A Re-Inventory after 12 Years—Increase in Red Wood Ant Nests and Woodpecker Cavities in Nests in the West Eifel Volcanic Field despite Climatic Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Geologic Setting
2.2. Forest Owners
2.3. Standardized, Integrated Mapping Approach and Data Collection
2.4. Definition of Variable Site Factors
2.5. Data Analysis
3. Results
3.1. Physical Nest Parameters
3.2. Type and Composition of Forest Stands, Tree Age, and Clearing Plots
3.3. Herbaceous Layer
3.4. Woodpecker Cavities
4. Discussion
4.1. Pre-Requisites for (Re-)inventories of RWA Nests
4.2. Interconnection of Forest Composition, Physical RWA Nest Parameters, and Clearings
4.2.1. Tree Species and Age
4.2.2. Clearings
4.2.3. Herbaceous Layer
4.3. Interconnection of RWA Nests and Woodpeckers
4.4. GeoBio-Interactions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Descombes, P.; Leprieur, F.; Albouy, C.; Heine, C.; Pellissier, L. Spatial imprints of plate tectonics on extant richness of terrestrial vertebrates. J. Biogeogr. 2017, 44, 1185–1197. [Google Scholar] [CrossRef]
- Frouz, J.; Jilkova, V. The effect of ants on soil properties and processes (Hymenoptera: Formicidae). Myrm. News 2008, 11, 191–199. [Google Scholar]
- Frouz, J.; Rybnicek, M.; Cudlin, P.; Chmelikova, E. Influence of the wood ant Formica polyctena on soil nutrient and the spruce tree growth. J. Appl. Entom. 2008, 132, 281–284. [Google Scholar] [CrossRef]
- Robinson, E.J.H.; Stockan, J.A.; Iason, G.R. Wood Ants and Their Interaction with Other Organisms. In Wood Ant Ecology and Conservation; Stockan, J.A., Robinson, E.J.H., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 177–206. [Google Scholar]
- Berberich, G.M.; Berberich, M.B. Comparison of Geogases in Two Cenozoic Sedimentary Basins. Geosciences 2022, 12, 388. [Google Scholar] [CrossRef]
- Berberich, G.M.; Berberich, M.B.; Gibhardt, M. Red wood Ants (Formica rufa-group) prefer mature pine forests in Variscan granite environments(Hymenoptera: Formicidae). Fragm. Entomo. 2022, 54, 1–18. [Google Scholar] [CrossRef]
- Berberich, G.M.; Klimetzek, D.; Paraschiv, M.; Stancioiu, P.T.; Grumpe, A. Biogeostatistics confirm: Even a low total number of red wood ant nests provide new information on tectonics in the East Carpathian Orogen (Romania). Ecol. Indic. 2019, 101, 486–500. [Google Scholar] [CrossRef]
- Berberich, G.M.; Berberich, M.B.; Ellison, A.M.; Wöhler, C. Degassing Rhythms and Fluctuations of Geogenic Gases in A Red Wood-Ant Nest and in Soil in The Neuwied Basin (East Eifel Volcanic Field, Germany. Insects 2018, 9, 135. [Google Scholar] [CrossRef]
- Berberich, G.M.; Ellison, A.M.; Berberich, M.B.; Grumpe, A.; Becker, A.; Wöhler, C. Can a red wood-ant nest be a trap for fault-related CH4 micro-seepage? A case study from continuous short-term in-situ sampling. Animals 2018, 8, 46. [Google Scholar] [CrossRef]
- Berberich, G.M.; Dormann, C.F.; Klimetzek, D.; Berberich, M.B.; Sanders, N.J.; Ellison, A.M. Detection probabilities for sessile organisms. Ecosphere 2016, 7, e01546. [Google Scholar] [CrossRef]
- Berberich, G.M.; Sattler, T.; Klimetzek, D.; Benk, S.A.; Berberich, M.B.; Polag, D.; Schöler, H.F.; Atlas, E. Halogenation processes linked to red wood ant nests (Formica spp.) and tectonics. J. Atm. Chem. 2016, 74, 261–281. [Google Scholar] [CrossRef]
- Berberich, G.M.; Grumpe, A.; Berberich, M.B.; Klimetzek, D.; Wöhler, C. Are red wood ants (Formica rufa-group) tectonic indicators? A statistical approach. Ecol. Indic. 2016, 6, 968–979. [Google Scholar] [CrossRef]
- Berberich, G.; Klimetzek, D.; Wöhler, C.; Grumpe, A. Statistical Correlation between Red Wood Ant Sites and Tectonically Active Fault Structures. Mitt. Dtsch. Ges. Allg. Angew. Ent. 2014, 19, 45–50. [Google Scholar]
- Berberich, G. Identifikation Junger gasführender Störungszonen in der West- und Hocheifel mit Hilfe von Bioindikatoren. Ph.D. Thesis, University of Duisburg-Essen, Essen, Germany, 2010. [Google Scholar]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total fl ying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809-21. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.R.; Berenbaum, M.R.; Stopakd, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Nat. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef] [PubMed]
- Crist, T.O. Biodiversity, species interactions and functional role of ants (Hymenoptera: Formicidae) in fragmented landscapes: A review. Myrmec. News 2009, 12, 3–13. [Google Scholar]
- Çamlitepe, Y.; Aksoy, V. Distribution and conservation status of the European red wood ant species Formica pratensis. J. Entomol. Res. Soc. 2019, 21, 199–211. [Google Scholar]
- Stoschek, N.; Roch, T. Zentrale Erfassung von Waldameisen im Freistaat Sachsen. AFZ-Der Wald 2006, 61, 186–188. [Google Scholar]
- Wilson, P. Wood Ants of Wyre. Wyre For. Study Group Rev. 2011, 2011, 17–22. [Google Scholar]
- Sturm, P.; Distler, H. Rote Liste gefährdeter Ameisen (Hymenoptera: Formicoidea) Bayerns. BayLfU 2003, 166, 208–212. [Google Scholar]
- BNatSchG. Gesetz über Naturschutz und Landschaftspflege (Bundesnaturschutzgesetz-BNatSchG) vom 29. Juli 2009 (BGBl. I S.2542/FNA 791-9). Available online: https://www.gesetze-im-internet.de/bnatschg_2009/BJNR254210009.html (accessed on 6 February 2023).
