The Potential of Paulownia fortunei L. for the Phytoremediation of Pb
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experiment Method
2.2.1. Plant Growth Parameters
2.2.2. Plant Physiological and Biochemical Measures
2.2.3. Pb Content
2.2.4. Subcellular Distribution and Chemical Forms of Pb
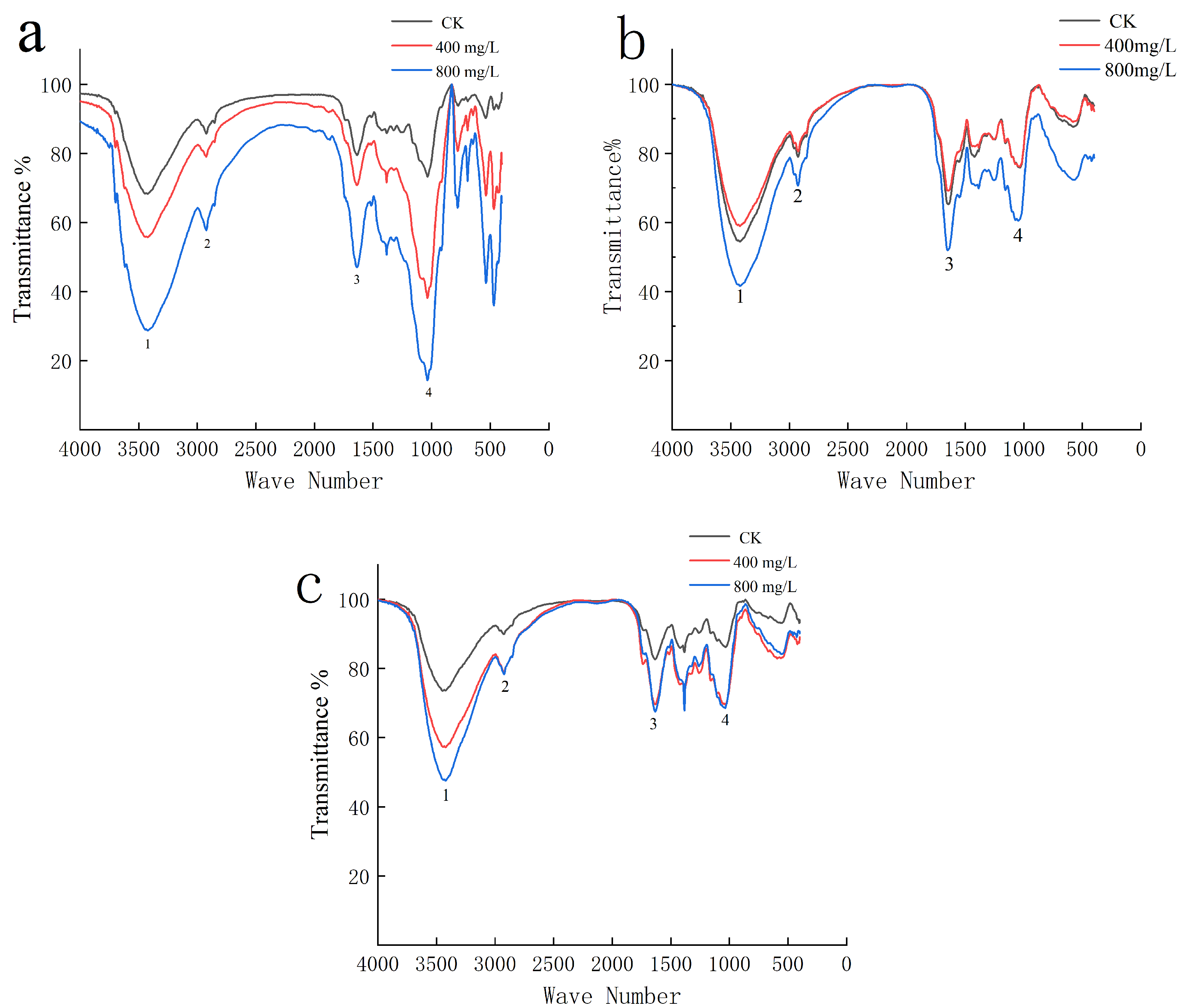
2.2.5. Plant Microstructure and Functional Group Composition
2.3. Data Analysis
3. Results
3.1. Influence of Pb Stress on the Growth of P. fortunei Seedlings
3.2. Pb Transport and Accumulation in P. fortunei Seedlings
3.3. Changes in Pb Contents in the Subcellular Components of P. fortunei Seedlings
3.4. Changes in the Chemical Morphology of Pb in P. fortunei Seedlings
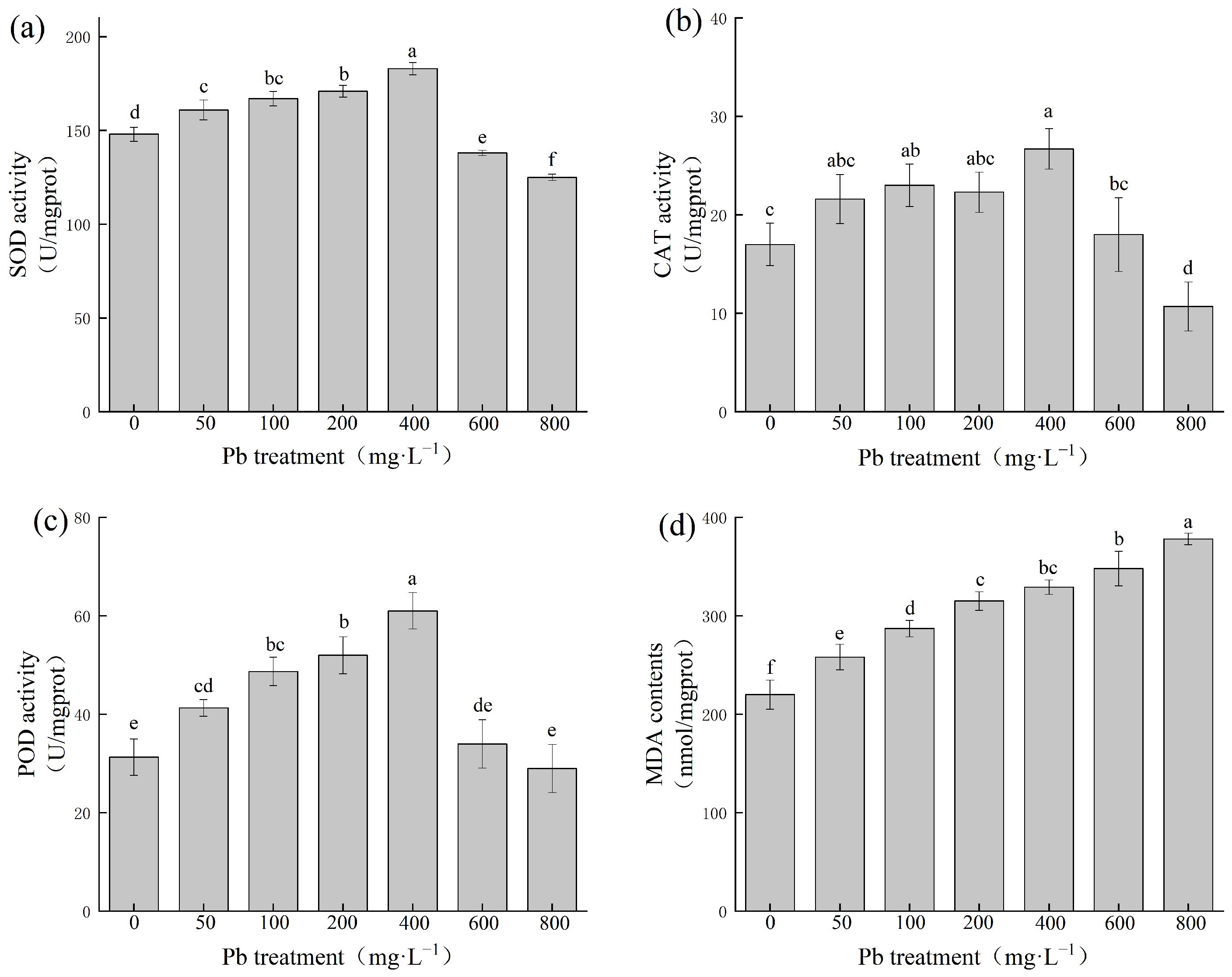
3.5. Physiological and Biochemical Effects of Pb Stress on P. fortunei Seedlings
3.6. Characterization of Roots, Stems, and Leaves of P. fortunei Seedlings under Pb Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Usman, K.; Abu-Dieyeh, M.H.; Zouari, N.; Al-Ghouti, M.A. Lead (Pb) bioaccumulation and antioxidative responses in Tetraena qataranse. Sci. Rep. 2020, 10, 17070. [Google Scholar] [CrossRef] [PubMed]
- Moshchenko, D.; Kolesnikov, S.I.; Kuzina, A.A.; Kazeev, K.S.; Minkina, T.M.; Mezhenkov, A.A.; Litvinov, Y.A.; Shende, S.S.; Mandzhieva, S.S.; Sushkova, S.N. Comparative Assessment of the Resistance to Lead (Pb) Pollution of Forest, Forest-Steppe, Steppe, and Mountain-Meadow Soils of the Central Ciscaucasia and the Caucasus Regions. Forests 2022, 13, 1528. [Google Scholar] [CrossRef]
- Bouchard, M.F.; Bellinger, D.C.; Weuve, J.; Matthews-Bellinger, J.; Gilman, S.E.; Wright, R.O.; Schwartz, J.; Weisskopf, M.G. Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in US young adults. Arch. Gen. Psychiatry 2009, 66, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Lanphear, B.P.; Hornung, R.; Khoury, J.; Yolton, K.; Baghurst, P.; Bellinger, D.C.; Canfield, R.L.; Dietrich, K.N.; Bornschein, R.; Greene, T.; et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ. Health Perspect. 2005, 113, 894–899. [Google Scholar] [CrossRef]
