Abstract
Nothofagus alessandrii is an endangered species with limited and fragmented distribution in the Maule coastal forest of central Chile. Understanding the factors and processes that influence the natural growth of this species is crucial for mitigating its ecological vulnerability. The primary objective of this research is to determine the spatial distribution pattern of N. alessandrii and its association with geomorphometric variables (slope, elevation, and exposure), as well as its association with other tree species in a representative forest located in the northernmost natural distribution range of the species. To achieve this, the coordinates (x, y, z) of all N. alessandrii individuals and accompanying tree species, along with their slope, elevation, and exposure, were obtained using a total station. A spatial analysis tool based on distance indices (SADIE) was used to quantify the spatial pattern of N. alessandrii and detect local aggregates, as well as determine the degree of spatial association between pairs of variables. The results showed that N. alessandrii trees had a random distribution pattern and a significant spatial association with the studied geomorphometric variables. An additional significant finding was the lack of spatial association observed between N. alessandrii and the accompanying species. In conclusion, our study provides valuable information on the spatial distribution and ecological correlates of the endangered N. alessandrii in a fragmented forest ecosystem of central Chile. The results highlight the importance of geomorphometric variables in shaping the distribution pattern of the species, which can be used to guide restoration and conservation efforts.
1. Introduction
Nothofagus alessandrii (Fagaceae), commonly known as ruil, is an endemic and native species of Chile that has been classified as an “endangered” species since 2007 [1] due to a significant reduction in its population, distribution area, habitat quality, and the number of mature individuals. The species is found in its natural form only in the coastal mountain range of the administrative region of Maule in central Chile, with a distribution of less than 100 km of latitudinal extension [2]. The area occupied by N. alessandrii in its natural distribution is highly fragmented and has been declining over time. According to the last published record [2], the species occupies an area of no more than 314 ha, which is distributed in 15 localities within fast-growing species forests [2]. A subsequent study indicates that 55% of these forests were affected by the mega-wildfire of 2017 [3]. Unlike other Nothofagus species, the distribution pattern of ruil is not continuous [4].
Various factors threaten fragmented ruil forests; among them is the invasion of surrounding vegetation, which mostly corresponds to Pinus radiata D. Don plantations [5]. Some sites with greater conservation of ruil present a good regeneration by seeds, but, in general, due to the alteration of the habitat and the species that are associated with it, most of the fragments do not show space recovery [6]. Given the limited and fragmented distribution of N. alessandrii, it is crucial to prioritize conservation actions to ensure the survival of this species and its coexistence with other native trees in the region of origin. Despite the limited information available regarding the spatial distribution of N. alessandrii, it is associated with steep slopes [7], elevations that range from 100 to 450 m above sea level [8], and shady exposures [7].
Understanding the spatial distribution of a species is crucial in order to grasp the dynamics of its population and how it is influenced by a range of biological, ecological, and biogeographic factors [9,10]. This analysis is also helpful in developing silvicultural practices and designing recovery and conservation plans [11]. The history, dynamics, and interactions between species in forests can be elucidated by analyzing the spatial patterns of their distribution [12,13]. These patterns can provide insights into the processes and mechanisms that influence the coexistence of different species [14]. Additionally, they can help identify seed dispersal mechanisms [15,16], recognize interspecific associations in populations, and understand species’ responses to various disturbances [17]. The patterns of spatial distribution can be classified into three types: random, aggregate, and uniform [18]. These types of distribution can offer insights into the interactions between organisms in a particular environment. Various factors can influence the spatial distribution of plants, such as competition or facilitation [19], as well as geomorphometric variables such as relief conditions [20,21], slope exposure, elevation, and the physical–chemical properties of the soil [22,23,24]. All of these factors significantly impact the distribution of vegetation [20,21].
Spatial analysis is a widely used tool in various disciplines, including ecology, geography, geology, and physics. In ecology, several tools for spatial analysis are available [18], one of which is the Spatial Analysis by Distance Indices (SADIE). SADIE performs a spatial analysis based on distance indices and allows for the quantification of spatial heterogeneity. This technique has been used in various ecological studies, including the analysis of spatial patterns of survival in afforestation, vegetation cover restoration processes, and plant-to-plant interaction studies [25,26,27,28]. Additionally, SADIE has been used in the field of phytopathology [29]. The methodology used in SADIE enables the quantification of the spatial pattern of a variable or object under study, detecting the local aggregates of the variable, and quantifying the degree of association or spatial dissociation between pairs of variables. An advantage of this tool is that it can handle data of different natures [30].
Therefore, the aim of this study is to determine the distribution pattern of N. alessandrii and analyze its association with the aforementioned geomorphometric variables as well as other tree species in a fragmented forest in a N. alessandrii stand of its northern distribution. The findings of this study will provide insights into the ecological requirements of N. alessandrii and the factors that influence its distribution pattern, contributing to the design of conservation strategies for this species.