- BArtSchV. Verordnung zum Schutz Wild Lebender Tier- und Pflanzenarten. Bundesartenschutzverordnung (BArtSchV) vom 16. Februar 2005 (BGBl. I S. 258, 896), Die Zuletzt Durch Artikel 10 des Gesetzes vom 21. Januar 2013 (BGBl. I S. 95) Geändert Worden Ist. Available online: https://www.gesetze-im-internet.de/bartschv_2005/BArtSchV.pdf (accessed on 6 February 2023).
- AP Insektenschutz, 2019. Aktionsprogramm Insektenschutz der Bundesregierung–Gemeinsam Wirksam Gegen das Insektensterben. Drucksache 19/13031. 09.09. 2019. Available online: https://dserver.bundestag.de/btd/19/130/1913031.pdf (accessed on 6 February 2023).
- Schmincke, H.U. The Quaternary Volcanic Fields of the East and West Eifel (Germany). In Mantle Plumes—A Multidisciplinary Approach; Ritter, R., Christensen, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 241–322. [Google Scholar]
- KWIS-RLP. Klimawandelinformationssystem Rheinland-Pfalz. 2021. Available online: www.kwis-rlp.de/anpassungsportal/regionale-informationen/osteifel/ (accessed on 22 October 2021).
- Data Europe EU: Digital Elevation Model über Europa aus dem GMES RDA-Projekt (EU-DEM) ist ein Digital SurfaceModel (DSM), The Official Portal for European Data 2021. Available online: https://land.copernicus.eu/imagery-in-situ/eu-dem/eu-dem-v1-0-and-derived-products/slope (accessed on 20 June 2021).
- Keil, P.; Storch, D.; Jetz, W. On the decline of biodiversity due to area loss. Nat. Com. 2015, 6, 8837. [Google Scholar] [CrossRef]
- Balzani, P.; Dekoninck, W.; Feldhaar, H.; Freitag, A.; Frizzi, F.; Frouz, J.; Masoni, A.; Robinson, E.; Sorvari, J.; Santini, G. Challenges and a call to action for protecting European red wood ants. Conserv. Biol. 2022, 36, e13959. [Google Scholar] [CrossRef] [PubMed]
- Reimann, H. Zustand eines Waldameisenvorkommens 2010 und 2021. Ameisenschutz Aktuell 2021, 35, 74–78. [Google Scholar]
- Sondeij, I.; Domisch, T.; Finér, L.; Czechowski, W. Wood ants in the Białowieża Forest and factors affecting their distribution. Ann. Zool. Fen. 2018, 55, 103–114. [Google Scholar] [CrossRef]
- Klimetzek, D.; Stancioiu, P.T.; Paraschiv, M.; Nita, M.D. Ecological Monitoring with Spy Satellite Images—The Case of Red Wood Ants in Romania. Remote Sens. 2021, 13, 520. [Google Scholar] [CrossRef]
- Véle, A.; Frouz, J. Bark Beetle Attacks Reduce Survival of Wood Ant Nests. Forests 2023, 14, 199. [Google Scholar] [CrossRef]
- Bär, R.; (Ameisenschutzwarte NRW, Siegen, Germany). Personal communication, 2022.
- Robinson, N.A.; Robinson, E.J.H. The population of the red wood ant Formica rufa L. (Hymenoptera: Formicidae) at Gait Barrows National Nature Reserve, Lancashire, England over the 20 year period 1986–2006: Nest longevity, reproduction and the effect of management. Br. J. Entomol. Nat. Hist. 2008, 21, 225–241. [Google Scholar]
- Dekoninck, W.; Hendrickx, F.; Grootaert, P.; Maelfait, J.-P. Present conservation status of red wood ants in northwestern Belgium: Worse than previously, but not a lost cause. Eur. J. Entomol. 2010, 107, 209–218. [Google Scholar] [CrossRef]
- Mabelis, A.A.; Korczyńska, J. Long term impact of agriculture on the survival of wood ants of the Formica rufa group (Formicidae). J. Insect Conserv. 2016, 20, 621–628. [Google Scholar] [CrossRef]
- Van Buggenum, H.J.M. Presence after three decades of red wood ants (Formica rufa group; Hymenoptera: Formicidae) in forests in an agricultural landscape. Eur. J. Entomol 2022, 119, 85–91. [Google Scholar] [CrossRef]
- Bretz, D. Gewaltiger Waldameisenrückgang im Forstamt Weilburg: Dramatische Bilanz nach 25 Jahren Waldameisen-Kartierung. Ameisenschutz Aktuell 2020, 92–110. [Google Scholar]
- BMEL. Der Wald in Deutschland. Ausgewählte Ergebnisse der Dritten Bundeswaldinventur. Bundesministerium für Ernährung und Landwirtschaft (BMEL). 2023. Available online: www.bundeswaldinventur.de (accessed on 6 February 2023).