- Pourrut, B.; Jean, S.; Silvestre, J.; Pinelli, E. Lead-induced DNA damage in Vicia faba root cells: Potential involvement of oxidative stress. Mutat. Res. -Genet. Toxicol. Environ. Mutagen. 2011, 726, 123–128. [Google Scholar] [CrossRef]
- Tauqeer, H.M.; Ali, S.; Rizwan, M.; Ali, Q.; Saeed, R.; Iftikhar, U.; Ahmad, R.; Farid, M.; Abbasi, G.H. Phytoremediation of heavy metals by Alternanthera bettzickiana: Growth and physiological response. Ecotoxicol. Environ. Saf. 2016, 126, 138–146. [Google Scholar] [CrossRef]
- Shahid, M.; Ferrand, E.; Schreck, E.; Dumat, C. Behavior and Impact of Zirconium in the Soil-Plant System: Plant Uptake and Phytotoxicity. Rev. Environ. Contam. Toxicol. 2013, 221, 107–127. [Google Scholar]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Menon, M.; Hermle, S.; Abbaspour, K.C.; Günthardt-Goerg, M.S.; Oswald, S.E.; Schulin, R. Water regime of metal-contaminated soil under juvenile forest vegetation. Plant Soil 2005, 271, 227–241. [Google Scholar] [CrossRef]
- Wu, Y.; Qi, L.; Wang, B.Y.; Medley, P.; Drake, J.; Vernon, J.; Ibeanusi, V.; Chen, G. Assess long-term As, Pb and Cr contamination and uptake by Eriocaulon decangulare in the Apalachicola National Forest. Sci. Total Environ. 2022, 838, 156040. [Google Scholar] [CrossRef]
- Wang, D.; Guo, W.; Zhang, G.; Zhou, L.; Wang, M.; Lu, Y.; Cai, D.; Wu, Z. Remediation of Cr (VI)-contaminated acid soil using a nanocomposite. ACS Sustain. Chem. Eng. 2017, 5, 2246–2254. [Google Scholar] [CrossRef]
- Rigoletto, M.; Calza, P.; Gaggero, E.; Malandrino, M.; Fabbri, D. Bioremediation methods for the recovery of lead-contaminated soils: A review. Appl. Sci. 2020, 10, 3528. [Google Scholar] [CrossRef]
- Su, R.; Ou, Q.; Wang, H.; Luo, Y.; Dai, X.; Wang, Y.; Chen, Y.; Shi, L. Comparison of Phytoremediation Potential of Nerium indicum with Inorganic Modifier Calcium Carbonate and Organic Modifier Mushroom Residue to Lead–Zinc Tailings. Int. J. Environ. Res. Public Health 2022, 19, 10353. [Google Scholar] [CrossRef] [PubMed]