2. Materials and Methods
2.1. Study Area
The study area was located near the town of Lo Ramírez, which is part of the Curepto municipality, Talca province, in the Chilean administrative region of Maule (Figure 1). It was situated at 35°10′ S latitude and 72°06′ W longitude, with an altitude of 385 m above sea level, and covered an area of 46.3 hectares, characterized by forests of Nothofagus alessandrii. Considering the low variability of the forest stand under investigation and following [27,31,32] a plot of 2475 square meters (55 m × 45 m) was selected based on its varying slopes, elevations, and exposures [2].
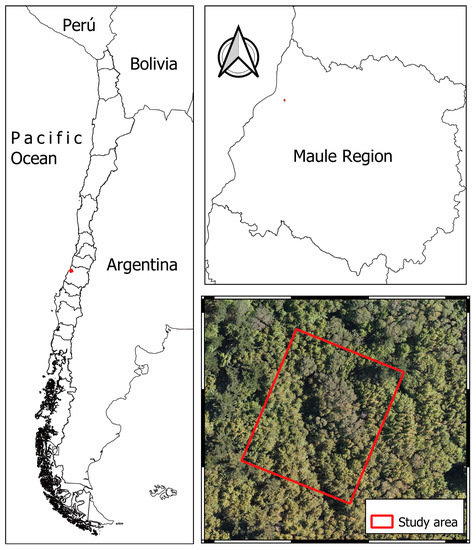
Figure 1.
Study area.
The study area is characterized by soil belonging to the Constitución series [6], which is of metamorphic origin and ranges from semi-deep to deep. The upper layers of the soil have a clay loam texture, while the lower layers have a silt loam texture. Overall, these soils have a moderately fine surface texture and good drainage [6,33].
The study area experiences a warm temperate climate with prolonged dry seasons and maritime influence [17]. The average annual temperature is 14.2 °C, and the accumulated annual precipitation in 2021 was 470.9 mm (https://www.cr2.cl/ accessed on 11 October 2022).
2.2. Identification of Tree Species and Survey of Points with Total Station
To detect the presence of Nothofagus alessandrii and other associated tree species in the study area, all woody species that grow to a minimum height of 5 m in their adult state under typical habitat conditions or less in environments that may restrict their growth, were taken into account.
To determine the coordinates of each N. alessandrii and associated tree species, we utilized a Leica model TS02 total station and its corresponding prism. The survey points were captured in local coordinates, commencing with marked reference points on the ground that were denoted with stakes. After leveling the total station, we obtained and recorded the coordinates of the reference points for later analysis. We then repeated this procedure for the tree species of interest, documenting information such as the point number, corresponding species, and any pertinent observations.
Following the completion of the topographic survey, the field data (points) were downloaded as a plain text file in CSV (Comma Separated Values) format onto a computer. We utilized the academic version of Civil 3D software to realign the points with the established reference points in UTM coordinates. After realignment, the points were exported in DXF (Drawing Exchange Format) for additional analysis.
2.3. Creation of Maps (Slope-Orientation) and Preparation of the Attribute Table
The CSV file obtained from the total station contained four columns that included the point identification and coordinates in X, Y, and Z. These coordinates were used to generate a new shapefile with all the registered points. A digital terrain model was created by performing spatial interpolation using the Triangulated Irregular Networks (TIN) method. The interpolation was carried out using QGIS (v3.18.3), and the slope and prevailing exposure maps were generated in the same program. The GDAL library’s Slope and Orientation tools were employed for this purpose. A raster layer was generated with values that spanned from 0 to 360 degrees for the purpose of computing the orientation. This layer was reclassified as follows: values between 0–45 degrees and 315–360 degrees were classified as north slope, values between 45–135 degrees as east slope, values between 135–225 degrees as south slope, and values between 225–315 degrees as west slope. Similarly, the raster layer with the slopes was also reclassified into five categories based on the range of values (less than 20%, 20%–40%, 40%–60%, 60%–80%, and more than 80%).
Following the acquisition of the slope and orientation maps, an attribute table was established, linking each recorded point or species with its corresponding X, Y, Z coordinates, and slope, elevation, and exposure measurements. Subsequently, the table was exported to Excel and converted into a CSV file.
2.4. Spatial Analysis by Distance Index for the Creation of Aggregation Maps, and for the Association between Nothofagus alessandrii and Other Native Tree Species
The association between the variables, such as species, slope, elevation, and exposure, was analyzed using the CSV file generated in SADIE (Spatial Analysis by Distance Indices) tool, implemented in the program “SADIEShell v2.0”. The analysis considered a two-dimensional plane with X and Y coordinates to represent the location of the ruil, other tree species, and geomorphometric variables such as slope, elevation, and exposure. Next, the total aggregation index (Ia) was calculated for each environmental variable, while the clustering index (v) was computed for each pair of datasets.