- Domisch, T.; Finér, L.; Jurgensen, M.F. Red wood ant mound densities in managed boreal forests. Ann. Zool. Fenn. 2005, 42, 277–282. [Google Scholar]
- MUFV. Waldzustandsbericht 2010. Ministerium für Umwelt, Forsten und Verbraucherschutz (MUFV). 2010. Available online: www.mufv.rlp.de (accessed on 6 February 2023).
- MKUEM Waldzustandsbericht 2021. (Ministerium für Klimaschutz, Umwelt, Energie und Mobilität). 2021, p. 82. Available online: www.mkuem.rlp.de (accessed on 6 February 2023).
- KHVO Eifel. Newsletter Nr. 2. Dezember 2019. Available online: www.holzvermarktung-eifel.de (accessed on 6 February 2023).
- Hýsek, Š.; Löwe, R.; Turcáni, M. What Happens to Wood after a Tree Is Attacked by a Bark Beetle? Forests 2021, 12, 1163. [Google Scholar] [CrossRef]
- Adlung, K.G. A Critical Evaluation of the European Research on Use of Red Wood Ants (Formica rufa Group) for the Protection of Forests against Harmful Insects. Z. Angew. Entom. 1966, 57, 167–189. [Google Scholar] [CrossRef]
- Seifert, B. Formica nigricans Emery 1909–An ecomorph of Formica pratensis Retzius, 1783 (Hymenoptera, Formicidae). Entomol. Fenn. 1992, 2, 217–226. [Google Scholar] [CrossRef]
- Gilliam, F.S. The Ecological Significance of the Herbaceous Layer in Temperate Forest Ecosystems. BioScience 2007, 57, 10. Available online: https://academic.oup.com/bioscience/article/57/10/845/232416 (accessed on 6 February 2023). [CrossRef]
- BMEL. Ergebnisse der Waldzustandserhebung 2020. Bundesministerium für Ernährung und Landwirtschaft (BMEL). 2021, p. 72. Available online: www.bmel.de (accessed on 6 February 2023).
- Zimmerer, V. Erfolgsmelder im Waldnaturschutz. Digitale Ausgabe Bayerisches Landwirtschaftliches Wochenblatt BLW 25-2021. Available online: www.digitalmagazin.de/marken/blw/hauptheft/2021-25/wald/026_erfolgsmelder-im-waldnaturschutz (accessed on 6 February 2023).
- Wübbenhorst, J.; Südbeck, P. Woodpeckers as Indicators for Sustainable Forestry? First Results of a Study in the EU/LIFE–Demonstration Areas Lüneburger Heide und Solling. Demonstration of Methods to Monitor Sustainable Forestry. 2001. EU/LIFE Project 1998–2001 (LIFE98ENV/S/000478). Available online: https://vdocument.in/woodpeckers-as-indicators-for-sustainable-forestryj-woodpeckers-as-indicators.html?page=1 (accessed on 9 June 2018).
- Froehlich-Schmitt, B. Spechte in der Hördter Rheinaue nach 40 Jahren. 28. Jahrestagung der Fachgruppe Spechte. Ornithol. Anz. 2018, 57, 67–70. [Google Scholar]
- Del Toro, I.; Berberich, G.M.; Ribbons, R.R.; Berberich, M.B.; Sanders, N.J.; Ellison, A.M. Nests of red wood ants (Formica rufa-group) are positively associated with tectonic faults: A double-blind test. PeerJ 2017, 5, e3903. [Google Scholar] [CrossRef]
- Kreemer, C.; Blewitt, G.; Davis, P.M. Geodetic evidence for a buoyant mantle plume beneath the Eifel volcanic area, NW Europe. Geophys. J. Int. 2020, 222, 1316–1332. [Google Scholar] [CrossRef]
- Wolf, C. Copernicus-Dienst Rhein-Mosel. Copernicus-Dienst zur Unterstützung von Gefährdungsanalysen und Regionalplanung im Rhein-Mosel-Gebiet; BGR-Nr. 05-3039-01; Bundesanstalt für Geowissenschaften und Rohstoffe (BGR): Hannover, Germany, 2016. [Google Scholar]
- BfS. GEOPORTAL. Bundesamt für Strahlenschutz (BfS), Salzgitter. 2020. Available online: www.imis.bfs.de/geoportal/ (accessed on 4 July 2021).
- Kemski, J.; Klingel, R.; Siehl, A.; Neznal, M.; Matolin, M. Erarbeitung Fachlicher Grundlagen zur Beurteilung der Vergleichbarkeit Unterschiedlicher Messmethoden zur Bestimmung der Radonbodenluftkonzentration. Vorhaben 3609S10003. Bd. 2 Sachstandsbericht “Radonmessungen in der Bodenluft. Einflussfaktoren, Messverfahren, Bewertung”. 2012. BfS-RESFOR-63/12-Bd.2. urn:nbn:de:0221-201203237830. Available online: https://doris.bfs.de/jspui/handle/urn:nbn:de:0221-201203237830 (accessed on 6 February 2023).