- Mohtadi, A.; Ghaderian, S.M.; Schat, H. A comparison of lead accumulation and tolerance among heavy metal hyperaccumulating and non-hyperaccumulating metallophytes. Plant Soil 2012, 352, 267–276. [Google Scholar] [CrossRef]
- Jaffré, T.; Pillon, Y.; Thomine, S.; Merlot, S. The metal hyperaccumulators from New Caledonia can broaden our understanding of nickel accumulation in plants. Front. Plant Sci. 2013, 4, 279. [Google Scholar] [CrossRef] [PubMed]
- Capuana, M. Heavy metals and woody plants-biotechnologies for phytoremediation. Iforest-Biogeosciences For. 2011, 4, 7. [Google Scholar] [CrossRef]
- Gallagher, F.J.; Pechmann, I.; Bogden, J.D.; Grabosky, J.; Weis, P. Soil metal concentrations and vegetative assemblage structure in an urban brownfield. Environ. Pollut. 2008, 153, 351–361. [Google Scholar] [CrossRef]
- Kong, S.X.; Li, H.K.; Chao, J.; Cui, X.S.; Guo, Y.H. Effects of Pb Stress on Photosynthetic Pigment Biosynthesis and Growth of Rabdosia rubescens. Zhong Yao Cai 2015, 38, 215–220. [Google Scholar]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef]
- Peng, X.; Yang, B.; Deng, D.; Dong, J.; Chen, Z. Lead tolerance and accumulation in three cultivars of Eucalyptus urophyllaXE. grandis: Implication for phytoremediation. Environ. Earth Sci. 2012, 67, 1515–1520. [Google Scholar] [CrossRef]
- Kanwal, U.; Ibrahim, M.; Abbas, F.; Yamin, M.; Jabeen, F.; Shahzadi, A.; Farooque, A.A.; Imtiaz, M.; Ditta, A.; Ali, S. Phytoextraction of Lead Using a Hedge Plant [Alternanthera bettzickiana (Regel) G. Nicholson]: Physiological and Biochemical Alterations through Bioresource Management. Sustainability 2021, 13, 5074. [Google Scholar] [CrossRef]
- Silbergeld, E.K. Implications of new data on lead toxicity for managing and preventing exposure. Environ. Health Perspect. 1990, 89, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.N.; Chen, Y.H.; Du, L.; Zhang, M.Y.; Han, L.Z. Accumulation and subcellular distribution of heavy metal in Paulownia fortunei cultivated in lead-zinc slag amended with peat. Int. J. Phytoremediation 2019, 21, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Chen, G.; Xiong, L.; Liu, Q.; Yang, W. Accurate digitization of the chlorophyll distribution of