The aggregation index is a statistical measure that assesses the degree to which a set of objects or phenomena is clustered or dispersed relative to a theoretical random distribution. It is commonly used in ecology to measure the spatial pattern of organisms in a given area. The Ia provides information on the overall spatial pattern of each environmental variable. According to the study by Quero et al. (2006), the spatial pattern is aggregated if Ia > 1, random if Ia is close to one, and regular if Ia < 1. The Aggregation Index (Ia) was also computed for accompanying tree species.
The clustering index, v, measures the degree of clustering of data into patches (mean vi) and gaps (mean vj), where high values correspond to areas of high concentration of the target variable and low values to areas of low concentration. To visualize the spatial distribution of patches (v > 1.5) and gaps (v < −1.5), v was contoured by kriging in a two-dimensional map using Surfer 16.3.408 (Golden Software, Golden, Colorado, CO, USA). This method allows for the identification of characteristics of the data that may not be evident when analyzing the correlation coefficient of the variables [34], revealing areas with the greatest presence of the variable or the locations where two pairs of data coincide.
To assess the association between two variables, the association index (χ) was calculated. If the χ value is >0, the variables are considered associated, if it is <0, they are not associated, and if it is equal to 1, the arrangement is random. The level of probability, indicated by the value of p, was also obtained. Association maps were generated using Surfer 16.3.408 (Golden Software, Golden, Colorado, CO, USA).
3. Results
3.1. Total and Local Aggregation Analysis for Nothofagus alessandrii and Accompanying Tree Species
The following accompanying species were found in the study area: Nothofagus glauca (Phil.) Krasser, Crytocarya alba (Molina) Looser, Lithraea caustica (Molina) Hook. et Arn., Peumus boldus Molina, Azara dentata Ruiz and Pav., Luma apiculata (DC.) Burret, Aextoxicon punctatum Ruiz et Pav and Lomatia hirsuta (Lam.) Diels ex J.F. macbr.
The values of the total aggregation index (Ia) and the local aggregation indices vj and vi provided by SADIE (Table 1) show that Nothofagus alessandrii has a random distribution pattern with no clear or significant patches. Most of the accompanying tree species showed a random distribution pattern in their total aggregation indices. However, N. glauca, C. alba, and L. hirsuta had an aggregated distribution pattern, forming significant patches and clearings with areas of greater or lesser presence of the species. No gaps or patches were observed in the rest of the species.

Table 1.
Total aggregation indices (Ia), means of local aggregation indices (vj and vi), and statistical significance (p-value) for Nothofagus alessandrii and accompanying tree species in the study area.
Figure 2 displays the distribution of N. alessandrii in the study area, with the darker red areas indicating a higher density of the species.
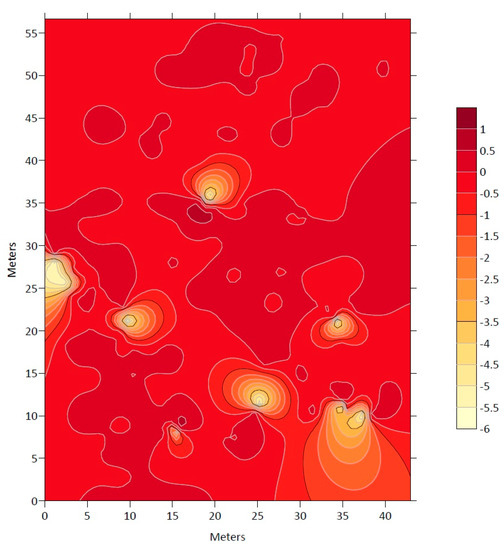
Figure 2.
Map of the grouping index (ν) with the presence of Nothofagus alessandrii in the study area.
3.2. Association between Areas with the Presence of Nothofagus alessandrii and Geomorphometric Variables (Slope, Elevation, and Exposure) and Association between Nothofagus alessandrii and Accompanying Species
The association analysis between Nothofagus alessandrii and the different geomorphometric variables consistently showed a significant relationship, as indicated by the association indices (χ) (Table 2). The presence of ruil was found to be significantly associated with slope, elevation, and exposure.

Table 2.
Association index (χ) and level of significance (p-value) between Nothofagus Alessandrii and the geomorphometric variables: slope, elevation, and exposure.
Figure 3 displays association maps between N. alessandrii and the different geomorphometric variables. Figure 3a delineates the areas of significant association with black lines and darker tones indicating the presence of N. alessandrii. Within these areas, 75% of the individuals were associated with slopes ranging from 20% to 40%. In Figure 3b, the association with elevation shows that N. alessandrii was present at elevations ranging from 387.6 to 406.1 m above sea level, with 95% of individuals found within this range. Figure 3c reveals that exposure was also associated with the distribution of N. alessandrii, with a predominance of southern exposure. Approximately 60% of individuals were found in areas with exposure between 180–270 degrees (south-east).
