Legacy of Prior Management of Cropland after Afforestation with Populus x euroamericana (Dode) Guinier: Effects on Soil Respiration
Abstract
:1. Introduction
- As changes in land use (cropped vs. forest) affect SOC dynamics, SR will also be affected by change in the same direction.
- Differences in the prior management of soils and in crop rotations (maize–pasture legumes–fallow; maize–fallow) will affect differences in SR between pairs of plots.
- Soil temperature (Ts) and moisture (water) content (W) affect the biological activity and diffusion of gases in the soil, so that the variations in SR will mainly be associated with individual variations in these parameters or their interactions.
2. Materials and Methods
2.1. Description of the Study Site
2.2. Measurement of CO2 Flow
2.3. Soil Parameters
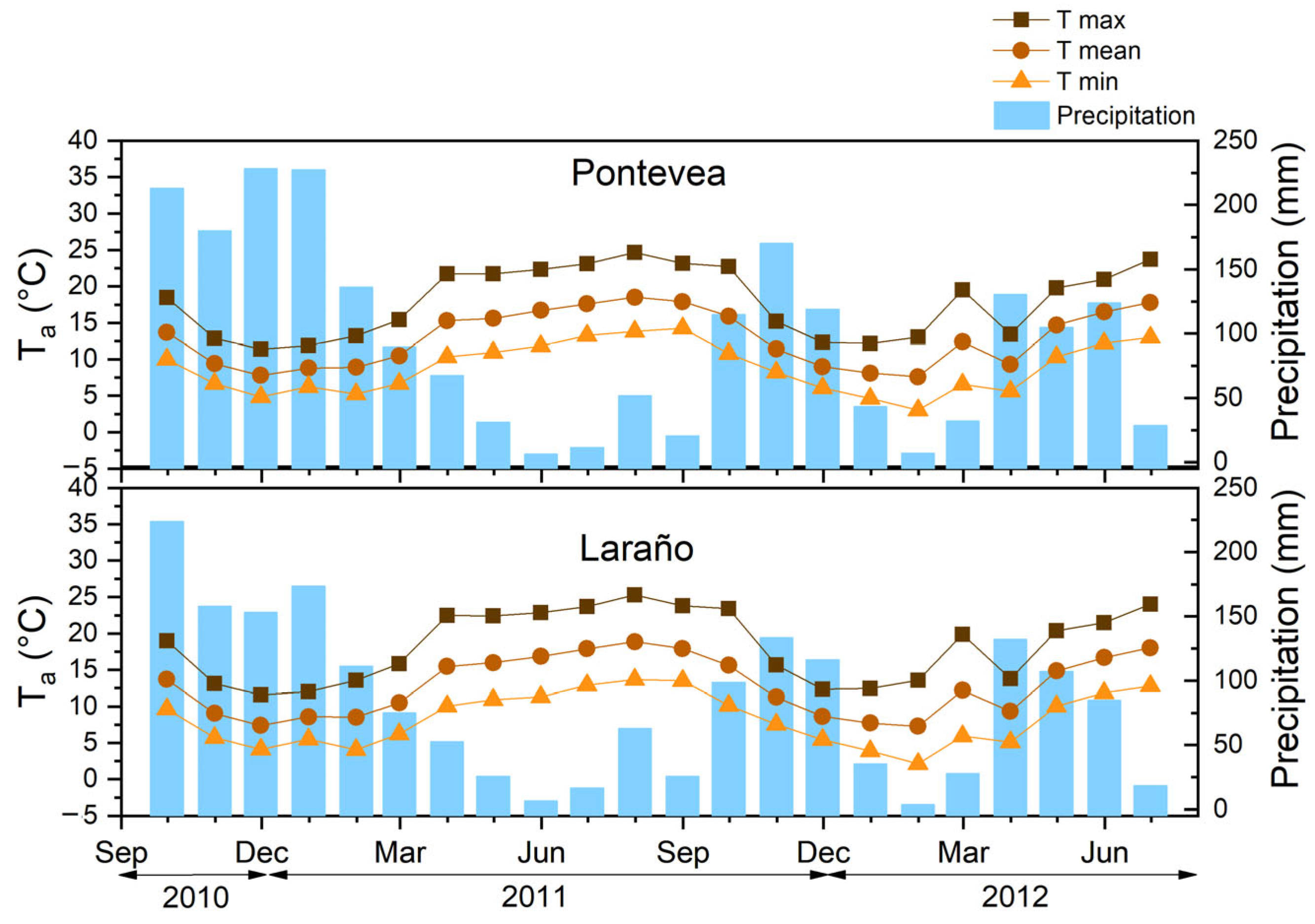
2.4. Climatological Data
2.5. Statistical Analysis
3. Results
3.1. Effect of Afforestation on Soil Characteristics
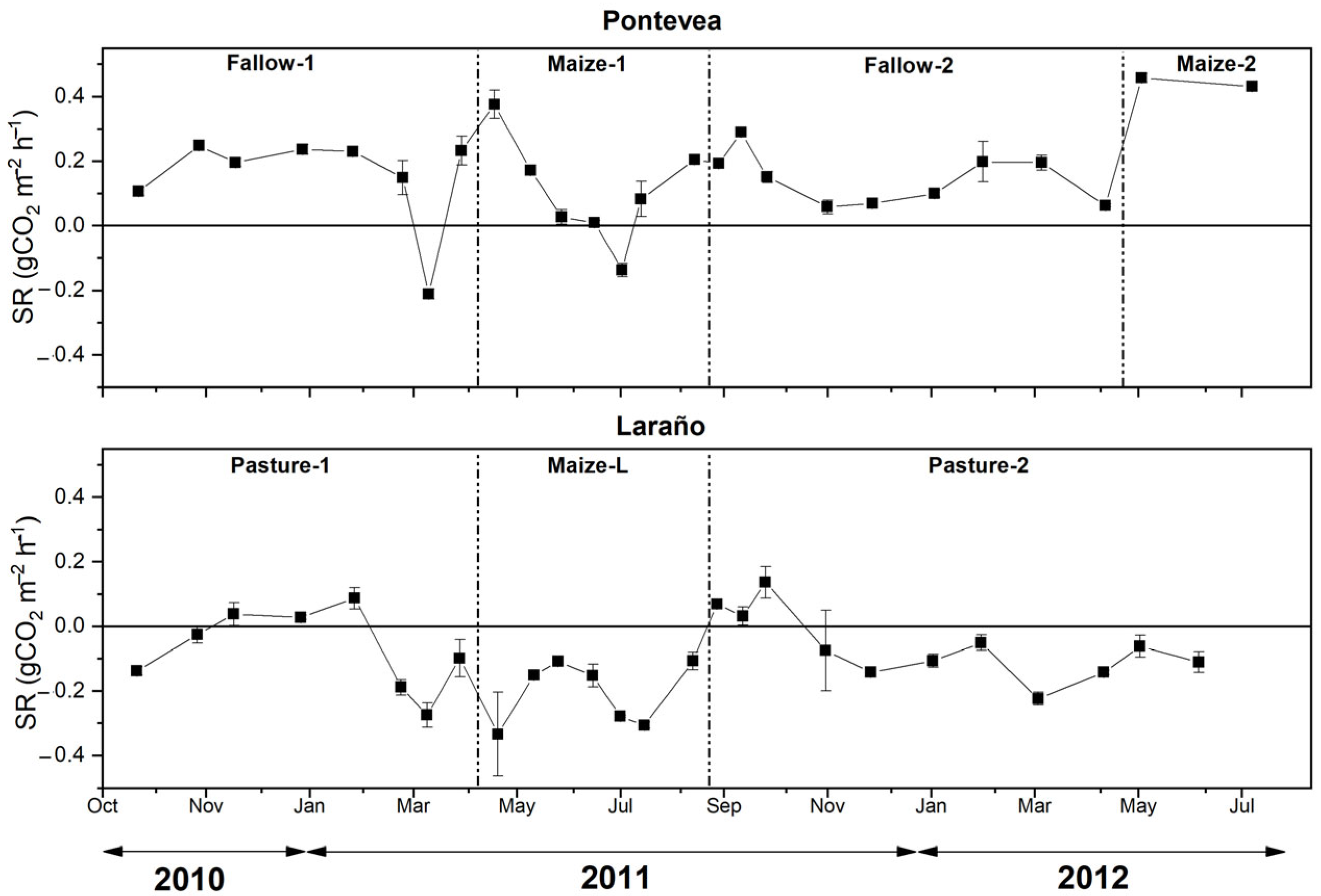
3.2. Temporal and Spatial Variation in Soil Respiration
3.3. Effect of Afforestation on Soil Respiration
3.4. Temporal Variation in Soil Temperature and Moisture Content
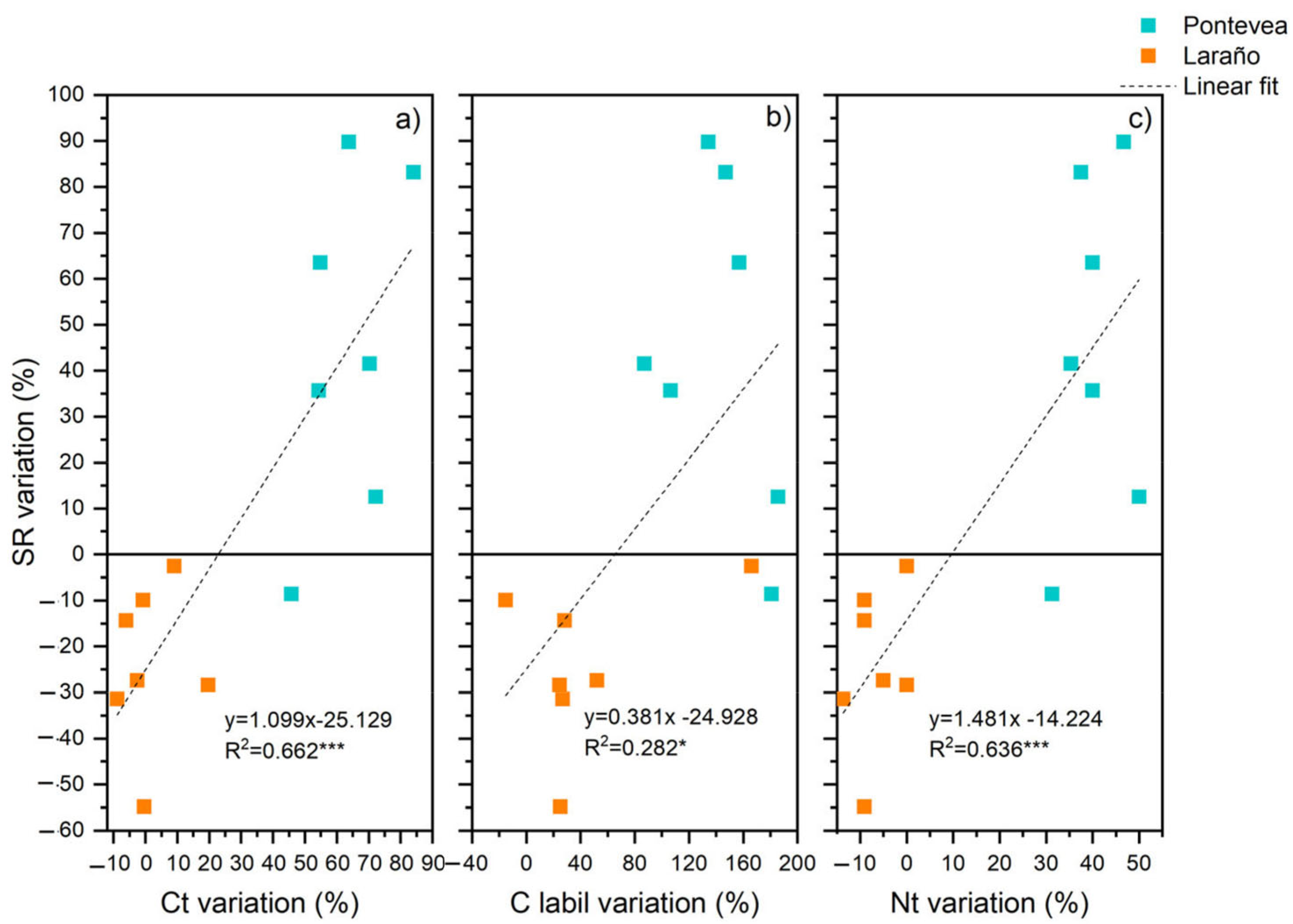
3.5. Relationships between Soil Respiration and Soil Temperature and Moisture Content
4. Discussion
4.1. Effect of Afforestation on Soil Respiration
4.2. Relationship between Soil Respiration and Soil Temperature and Moisture Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirschbaum, M.U.F.; Guo, L.B.; Gifford, R.M. Why does rainfall affect the trend in soil carbon after converting pastures to forests? A possible explanation based on nitrogen dynamics. For. Ecol. Manag. 2008, 255, 2990–3000. [Google Scholar] [CrossRef]