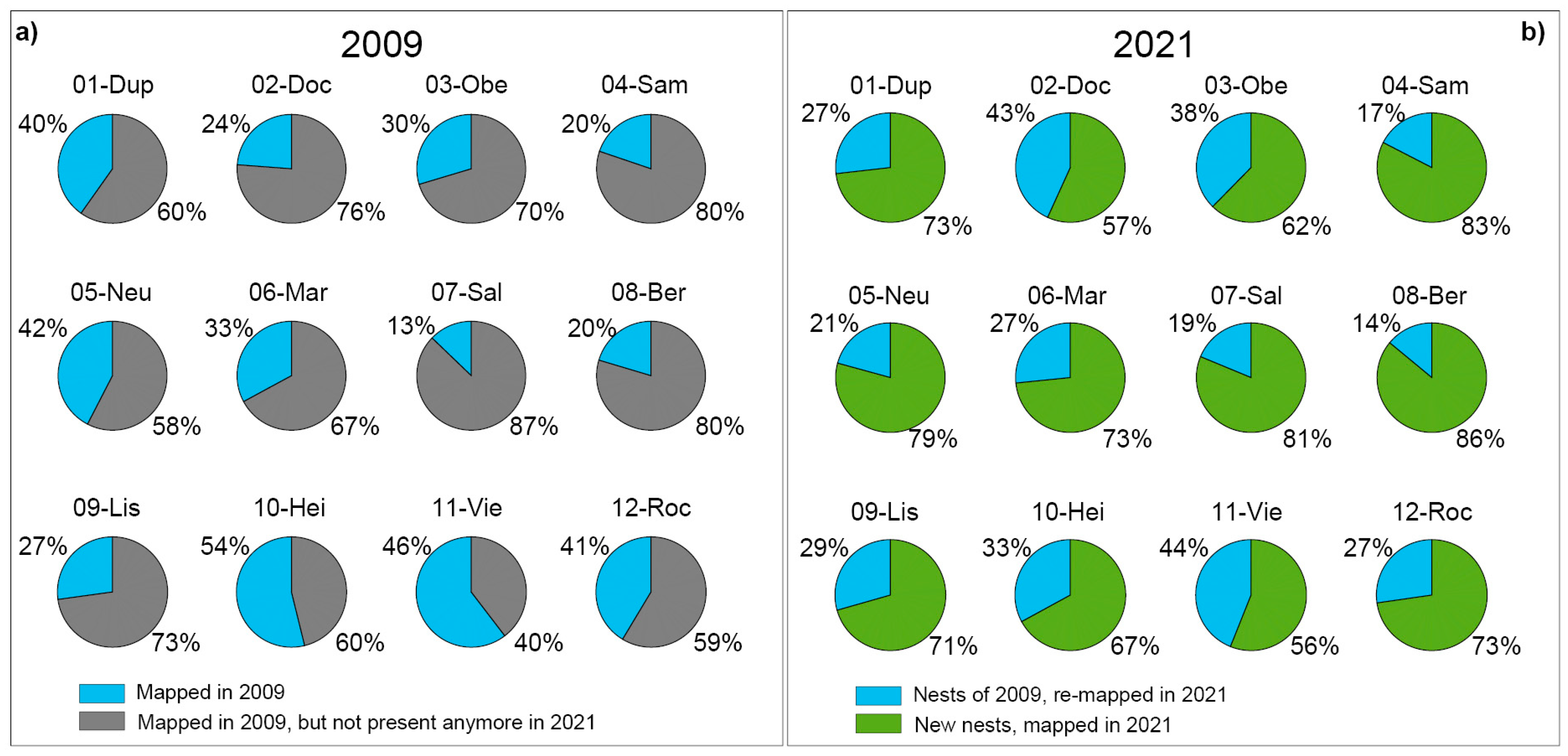
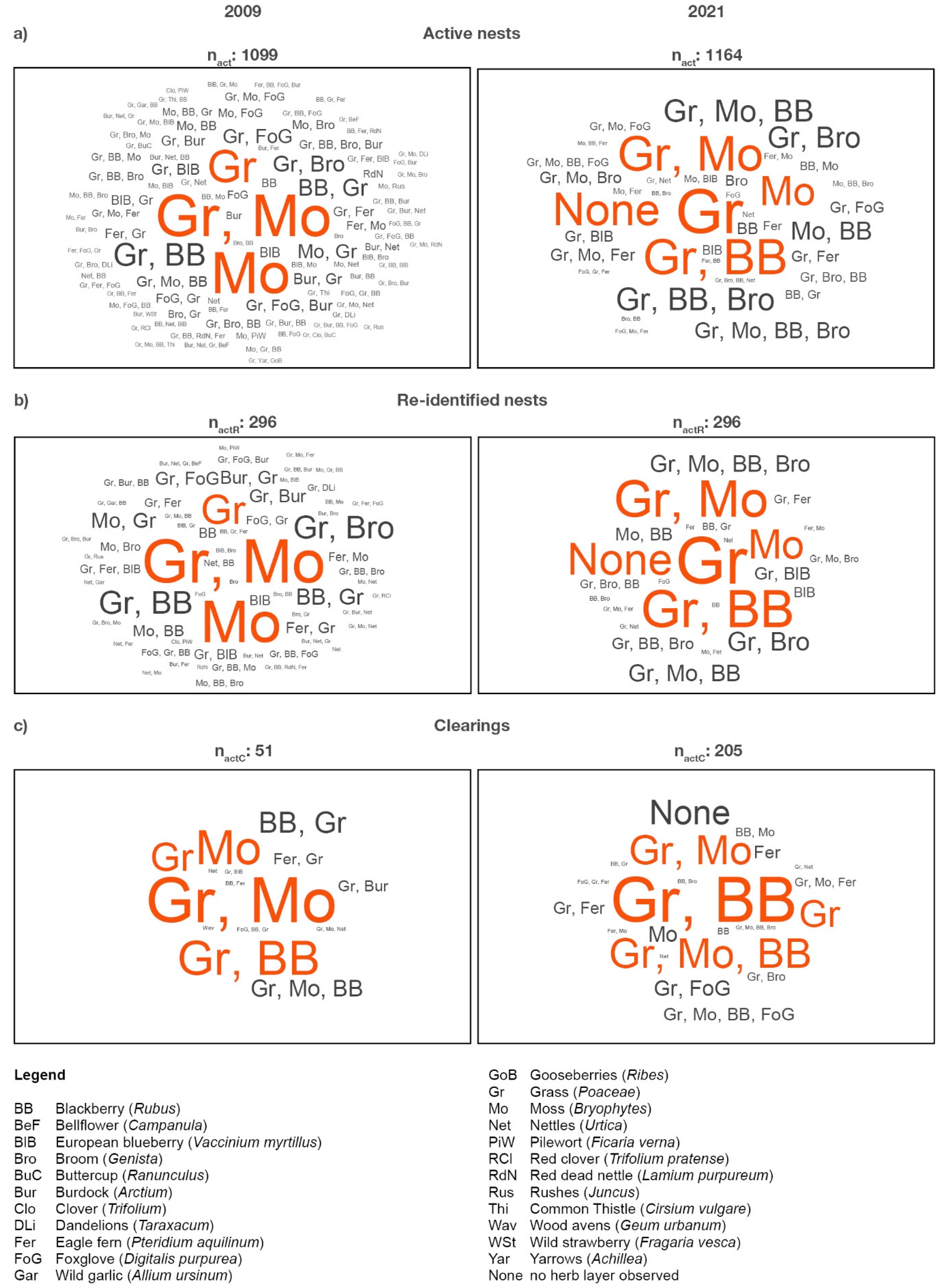

| (a) 2009 | (a) 2021 | ||||||||||
| Source | SS | df | MS | Chi-sq | Prob > Chi-sq | Source | SS | df | MS | Chi-sq | Prob > Chi-sq |
| Groups | 4503029613 | 3 | 1501009871 | 2974.7 | 0.00 | Groups | 3916176557 | 3 | 1305392186 | 2418.48 | 0.00 |
| Error | 2003097694 | 4295 | 466,379.0 | Error | 3287961357 | 4446 | 739,532.47 | ||||
| Total | 6506127307 | 4298 | Total | 7204137914 | 4449 | ||||||
| (b) 2009 | (b) 2021 | ||||||||||
| Group A | Group B | Lower Limit | A-B | Upper Limit | p-Value | Group A | Group B | Lower Limit | A-B | Upper Limit | p-Value |
| NH | ND | −525.5 | −390.7 | −255.8 | 0.0000 | NH | ND | −711.1 | −575.5 | −440.0 | 0.0000 |
| NH | WpC | 758.8 | 893.6 | 1028.5 | 0.0000 | NH | WpC | −362.1 | −226.6 | −91.1 | 0.0001 |
| NH | mTA | −2119.8 | −1981.8 | −1843.7 | 0.0000 | NH | mTA | −2634.6 | −2492.0 | −2349.4 | 0.0000 |
| ND | WpC | 1149.5 | 1284.3 | 1419.1 | 0.0000 | ND | WpC | 213.4 | 348.9 | 484.5 | 0.0000 |
| ND | mTA | −1729.2 | −1591.1 | −1453.1 | 0.0000 | ND | mTA | −2059.1 | −1916.5 | −1773.9 | 0.0000 |
| WpC | mTA | −3013.5 | −2875.4 | −2737.4 | 0.0000 | WpC | mTA | −2408.0 | −2265.4 | −2122.8 | 0.0000 |
| 2009 | 2021 | 2009–2021 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nest Height (NH) Classes of Active Nests (nact) | |||||||||||||||||||
| No | Study Site | Numbers | n | Numbers | n | nact | % | ||||||||||||
| ntot | nact | Start-Ups | Short | Medium | Tall | Very Tall | Extra Tall | ntot | nact | Start-Ups | Short | Medium | Tall | Very Tall | Extra Tall | ||||
| 0.01–0.10 | 0.11–0.50 | 0.51–1.00 | 1.01–1.50 | 1.51–2.00 | >2.01 | 0.01–0.10 | 0.11–0.50 | 0.51–1.00 | 1.01–1.50 | 1.51–2.00 | >2.01 | ||||||||
| 01 | Dup | 102 | 97 | 1 | 26 | 26 | 18 | 26 | – | 153 | 142 | 14 | 44 | 40 | 32 | 12 | – | 45 | 46 |
| 02 | Doc | 147 | 142 | 10 | 40 | 74 | 5 | 13 | – | 81 | 57 | 10 | 24 | 16 | 5 | 1 | 1 | −85 | −60 |
| 03 | Obe | 216 | 210 | 44 | 74 | 37 | 3 | 52 | – | 170 | 163 | 23 | 77 | 46 | 14 | 2 | 1 | −47 | −22 |
| 04 | Sam | 96 | 93 | 7 | 31 | 42 | 9 | 4 | – | 109 | 103 | 2 | 59 | 30 | 11 | 1 | – | 10 | 11 |