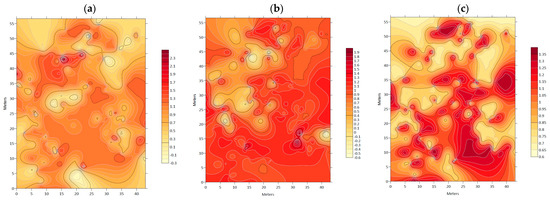
Figure 3.
Association index (χ) maps between: (a) Nothofagus alessandrii and slope; (b) Nothofagus alessandrii and exposure; (c) Nothofagus alessandrii and elevation.
The association indices (χ) were examined between N. alessandrii and other accompanying tree species in the study area, which revealed that ruil individuals did not exhibit any significant association with the presence of other tree species (as shown in Table 3).

Table 3.
Association index (χ) and probability level (p) of Nothofagus alessandrii and accompanying tree species in the study area.
Figure 4 and Figure 5 display maps indicating lower association levels between N. alessandrii and each accompanying tree species, as represented by lighter color tones and delimited with a black line. These areas are characterized by lower values of the respective studied variables.
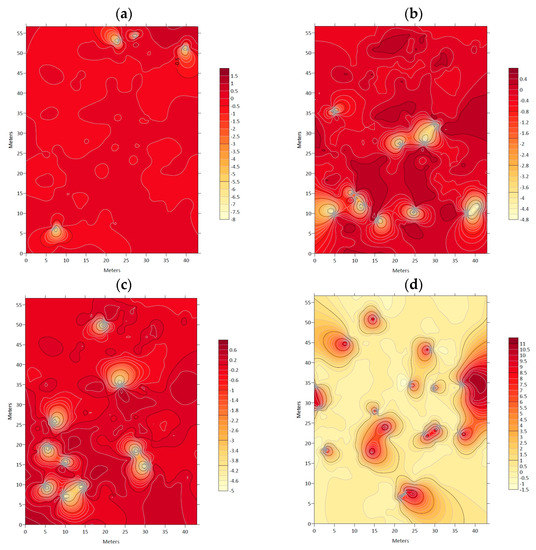
Figure 4.
Association index (χ) maps between: (a) Nothofagus alessandrii and Luma apiculata; (b) Nothofagus alessandrii and Peumus boldo; (c) Nothofagus alessandrii and Azara dentata; (d) Nothofagus alessandrii and Nothofagus glauca.
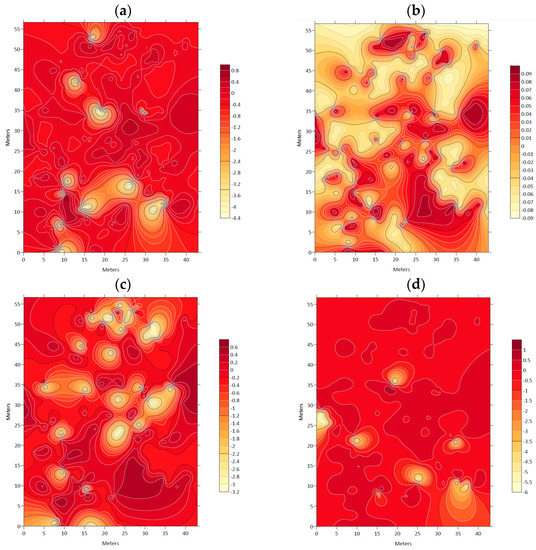
Figure 5.
Association index (χ) maps between: (a) Nothofagus alessandrii and Lithraea caustica; (b) Nothofagus alessandrii and Aextoxicon punctatum; (c) Nothofagus alessandrii and Crytocarya alba; (d) Nothofagus alessandrii and Lomatia hirsuta.
4. Discussion
The comprehensive analysis of the distribution patterns of Nothofagus alessandrii conducted in this study revealed that the species are randomly distributed in the study area, which has significant implications for understanding the ecology and conservation of the species. The analysis of local aggregation indices did not detect any significant patches of species aggregation, indicating a lack of local fragmentation of N. alessandrii in the study area. However, it is noteworthy that the shape and surface area of ruil populations vary throughout their distribution in Chile, which implies fragmentation at the regional level, as previously observed in studies [2,6].