- Aertsens, J.; De Nocker, L.; Gobin, A. Valuing the carbon sequestration potential for European agriculture. Land Use Pol. 2013, 31, 54–94. [Google Scholar] [CrossRef]
- Watson, R.T.; Noble, I.R.; Bolin, B.; Ravindranath, N.H.; Verardo, D.J.; Dokken, D.J. A Special Report of the IPCC. In Land Use, Land-Use Change and Forestry; IPCC: Cambridge, UK, 2000; pp. 1–20. [Google Scholar]
- Trenberth, K.E.; Jones, P.D.; Ambenje, P.; Bojariu, R.; Easterling, D.; Klein Tank, A.; Parker, D.; Rahimzadeh, F.; Renwick, J.A.; Rusticucci, M.; et al. Observations: Surface and Atmospheric climate change. In Climate Change 2007: The Physical Science Basis Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; pp. 237–336. [Google Scholar]
- Ovando, P.; Caparrós, A. Land use and carbon mitigation in Europe: A survey of the potentials of different alternatives. Energy Policy 2009, 37, 992–1003. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks—A meta-analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Meireles, C.I.R.; Gomes, C.J.P.; Ribeiro, N.M.C.A. Forest contribution to climate change mitigation: Management oriented to carbon capture and storage. Climate 2020, 8, 21. [Google Scholar] [CrossRef]
- Xu, X.; Li, D.; Cheng, X.; Ruan, H.; Luo, Y. Carbon: Nitrogen stoichiometry following afforestation: A global synthesis. Sci. Rep. 2016, 6, 19117. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Sensitivity of soil organic carbon stocks and fractions to different land-use changes across Europe. Geoderma 2013, 192, 189–201. [Google Scholar] [CrossRef]
