carbon in Subtropical Forests of China. Forests 2023, 14, 1023. https://doi.org/10.3390/f14051023
Zhou T, Lv Y, Xie B, Xu L, Zhou Y, Mei T, Li Y, Yuan N, Shi Y. Topography and Soil Organic Carbon in Subtropical Forests of China. Forests. 2023; 14(5):1023. https://doi.org/10.3390/f14051023
Chicago/Turabian StyleZhou, Tao, Yulong Lv, Binglou Xie, Lin Xu, Yufeng Zhou, Tingting Mei, Yongfu Li, Ning Yuan, and Yongjun Shi. 2023. "Topography and Soil Organic Carbon in Subtropical Forests of China" Forests 14, no. 5: 1023. https://doi.org/10.3390/f14051023