The spatial analysis revealed no significant clusters of N. alessandrii, indicating a random distribution of the species that is independent of other organisms and environmental conditions, as reported in studies of other Nothofagus species, such as N. dombeyi and N. obliqua [35]. These findings suggest that the spatial distribution of N. alessandrii is associated with geomorphometric variables, including slope, elevation, and exposure. In mountainous regions with steep slopes, such as those where the ruil is naturally distributed, the presence of N. alessandrii was found on slopes ranging from 13.5% to 49.5%, with 95% of individuals falling within this range. Further analysis of this interval revealed that 75.1% of individuals were found on slopes between 20% and 40%, with 47.2% on slopes between 20% and 30%, and 30.5% between 25% and 30%. These results are consistent with other studies of Nothofagus species such as N. glauca, which also grows on steep slopes [36], and N. alpina, where slope conditions of around 43% have been identified [37].
The information available indicates that N. alessandrii is predominantly located in mountainous regions below 500 m in elevation, akin to other Nothofagus species such as N. glauca, which thrives at elevations ranging from 300 to 553 m [38]. Nevertheless, N. alessandrii has also been observed at higher elevations exceeding 600 m above sea level [39], where there is an expected occurrence of high winter rainfall. In such areas, rainfall is generally concentrated at the highest elevations and in coastal mountains, with a predominantly southern exposure and the possibility of moving in a southwest or southeast direction, but never northward [6]. These findings are consistent with previous research on Nothofagus species and their relationship with elevation. In this study, the presence of N. alessandrii was mainly associated with elevations ranging from 388 to 406 m above sea level, which accounted for 95% of the species distribution.
The study found that the spatial distribution of N. alessandrii was predominantly associated with southern exposures, with most exposure values ranging between 135 and 270 degrees as southern slopes. Data between 45–135 degrees were closer to south-east slopes, and values between 225–315 degrees were closer to south-west slopes. The association between N. alessandrii and the southern exposure confirms previous reports that ruil trees thrive in shaded conditions [40], in specific areas influenced by environmental variables, where the temperature has a direct relationship with the tree’s presence, validating one of the known characteristics of ruil, which is taking refuge in shaded places [2]. These results suggest that N. alessandrii individuals can be established in southern exposures under shaded and semi-shaded conditions. Studies have shown that optimal recruitment of ruil can be achieved in light gaps that provide intermediate shade conditions [41]. Furthermore, the association between N. alessandrii and southern exposure reaffirms that shade-tolerant species benefit from protection [42,43]. While ruil prefers to grow in shady places, further studies are required to understand its response to different shade conditions, given that the species has been classified as shade intolerant, although it can tolerate shade in its early growth stages [8,44]. Additionally, other studies suggest that protectors or shelters should not be limited to the first stages of the species’ development, but may also be beneficial for its growth in shaded or semi-shaded areas [2].
The results of this study showed that the ruil is influenced by the slope, elevation, and exposure, which were identified using SADIE’s ability to relate the positioning of one variable’s patches with the positioning of another variable’s patches in the same area [10]. The presence of the ruil at the elevations and exposures identified in this study confirms previous knowledge about the species’ affinity for shady conditions, emphasizing the need to consider these variables in future forest management and conservation strategies.
The results of the association indices between N. alessandrii and other tree species suggest that ruil individuals do not show a correlation with any accompanying tree species present in the study area, indicating a possible competitive relationship between N. alessandrii and other tree species [45]. These findings are in line with previous research identifying changes in the composition structure of ruil stands [46]. Interestingly, this study also identified foreign species in the N. alessandrii fragments, mainly belonging to the sclerophyllous forest, such as C. alba, P. boldus, and L. caustica, among others [45]. These results highlight the significance of comprehending the ecological dynamics and competitive interactions among tree species in the ruil stands for effective conservation and forest management.
In the studied forest, the presence of N. alessandrii individuals exceeded that of other sclerophyllous forest species. Even if 50% of the trees in the area are N. alessandrii, it is still considered a “conserved” stand [6]. The absence of association between N. alessandrii and other tree species is crucial for its management and conservation since it indicates possible factors affecting its coexistence with other species. This information is particularly relevant for forest restoration efforts since it is necessary to understand the dynamics of their generation [41]. Moreover, this knowledge can support the development of recovery and conservation programs, which are critical given the absence of specific protocols to establish ruil in the field and ensure its survival [47].
Since the composition and structure of ruil stands have been altered, it is crucial to implement protective measures that consider the species’ natural habitat. This may include factors such as slope, elevation, and exposure, which can impact the health and growth of the species [2]. Moreover, it is important to note that the analyses conducted do not provide evidence of positive interactions between N. alessandrii and other accompanying tree species, highlighting the need for further exploration of potential factors that may be contributing to the lack of association between ruil and other tree species in the study area.