- Amadi, C.C.; Van Rees, K.C.J.; Farrell, R.E. Soil-atmosphere exchange of carbon dioxide, methane and nitrous oxide in shelterbelts compared with adjacent cropped fields. Agric. Ecosyst. Environ. 2016, 223, 123–134. [Google Scholar] [CrossRef]
- Mazza, G.; Agnelli, A.E.; Cantiani, P.; Chiavetta, U.; Doukalianou, F.; Kitikidou, K.; Milios, E.; Orfanoudakis, M.; Radoglou, K.; Lagomarsino, A. Short-term effects of thinning on soil CO2, N2O and CH4 fluxes in Mediterranean forest ecosystems. Sci. Total Environ. 2019, 651, 713–724. [Google Scholar] [CrossRef]
- Prentice, I.C.; Farquhar, G.D.; Fasham, M.J.R.; Goulden, M.L.; Heimann, M.; Jaramillo, V.J.; Kheshgi, H.S.; Le Quéré, C.; Scholes, R.J.; Wallace, D.W.R. The carbon cycle and atmospheric carbon dioxide. In Climate Change 2001: The Scientific Basis; Pitelka, L., Ramirez Rojas, A., Eds.; Cambidge University Press: Cambridge, UK, 2001; pp. 183–237. [Google Scholar]
- Da Silva Teixeira, R.; Cardoso Fialho, R.; Costa, D.C.; Nogueira de Sousa, R.; Silva Santos, R.; Mendes Teixeira, A.P.; Guimarães Reis, T.; Ribeiro da Silva, I. Land-use change with pasture and short rotation eucalypts impacts the soil C emissions and organic C stocks in the Cerrado biome. Land Degrad. Dev. 2019, 31, 909–923. [Google Scholar] [CrossRef]
- Qubaja, R.; Tatarinov, F.; Rotenberg, E.; Yakir, D. Partitioning of canopy and soil CO2 fluxes in a pine forest at the dry timberline across a 13-year observation period. Biogeosciences 2020, 17, 699–714. [Google Scholar] [CrossRef]