| 05 | Neu | 66 | 64 | 10 | 19 | 27 | 7 | 1 | – | 135 | 127 | 11 | 74 | 37 | 5 | – | – | 63 | 98 |
| 06 | Mar | 76 | 71 | 18 | 17 | 17 | 19 | – | – | 94 | 93 | 4 | 56 | 22 | 11 | – | – | 22 | 31 |
| 07 | Sal | 117 | 108 | 17 | 57 | 21 | 13 | – | – | 80 | 78 | 2 | 53 | 22 | 1 | – | – | −30 | −28 |
| 08 | Ber | 103 | 100 | 22 | 56 | 22 | – | – | – | 150 | 142 | 31 | 96 | 15 | – | – | – | 42 | 42 |
| 09 | Lis | 92 | 85 | 9 | 34 | 24 | 18 | – | – | 85 | 78 | 5 | 54 | 11 | 8 | – | – | −7 | −8 |
| 10 | Hei | 52 | 52 | 5 | 5 | 28 | 14 | – | – | 85 | 76 | 3 | 62 | 10 | 1 | – | – | 24 | 46 |
| 11 | Vie | 48 | 48 | – | 11 | – | 14 | 23 | – | 66 | 63 | 2 | 17 | 21 | 12 | 10 | 1 | 15 | 31 |
| 12 | Roc | 29 | 29 | – | 3 | 10 | 16 | – | – | 44 | 42 | 1 | 29 | 9 | 2 | 1 | – | 13 | 45 |
| Total | 1144 | 1099 | 143 | 373 | 328 | 136 | 119 | 0 | 1252 | 1164 | 108 | 645 | 279 | 102 | 27 | 3 | 65 | 6 | |
| Nest Diameter (ND) Classes of Active Nests (nact) | |||||||||||||||||||
| No | Study Site | Numbers | n | Numbers | n | nact | % | ||||||||||||
| ntot | nact | Small | Medium | Large | Very Large | Extra-Large | – | ntot | nact | Small | Medium | Large | Very Large | Extra-Large | – | ||||
| 0.01–0.50 | 0.51–1.00 | 1.01–1.50 | 1.51–2.00 | >2.01 | – | 0.01–0.50 | 0.51–1.00 | 1.01–1.50 | 1.51–2.00 | >2.01 | – | ||||||||
| 01 | Dup | 102 | 97 | 28 | 19 | 16 | 16 | 18 | – | 153 | 142 | 28 | 29 | 29 | 22 | 34 | – | 45 | 46 |
| 02 | Doc | 147 | 142 | 16 | 44 | 49 | 24 | 9 | – | 81 | 57 | 15 | 16 | 12 | 6 | 8 | – | −85 | −60 |
| 03 | Obe | 216 | 210 | 76 | 58 | 35 | 19 | 22 | – | 170 | 163 | 62 | 45 | 32 | 16 | 8 | – | −47 | −22 |
| 04 | Sam | 96 | 93 | 8 | 21 | 28 | 14 | 22 | – | 109 | 103 | 17 | 32 | 37 | 13 | 4 | – | 10 | 11 |
| 05 | Neu | 66 | 64 | 15 | 12 | 13 | 15 | 9 | – | 135 | 127 | 38 | 44 | 31 | 13 | 1 | – | 63 | 98 |
| 06 | Mar | 76 | 71 | 25 | 13 | 10 | 6 | 17 | – | 94 | 93 | 33 | 31 | 26 | 3 | – | – | 22 | 31 |
| 07 | Sal | 117 | 108 | 50 | 23 | 15 | 7 | 13 | – | 80 | 78 | 28 | 32 | 12 | 3 | 3 | – | −30 | −28 |
| 08 | Ber | 103 | 100 | 36 | 39 | 19 | 4 | 2 | – | 150 | 142 | 81 | 33 | 17 | 7 | 4 | – | 42 | 42 |