5. Conclusions
The study found that the spatial distribution of Nothofagus alessandrii was random, without significant patches or gaps, but distinguishable among individuals of the species. The results also showed a significant association between the spatial distribution of N. alessandrii and geomorphometric variables, such as slope, elevation, and exposure. This association is crucial for understanding the natural growth conditions of the species and is thus important for conservation programs. However, no significant association was found between the presence of N. alessandrii and accompanying tree species, indicating possible competition between them. This interaction can have implications for the functioning of the forest and its management such as the identification of suitable habitats and areas for restoration. The findings on the spatial distribution of N. alessandrii highlight the existence of interactions among individuals of the same species. Finally, the findings of this study provide valuable information for understanding the dynamics of the species’ population and can help in the development of conservation and restoration programs.
Author Contributions
Conceptualization, A.M.C.-A.; methodology, A.M.C.-A., R.S.-M. and C.P.-R.; software, A.M.C.-A. and S.O.-M.; validation, A.M.C.-A., R.S.-M. and F.M-P.; formal analysis, A.M.C.-A., S.O.-M. and R.S.-M.; investigation, A.M.C.-A., S.O.-M. and R.S.-M.; resources, A.M.C.-A., R.S.-M. and F.M-P.; data curation, S.O.-M.; writing—original draft preparation, S.O.-M. and A.M.C.-A.; writing—review and editing, A.M.C.-A., R.S.-M. and F.M.-P.; visualization, A.M.C.-A. and S.O.-M.; supervision, A.M.C.-A. and R.S.-M.; project administration, A.M.C.-A. and R.S.-M.; funding acquisition, A.M.C.-A., R.S.-M. and C.P.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Acknowledgments
F.M.-P. acknowledges the support from ANID FONDECYT grant No. 1201973 and 1231681.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barstow, M.; Echeverría, C.; Baldwin, H.; Rivers, M.C. Nothofagus alessandrii. The IUCN Red List of Threatened Species 2017: E.T32033A2808995. Available online: http://www.iucnredlist.org/details/32033/0 (accessed on 2 March 2022).
- Santelices, R.; Drake, F.; Navarro-Cerrillo, R.M. Establishment of a Nothofagus alessandrii plantation using different levels of shade and weed control methods in Talca province, central Chile. South For. 2012, 74, 71–76. [Google Scholar] [CrossRef]
- Valencia, D.; Saavedra, J.; Brull, J.; Santelices, R. Severidad del daño causado por los incendios forestales en los bosques remanentes de Nothofagus alessandrii Espinosa en la región del Maule de Chile. Gayana Bot. 2018, 75, 531–534. [Google Scholar] [CrossRef]
- Santelices, R.; Navarro-Cerrillo, R.M.; Drake, F. Caracterización del material forestal de reproducción de cinco procedencias de Nothofagus alessandrii Espinosa una especie en peligro de extinción. Interciencia 2009, 34, 113–119. [Google Scholar]
- Nuñez, M.A.; Pauchard, A.; Langdon, B.; Jimenez, A.; Cavieres, L.A.; Peña, E. Pináceas invasoras en el sur de Sudamérica: Patrones, mecanismos e impactos potenciales. In Invasiones Biológicas en Chile: Causas Globales e Impactos Locales; Jaksic, F., Castro, S., Eds.; Ediciones UC: Santiago, Chile, 2014; pp. 283–308. [Google Scholar]
- Olivares, P.; San Martín, J.; Santelices, R. Ruil (Nothofagus alessandrii): Estado del Conocimiento y Desafíos Para su Conservación; Comisión Nacional del Medioambiente (CONAMA): Talca, Chile, 2005; p. 55. [Google Scholar]
- Weber, S. Estado de Desarrollo de Nothofagus alessandrii Espinosa, Nothofagus glauca (Phil.) Krasser y Nothofagus leonii Espinosa Ex–Situ, en Valdivia; Ingeniería Forestal, Universidad Austral de Chile: Valdivia, Chile, 2004. [Google Scholar]
- San Martín, J.; Santelices, R.; Henríquez, R. Nothofagus alessandrii Espinosa, Ruil. Familia: Nothofagaceae. In Las Especies Arbóreas de los Bosques Templados de Chile y Argentina: Autoecología, 2nd ed.; Donoso, C., Ed.; Marisa Cuneo Ediciones: Valdivia, Chile, 2013; pp. 391–401. [Google Scholar]
- Zunino, M.; Zullini, A. Biogeografía: La Dimensión Espacial de la Evolución; Fondo de Cultura Económica: México, Mexico, 2003; p. 359. [Google Scholar]
- Montero, D.; García, Ó. Análisis espacial por indices de distancia (SADIE) de Lophophora williamsii en tres parcelas con diferentes grado de perturbación en San Luis de Potosí. In Proceedings of the VII Simposio Internacional sobre la Flora Silvestre en Zonas Áridas Ecología, Manejo y Conservación, Sonora, Mexico, 17–19 March 2010; p. 14. [Google Scholar]
- Ambiente, M.d.M. Aprueba, Reglamento Para la Elaboración de Planes de Recuperación, Conservación y Gestión de Especies. Available online: https://www.bcn.cl/leychile/navegar?i=1066896&f=2014-09-22&p= (accessed on 5 April 2023).