- Dhadli, H.S.; Brar, B.S.; Black, T.A. Influence of crop growth and weather variables on soil CO2 emissions in a maize-wheat cropping system. Agric. Res. J. 2015, 52, 28. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Moyano, F.E.; Manzoni, S.; Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 2013, 59, 72–85. [Google Scholar] [CrossRef]
- Nazaries, L.; Tottey, W.; Robinson, L.; Khachane, A.; Al-Soud, W.A.; Sørensen, S.; Singh, B.K. Shifts in the microbial community structure explain the response of soil respiration to land-use change but not to climate warming. Soil Biol. Biochem. 2015, 89, 123–134. [Google Scholar] [CrossRef]
- Lal, R. Offsetting global CO2 emissions by restoration of degraded soils and intensification of world agriculture and forestry. Land Degrad. Dev. 2003, 322, 309–322. [Google Scholar] [CrossRef]
- Barretto de Figueiredo, E.; Panosso, A.R.; de Oliveira Bordonal, R.; De Bortoli Teixeira, D.; Berchielli, T.T.; La Scala, N., Jr. Soil CO2–C emissions and correlations with soil properties in degraded and managed pastures in Southern Brasil. Land Degrad. Dev. 2016, 28, 1263–1273. [Google Scholar] [CrossRef]
- Berthrong, S.T.; Piñeiro, G.; Jobbágy, E.G.; Jackson, R.B. Soil C and N changes with afforestation of grasslands across gradients of precipitation and plantation age. Ecol. Appl. 2012, 22, 76–86. [Google Scholar] [CrossRef]
- Mäkipää, R.; Abramoff, R.; Adamczyk, B.; Baldy, V.; Biryol, C.; Bosela, M.; Casals, P.; Curiel Yuste, J.; Dondini, M.; Filipek, S.; et al. How does management affect soil C sequestration and greenhouse gas fluxes in boreal and temperate forests?—A review. For. Ecol. Manag. 2023, 529, 120637. [Google Scholar] [CrossRef]