| 09 | Lis | 92 | 85 | 29 | 20 | 15 | 10 | 11 | – | 85 | 78 | 22 | 32 | 15 | 6 | 3 | – | −7 | −8 |
| 10 | Hei | 52 | 52 | 14 | 18 | 13 | 3 | 4 | – | 85 | 76 | 16 | 32 | 15 | 8 | 5 | – | 24 | 46 |
| 11 | Vie | 48 | 48 | 3 | 12 | 9 | 11 | 13 | – | 66 | 63 | 7 | 11 | 23 | 10 | 12 | – | 15 | 31 |
| 12 | Roc | 29 | 29 | 5 | 2 | 12 | 5 | 5 | – | 44 | 42 | 5 | 18 | 11 | 8 | – | – | 13 | 45 |
| Total | 1144 | 1099 | 305 | 281 | 234 | 134 | 145 | – | 1252 | 1164 | 352 | 355 | 260 | 115 | 82 | – | 65 | 6 | |
| No | Study Site | Mapped Area | State Forest (SF) | Municipal Forest (MF) | Private Forest (PF) | Number of Nests (nact) 2009 | Number of Nests (nact) 2021 | Clearing Plots with RWA Nests | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2021 | |||||||||||||||||
| ha | ha | % | ha | % | ha | % | SF | MF | PF | SF | MF | PF | ha * | nact | ha * | nact | ||
| 01 | Dup | 72.4 | 38.2 | 52.8 | 4.9 | 6.8 | 29.3 | 40.5 | 66 | – | 31 | 104 | – | 38 | 2.13 | 2 | 0.38 | 16 |
| 02 | Doc | 59.3 | – | – | 59.3 | 100.0 | – | – | – | 142 | – | – | 57 | – | – | – | 0.28 | – |
| 03 | Obe | 408.5 | – | 35.9 | 261.7 | 64.1 | – | – | 118 | 92 | – | 108 | 55 | – | 1.38 | 26 | 2.69 | 61 |
| 04 | Sam | 46.7 | – | – | 33.9 | 72.6 | 12.8 | 27.4 | – | 85 | 8 | – | 98 | 5 | 0.87 | 16 | 3.4 | 58 |
| 05 | Neu | 92.9 | – | – | 60.6 | 65.2 | 32.3 | 34.8 | – | 34 | 30 | – | 95 | 32 | – | – | 0.03 | 1 |
| 06 | Mar | 124.7 | 0.8 | 0.6 | 82.8 | 66.4 | 41.1 | 33.0 | – | 63 | 8 | – | 77 | 16 | – | – | 0.38 | 1 |
| 07 | Sal | 56.5 | 50.7 | 89.7 | – | – | 5.8 | 10.3 | 108 | – | – | 78 | – | – | – | – | 1.36 | 17 |
| 08 | Ber | 85.2 | – | – | – | 100.0 | – | – | – | 100 | – | – | 142 | – | 0.25 | 4 | 1.24 | 29 |
| 09 | Lis | 176.6 | – | – | 171.2 | 96.9 | – | 3.1 | – | 77 | 8 | – | 73 | 5 | – | – | 0.64 | 10 |
| 10 | Hei | 21.4 | – | – | 3.7 | 17.3 | 17.7 | 82.7 | – | 28 | 24 | – | 37 | 39 | – | – | 0.43 | 12 |
| 11 | Vie | 14.1 | – | – | 6.3 | 44.7 | 7.8 | 55.3 | – | 25 | 23 | – | 33 | 30 | 0.15 | 3 | – | – |
| 12 | Roc | 122.9 | – | – | 105.1 | 85.5 | 17.8 | 14.5 | – | 29 | – | – | 42 | – | – | – | – | |