- Law, R.; Illian, J.; Burslem, D.F.R.P.; Gratzer, G.; Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N. Ecological information from spatial patterns of plants: Insights from point process theory. J. Ecol. 2009, 97, 616–628. [Google Scholar] [CrossRef]
- Perry, G.L.W.; Miller, B.P.; Enright, N.J. A Comparison of Methods for the Statistical Analysis of Spatial Point Patterns in Plant Ecology. Plant Ecol. 2006, 187, 59–82. [Google Scholar] [CrossRef]
- Getzin, S.; Dean, C.; He, F.; Trofymow, J.A.; Wiegand, K.; Wiegand, T. Spatial patterns and competition of tree species in a Douglas-fir chronosequence on Vancouver Island. Ecography 2006, 29, 671–682. [Google Scholar] [CrossRef]
- Nathan, R.; Muller-Landau, H.C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 2000, 15, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Vásquez Fernández, I.A. Patrones Espaciales de Reclutamiento y Dispersión de Semillas del Árbol Persea lingue (Miers ex Bertero) Ness en el Bosque del Valle del Sur de Chile, Efectos del Hábitat y un Corredor; Universidad de Chile: Santiago, Chile, 2011. [Google Scholar]
- Ne’eman, G.; Lahav, H.; Izhaki, I. Spatial pattern of seedlings 1 year after fire in a Mediterranean pine forest. Oecologia 1992, 91, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Dale, M.R.T. Spatial Pattern Analysis in Plant Ecology; Cambridge University Press: Cambridge, UK, 1999; p. 326. [Google Scholar]
- Lara-Romero, C.; de la Cruz, M.; Escribano-Ávila, G.; García-Fernández, A.; Iriondo, J.M. What causes conspecific plant aggregation? Disentangling the role of dispersal, habitat heterogeneity and plant–plant interactions. Oikos 2016, 125, 1304–1313. [Google Scholar] [CrossRef]
- McAuliffe, J.R. Landscape Evolution, Soil Formation, and Ecological Patterns and Processes in Sonoran Desert Bajadas. Ecol. Monogr. 1994, 64, 112–148. [Google Scholar] [CrossRef]
- Valverde, P.L.; Zavala-Hurtado, J.A.; Montaña, C.; Ezcurra, E. Numerical analyses of vegetation based on environmental relationships in the southern Chihuahuan Desert. Southw Nat. 1996, 41, 424–433. [Google Scholar]
- Otto, R.; Fernández-Palacios, J.M.; Krüsi, B.O. Variation in Species Composition and Vegetation Structure of Succulent Scrub on Tenerife in Relation to Environmental Variation. J. Veg. Sci. 2001, 12, 237–248. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; de Nicolás, J.P. Altitudinal Pattern of Vegetation Variation on Tenerife. J. Veg. Sci. 1995, 6, 183–190. [Google Scholar] [CrossRef]
- Abd El-Ghani, M.; Soliman, A.; Abd El-Fattah, R. Spatial distribution and soil characteristics of the vegetation associated with common succulent plants in Egypt. Turk. J. Bot. 2014, 38, 550–565. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L. Análisis espacial mediante índices de distancia. In Introducción al Análisis Espacial de Datos en Ecología y Ciencias Ambientales: Métodos y Aplicaciones; Maestre, F.T., Escudero, A., Bonet, A., Eds.; Universidad Rey Juan Carlos: Madrid, Spain, 2008; pp. 130–182. [Google Scholar]
- Zúñiga, B.; Malda, G.; Suzán, Y.H. Interacciones Planta-Nodriza en Lophophora diffusa (Cactaceae) en un Desierto Subtropical de México. Biotropica 2005, 37, 351–356. [Google Scholar] [CrossRef]
- Sánchez-Cuesta, R.; Navarro-Cerrillo, R.M.; Quero, J.L.; Ruiz-Gómez, F.J. Small-Scale Abiotic Factors Influencing the Spatial Distribution of Phytophthora cinnamomi under Declining Quercus ilex Trees. Forests 2020, 11, 375. [Google Scholar] [CrossRef]
- Sánchez-Cuesta, R.; González-Moreno, P.; Cortés-Márquez, A.; Navarro-Cerrillo, R.M.; Ruiz-Gómez, F.J. Soil distribution of Phytophthora cinnamomi inoculum in oak afforestation depends on site characteristics rather than host availability. New For. 2022. [Google Scholar] [CrossRef]
- Xu, X.; Madden, L.V. Interrelationships Among SADIE Indices for Characterizing Spatial Patterns of Organisms. Phytopathology 2005, 95, 874–883. [Google Scholar] [CrossRef]