- Thomas, A.D.; Stringer, L.C.; Dougill, A.J.; Elliott, D.R.; Robert, S.; Robin, H. The influence of trees, shrubs, and grasses on microclimate, soil carbon, nitrogen, and CO2 efflux: Potential implications of shrub encroachment for Kalahari rangelands. Land Degrad. Dev. 2018, 29, 1306–1316. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S.; Gil-Sotres, F. Biochemical properties in managed grassland soils in a temperate humid zone: Modifications of soil quality as a consequence of intensive grassland use. Biol. Fertil. Soils 2009, 45, 711–722. [Google Scholar] [CrossRef]
- Peichl, M.; Leava, N.A.; Kiely, G. Above- and belowground ecosystem biomass, carbon and nitrogen allocation in recently afforested grassland and adjacent intensively managed grassland. Plant Soil 2012, 350, 281–296. [Google Scholar] [CrossRef]
- Díaz-Fierros, F.; Gil-Sotres, F. Capacidad Productiva de los Suelos de Galicia, Mapa 1.200.000; Editorial USC: Santiago de Compostela, Spain, 1984; 82p, +maps. [Google Scholar]
- ISSS Working Group WRB. World Reference Base for Soil Resources 2006; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2006; 132p.
- García-Campos, E.; Zorita, F.; Leirós, M.C.; Gil-Sotres, F.; Trasar-Cepeda, C. Evaluation of C Stocks in Afforested High Quality Agricultural Land. Forests 2022, 13, 2055. [Google Scholar] [CrossRef]
- Soil Survey Laboratory Staff (SSLS). Soil Survey Field and Laboratory Methods Manual, Version 2.0; Soil; USDA-NRCS: Washington, DC, USA, 2014; 257p.
- Guitián-Ojea, F.; Carballas-Fernández, T. Técnicas de Análisis de Suelos, 2nd ed.; Editorial Pico Sacro: Santiago de Compostela, Spain, 1976; 288p. [Google Scholar]
- USDA. Soil Survey Manual. Agriculture Handbook No. 18; USDA: Washington, DC, USA, 1952; Volume 34, 145p.