| Total | 1281.2 | 236.5 | 18.5 | 874.7 | 68.3 | 170.0 | 13.3 | 292 | 675 | 132 | 290 | 709 | 165 | 4.78 | 51 | 10.83 | 205 | |
| No | Study Site | Mapped Nests (nact) | Numbers of WpC (n) in nact | Ratio WpC/Nest | Nests (n) with WPC Classes | Nests (n) with WPC Classes (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1–4 | 5–10 | >10 | 0 | 1–4 | 5–10 | >10 | |||||
| (a) 2009: 224 Nests with WpC | ||||||||||||
| 01 | Dup | 97 | 33 | 0.3 | 84 | 13 | 0 | 0 | 86.6 | 13.4 | 0.0 | 0.0 |
| 02 | Doc | 142 | 159 | 1.1 | 80 | 52 | 10 | 0 | 56.3 | 36.6 | 7.0 | 0.0 |
| 03 | Obe | 210 | 34 | 0.2 | 195 | 13 | 2 | 0 | 92.9 | 6.2 | 1.0 | 0.0 |
| 04 | Sam | 93 | 128 | 1.4 | 62 | 25 | 3 | 3 | 66.7 | 26.9 | 3.2 | 3.2 |
| 05 | Neu | 64 | 55 | 0.8 | 46 | 15 | 3 | 0 | 71.9 | 23.4 | 4.7 | 0.0 |
| 06 | Mar | 71 | 116 | 1.6 | 47 | 14 | 8 | 2 | 66.2 | 19.7 | 11.3 | 2.8 |
| 07 | Sal | 108 | 113 | 0.9 | 79 | 24 | 3 | 2 | 72.2 | 22.2 | 3.7 | 1.9 |
| 08 | Ber | 100 | 0 | 0.0 | 100 | 0 | 0 | 0 | 100.0 | 0.0 | 0.0 | 0.0 |
| 09 | Lis | 85 | 43 | 0.5 | 69 | 14 | 2 | 0 | 81.2 | 16.5 | 2.4 | 0.0 |
| 10 | Hei | 52 | 14 | 0.3 | 48 | 4 | 0 | 0 | 92.3 | 7.7 | 0.0 | 0.0 |
| 11 | Vie | 48 | 12 | 0.3 | 42 | 5 | 1 | 0 | 87.5 | 10.4 | 2.1 | 0.0 |
| 12 | Roc | 29 | 12 | 0.4 | 23 | 5 | 1 | 0 | 79.3 | 17.2 | 3.4 | 0.0 |
| Sum | 1099 | 699 | 0.6 | 875 | 184 | 33 | 7 | 79.6 | 16.7 | 3.0 | 0.6 | |
| (b) 2021: 624 nests with WpC | ||||||||||||
| 01 | Dup | 142 | 313 | 2.2 | 60 | 57 | 24 | 1 | 42.3 | 40.1 | 16.9 | 0.7 |
| 02 | Doc | 57 | 50 | 0.9 | 39 | 15 | 3 | 0 | 68.4 | 26.3 | 5.3 | 0.0 |
| 03 | Obe | 163 | 339 | 2.1 | 73 | 64 | 22 | 4 | 44.8 | 39.3 | 13.5 | 2.5 |
| 04 | Sam | 103 | 343 | 3.3 | 26 | 49 | 23 | 5 | 25.2 | 47.6 | 22.3 | 4.9 |
| 05 | Neu | 127 | 429 | 3.4 | 43 | 49 | 29 | 6 | 33.9 | 38.6 | 22.8 | 4.7 |
| 06 | Mar | 93 | 150 | 1.6 | 38 | 48 | 7 | 0 | 40.9 | 51.6 | 7.5 | 0.0 |
| 07 | Sal | 78 | 121 | 1.6 | 38 | 32 | 8 | 0 | 48.7 | 41.0 | 10.3 | 0.0 |
| 08 | Ber | 142 | 75 | 0.5 | 114 | 24 | 4 | 0 | 80.3 | 16.9 | 2.8 | 0.0 |
| 09 | Lis | 78 | 156 | 2.0 | 32 | 35 | 11 | 0 | 41.0 | 44.9 | 14.1 | 0.0 |
| 10 | Hei | 76 | 85 | 1.1 | 41 | 33 | 2 | 0 | 53.9 | 43.4 | 2.6 | 0.0 |