- Perry, J.N.; Winder, L.; Holland, J.M.; Alston, R.D. Red–blue plots for detecting clusters in count data. Ecol. Lett. 1999, 2, 106–113. [Google Scholar] [CrossRef]
- Maestre, F.T.; Cortina, J. Spatial patterns of surface soil properties and vegetation in a Mediterranean semi-arid steppe. Plant Soil 2002, 241, 279–291. [Google Scholar] [CrossRef]
- González-Rodríguez, V.; Villar, R.; Casado, R.; Suárez-Bonnet, E.; Quero, J.L.; Navarro-Cerrillo, R.M. Spatio-temporal heterogeneity effects on seedling growth and establishment in four Quercus species. Ann. For. Sci. 2011, 68, 1217–1232. [Google Scholar] [CrossRef]
- Donoso, C.; Landaeta, E. Ruil (Nothofagus alessandrii), a threatened Chilean tree species. Environ. Conserv. 1983, 10, 159–162. [Google Scholar] [CrossRef]
- Scharf, H. Local Indicators of Spatial Association (LISA). In Wiley StatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Soto, D.P.; Salas, C.; Donoso, P.J.; Uteau, D. Heterogeneidad estructural y espacial de un bosque mixto dominado por Nothofagus dombeyi después de un disturbio parcial. Rev. Chil. Hist. Nat. 2010, 83, 335–347. [Google Scholar] [CrossRef]
- Doll, U.; Araya, P.; Soto-Cerda, L.; Aedo, D.; Vizcarra, G. Producción y composición de la hojarasca en un renoval pre andino de Nothofagus glauca de la región del Maule. Bosque 2018, 39, 151–156. [Google Scholar] [CrossRef]
- Litton, C.M.; Orellana, M.; Bustamante, E. Estudio de la vegetación arbórea de una población relicto de Nothofagus alpina (P. et E.) Oerst. en la precordillera andina de la VII Región de Chile. Rev. Cien. For. 2000, 14–15, 38–49. [Google Scholar]
- Santelices-Moya, R.; Vergara, R.; Cabrera-Ariza, A.; Espinoza-Meza, S.; Silva-Flores, P. Variación intra-específica en Nothofagus glauca una especie endémica de los bosques mediterráneos de Chile. Bosque 2020, 41, 221–231. [Google Scholar] [CrossRef]
- Litton, C.M.; Santelices, R. Comparación de las comunidades vegetales en bosques de Nothofagus glauca (Phil.) Krasser en la Séptima Región de Chile. Bosque 1996, 17, 77–86. [Google Scholar] [CrossRef]
- Santelices, R.; Riquelme, M. Antecedentes dasométricos de Nothofagus alessandrii de la procedencia Coipué. Bosque 2007, 28, 281–287. [Google Scholar] [CrossRef]
- Torres-Díaz, C.; Valladares, M.A.; Acuña-Rodríguez, I.S.; Ballesteros, G.I.; Barrera, A.; Atala, C.; Molina-Montenegro, M.A. Symbiotic Interaction Enhances the Recovery of Endangered Tree Species in the Fragmented Maulino Forest. Front. Plant Sci. 2021, 12, 663017. [Google Scholar] [CrossRef]
- Puértolas, J.; Oliet, J.A.; Jacobs, D.F.; Benito, L.F.; Peñuelas, J.L. Is light the key factor for success of tube shelters in forest restoration plantings under Mediterranean climates? For. Ecol. Manag. 2010, 260, 610–617. [Google Scholar] [CrossRef]
- Oliet, J.A.; Blasco, R.; Valenzuela, P.; Melero de Blas, M.; Puértolas, J. Should we use meshes or solid tube shelters when planting in Mediterranean semiarid environments? New For. 2019, 50, 267–282. [Google Scholar] [CrossRef]
- Donoso, C. Bosques Templados de Chile y Argentina. Variación, Estructura y Dinámica; Editorial Universitaria: Santiago, Chile, 1993; p. 484. [Google Scholar]
- Silva, H. Análisis de la Distribución Espacial de los Árboles en los Bosques de Belloto del Norte (Beilschmiedia Miersii (Gay) Kosterm.) en el Cordón de Cantillana, Región Metropolitana, Chile; Universidad de Chile: Santiago, Chile, 2014. [Google Scholar]
- Bustamante, R.; Grez, A. Consecuencias ecológicas de la fragmentación de los bosques nativos. Ambiente y Desarrollo 1995, 11, 58–63. [Google Scholar]
- Acevedo, M.; Álvarez, C.; Cartes, E.; Dumroese, R.K.; González, M. Production and establishment techniques for the restoration of Nothofagus alessandrii, an endangered keystone species in a Mediterranean forest. New For. 2019, 51, 159–174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).