- Huang, Z.; Xu, Z.; Chen, C. Effect of mulching on labile soil organic matter pools, microbial community functional diversity and nitrogen transformations in two hardwood plantations of subtropical Australia. Appl. Soil Ecol. 2008, 40, 229–239. [Google Scholar] [CrossRef]
- Krause, K.; Niklaus, P.A.; Schleppi, P. Soil-atmosphere fluxes of the greenhouse gases CO2, CH4 and N2O in a mountain spruce forest subjected to long-term N addition and to tree girdling. Agric. For. Meteorol. 2013, 181, 61–68. [Google Scholar] [CrossRef]
- Ota, M.; Yamazawa, H. Forest floor CO2 flux estimated from soil CO2 and radon concentrations. Atmos. Environ. 2010, 44, 4529–4535. [Google Scholar] [CrossRef]
- Jacinthe, P.A. Carbon dioxide and methane fluxes in variably-flooded riparian forests. Geoderma 2015, 241–242, 41–50. [Google Scholar] [CrossRef]
- Nkongolo, N.V.; Hatano, R.; Kakembo, V. Diffusivity Models and Greenhouse Gases Fluxes from a Forest, Pasture, Grassland and Corn Field in Northern Hokkaido, Japan. Pedosphere 2010, 20, 747–760. [Google Scholar] [CrossRef]
- Schwen, A.; Jeitler, E.; Böttcher, J. Spatial and temporal variability of soil gas diffusivity, its scaling and relevance for soil respiration under different tillage. Geoderma 2015, 259–260, 323–336. [Google Scholar] [CrossRef]
- Bosco, S.; Volpi, I.; Antichi, D.; Ragaglini, G.; Frasconi, C. Greenhouse gas emissions from soil cultivated with vegetables in crop rotation under integrated, organic and organic conservation management in a Mediterranean environment. Agronomy 2019, 9, 446. [Google Scholar] [CrossRef]
- Raich, J.W.; Potter, C.S.; Bhagawati, D. Interannual variability in global soil respiration 1980–94. Glob. Chang. Biol. 2002, 8, 800–812. [Google Scholar] [CrossRef]
- Guttières, R.; Nunan, N.; Raynaud, X.; Lacroix, G.; Barot, S.; Barré, P.; Girardin, C.; Guenet, B.; Lata, J.-C.; Abbadie, L. Temperature and soil management effects on carbon fluxes and priming effect intensity. Soil Biol. Biochem. 2021, 153, 108103. [Google Scholar] [CrossRef]
- Curtin, D.; Wang, H.; Selles, F.; McConkey, B.G.; Campbell, C.A. Tillage Effects on Carbon Fluxes in Continuous Wheat and Fallow-Wheat Rotations. Soil Sci. Soc. Am. J. 2000, 64, 2080–2086. [Google Scholar] [CrossRef]
- Yao, Z.; Wolf, B.; Chen, W.; Butterbach-Bahl, K.; Brüggemann, N.; Wiesmeier, M.; Dannenmann, M.; Blank, B.; Zheng, X. Spatial variability of N2O, CH4 and CO2 fluxes within the Xilin River catchment of Inner Mongolia, China: A soil core study. Plant Soil 2010, 331, 341–359. [Google Scholar] [CrossRef]
- Gonsiorkiewicz Rigon, J.P.; Calonego, J.C.; Rosolem, C.A.; La Scala, N., Jr. Cover crop rotations in no-till system: Short-term CO2 emissions and soybean yield. Sci. Agric. 2018, 75, 18–26. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Cheng, K.; Yue, Q.; Chadwick, D.; Espenberg, M.; Truu, J.; Rees, R.M.; Smith, P. A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob. Chang. Biol. 2019, 25, 2530–2543. [Google Scholar] [CrossRef]