| 11 | Vie | 63 | 203 | 3.2 | 21 | 27 | 10 | 5 | 33.3 | 42.9 | 15.9 | 7.9 |
| 12 | Roc | 42 | 98 | 2.3 | 15 | 18 | 9 | 0 | 35.7 | 42.9 | 21.4 | 0.0 |
| Sum | 1164 | 2362 | 2.0 | 540 | 451 | 152 | 21 | 46.4 | 38.7 | 13.1 | 1.8 | |
| Nest Height (NH) Classes (m) | Woodpecker Cavities (WpC) in Nests | |||||||
|---|---|---|---|---|---|---|---|---|
| n | % | |||||||
| 0 | 1–4 | 5–10 | >10 | 0 | 1–4 | 5–10 | >10 | |
| (a) 2009 | ||||||||
| 0.01–0.10 | 143 | 0 | 0 | 0 | 13.0 | 0.0 | 0.0 | 0.0 |
| 0.11–0.50 | 322 | 45 | 2 | 2 | 29.2 | 4.1 | 0.2 | 0.2 |
| 0.51–1.00 | 246 | 79 | 15 | 2 | 22.4 | 7.2 | 1.4 | 0.2 |
| 1.01–1.50 | 64 | 31 | 11 | 3 | 5.8 | 2.8 | 1.0 | 0.3 |
| 1.51–2.00 | 100 | 29 | 5 | 0 | 9.1 | 2.6 | 0.5 | 0.0 |
| >2.00 | 143 | 0 | 0 | 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sum | 875 | 184 | 33 | 7 | 79.6 | 16.7 | 3.0 | 0.6 |
| (b) 2021 | ||||||||
| 0.01–0.10 | 108 | 0 | 0 | 0 | 9.3 | 0.0 | 0.0 | 0.0 |
| 0.11–0.50 | 333 | 253 | 54 | 5 | 28.6 | 21.7 | 4.6 | 0.4 |
| 0.51–1.00 | 73 | 137 | 62 | 6 | 6.3 | 11.8 | 5.3 | 0.5 |
| 1.01–1.50 | 21 | 47 | 29 | 6 | 1.8 | 4.0 | 2.5 | 0.5 |
| 1.51–2.00 | 5 | 14 | 7 | 4 | 0.4 | 1.2 | 0.6 | 0.3 |
| >2.00 | 0 | 0 | 0 | 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sum | 540 | 451 | 152 | 21 | 46.4 | 38.7 | 13.1 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berberich, G.M.; Berberich, M.B. A Re-Inventory after 12 Years—Increase in Red Wood Ant Nests and Woodpecker Cavities in Nests in the West Eifel Volcanic Field despite Climatic Changes. Forests 2023, 14, 985. https://doi.org/10.3390/f14050985
Berberich GM, Berberich MB. A Re-Inventory after 12 Years—Increase in Red Wood Ant Nests and Woodpecker Cavities in Nests in the West Eifel Volcanic Field despite Climatic Changes. Forests. 2023; 14(5):985. https://doi.org/10.3390/f14050985
Chicago/Turabian StyleBerberich, Gabriele M., and Martin B. Berberich. 2023. "A Re-Inventory after 12 Years—Increase in Red Wood Ant Nests and Woodpecker Cavities in Nests in the West Eifel Volcanic Field despite Climatic Changes" Forests 14, no. 5: 985. https://doi.org/10.3390/f14050985