- Rahman, M.A.; Moser, A.; Rötzer, T.; Pauleit, S. Microclimatic differences and their influence on transpirational cooling of Tilia cordata in two contrasting street canyons in Munich, Germany. Agric. For. Meteorol. 2017, 232, 443–456. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssen, I.A.; Luo, Y. On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Glob. Chang. Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Martins, C.S.C.; Nazaries, L.; Delgado-Baquerizo, M.; Macdonald, C.A.; Anderson, I.C.; Hobbie, S.E.; Venterea, R.T.; Reich, P.B.; Singh, B.K. Identifying environmental drivers of greenhouse gas emissions under warming and reduced rainfall in boreal–temperate forests. Funct. Ecol. 2017, 31, 2356–2368. [Google Scholar] [CrossRef]
- Juhász, C.; Huzsvai, L.; Kovács, E.; Kovács, G.; Tuba, G.; Sinka, L.; Zsembeli, J. Carbon Dioxide Efflux of Bare Soil as a Function of Soil Temperature and Moisture Content under Weather Conditions of Warm, Temperate, Dry Climate Zone. Agronomy 2022, 12, 3050. [Google Scholar] [CrossRef]
- Mcknight, J.Y.; Harden, C.P.; Schaeffer, S.M. Soil CO2 flux trends with differences in soil moisture among four types of land use in an Ecuadorian páramo landscape. Phys. Geogr. 2016, 38, 51–61. [Google Scholar] [CrossRef]







| Crop Soil | Afforested Soil | |||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SD | %CV | Min | Max | Mean ± SD | %CV | |
| PONTEVEA SOIL | ||||||||
| pH H2O | 5.08 | 5.62 | 5.33 ± 0.18 A | 3.3 | 5.26 | 5.46 | 5.40 ± 0.07 A | 1.32 |
| pH KCl | 4.32 | 4.46 | 4.38 ± 0.05 A | 1.1 | 4.27 | 4.45 | 4.33 ± 0.07 A | 1.6 |
| TOTAL C (%) | 1.44 | 1.99 | 1.69 ± 0.18 A | 10.6 | 2.4 | 3.26 | 2.75 ± 0.31 B | 11.1 |
| TOTAL N (%) | 0.14 | 0.16 | 0.15 ± 0.01 A | 5.1 | 0.21 | 0.24 | 0.22 ± 0.01 B | 5.2 |
| C/N | 9 | 14 | 11 ± 2 A | 14.4 | 11 | 15 | 13 ± 1 B | 8.7 |
| LABILE C (mg 100 g−1) | 49 | 70 | 59 ± 8 A | 13.3 | 116 | 177 | 142 ± 25 B | 17.3 |
| BULK DENSITY (g cm−3) | 1.04 ± 0.02 A | 0.95 ± 0.07 B | ||||||
| LARAñO SOIL | ||||||||
| pH H2O | 4.61 | 5.51 | 5.22 ± 0.34 A | 6.6 | 5.81 | 6.07 | 5.95 ± 0.10 B | 1.6 |
| pH KCl | 4.12 | 4.52 | 4.24 ± 0.13 A | 3.2 | 4.76 | 5.06 | 4.89 ± 0.10 B | 2.1 |
| TOTAL C (%) | 2.34 | 2.71 | 2.54 ± 0.12 A | 4.8 | 2.28 | 3.09 | 2.57 ± 0.28 A | 11.0 |
| TOTAL N (%) | 0.2 | 0.23 | 0.22 ± 0.01 A | 4.4 | 0.19 | 0.23 | 0.20 ± 0.02 A | 7.4 |
| C/N | 11 | 13 | 12 ± 1 A | 4.7 | 12 | 14 | 13 ± 1 B | 6.4 |
| LABILE C (mg 100 g−1) | 50 | 139 | 92 ± 27 A | 28.8 | 101 | 134 | 121 ± 12 B | 9.7 |
| BULK DENSITY (g cm−3) | 1.03 ± 0.02 A | 0.96 ± 0.05 B | ||||||
| Study Plot | Annual CO2 Emissions (kg CO2 kg−1 Ct) | |||
|---|---|---|---|---|
| Vegetation Cover | ||||
| Maize | Fallow | Pasture | ||
| AF-P | 1.95 | - | - | - |
| AF-L | 1.88 | - | - | - |
| CT-P | 2.09 | 2.79 | 1.59 | - |
| CT-L | 2.18 | 2.71 | - | 1.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priano, M.E.; Zorita, F.; Trasar-Cepeda, C. Legacy of Prior Management of Cropland after Afforestation with Populus x euroamericana (Dode) Guinier: Effects on Soil Respiration. Forests 2023, 14, 1048. https://doi.org/10.3390/f14051048
Priano ME, Zorita F, Trasar-Cepeda C. Legacy of Prior Management of Cropland after Afforestation with Populus x euroamericana (Dode) Guinier: Effects on Soil Respiration. Forests. 2023; 14(5):1048. https://doi.org/10.3390/f14051048
Chicago/Turabian StylePriano, María Eugenia, Félix Zorita, and Carmen Trasar-Cepeda. 2023. "Legacy of Prior Management of Cropland after Afforestation with Populus x euroamericana (Dode) Guinier: Effects on Soil Respiration" Forests 14, no. 5: 1048. https://doi.org/10.3390/f14051048