individual rice leaves using hyperspectral imaging and an integrated image analysis pipeline. Front. Plant Sci. 2017, 8, 1238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Chen, Y.; Du, L.; Wu, Y.; Liu, Z.; Han, L. The potential of Paulownia fortunei seedlings for the phytoremediation of manganese slag amended with spent mushroom compost. Ecotoxicol. Environ. Saf. 2020, 196, 110538. [Google Scholar] [CrossRef]
- Deng, H.; Xu, D.; Li, M.; Li, J. Comparison of different digestion methods in analyzing heavy metals content in soils. J. Guangxi Norm. Univ. Nat. Sci. Ed. 2010, 28, 80–83. [Google Scholar]
- Cai, B.; Chen, Y.; Du, L.; Liu, Z.; He, L. Spent mushroom compost and calcium carbonate modification enhances phytoremediation potential of Macleaya cordata to lead-zinc mine tailings. J. Environ. Manag. 2021, 294, 113029. [Google Scholar] [CrossRef]
- Han, L.; Chen, Y.; Chen, M.; Wu, Y.; Su, R.; Du, L.; Liu, Z. Mushroom residue modification enhances phytoremediation potential of Paulownia fortunei to lead-zinc slag. Chemosphere 2020, 253, 126774. [Google Scholar] [CrossRef]
- Feng, P.; Sun, L.; Shen, X.; Li, R.; Jiang, C.; Zheng, H.; Li, Z.; Li, Z.; Guo, W.; Han, X. Response and accumulation ability of perennial ryegrass to plumbum and cadmium stress. Fresenius Environ. Bull. 2017, 26, 598–606. [Google Scholar]
- Ma, L.; Sun, H.; Chen, L.; Zhao, J. Relation between heavy metal fraction in soils and plants enrichment in pilot scale experiment on land application of sewage sludge. J. Food Agric. Environ. 2011, 9, 967–973. [Google Scholar]
- Basile, A.; Sorbo, S.; Conte, B.; Cardi, M.; Esposito, S. Ultrastructural changes and Heat Shock Proteins 70 induced by atmospheric pollution are similar to the effects observed under in vitro heavy metals stress in Conocephalum conicum (Marchantiales-Bryophyta). Environ. Pollut. 2013, 182, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-X.; Xiao, H.; Guo, Q.J.; Zhang, Z.Y.; Yang, X.; Kong, J. Subcellular Distribution and Chemical Forms of Heavy Metals in Three Types of Compositae Plants from Lead-Zinc Tailings Area. Huan Jing Ke Xue Huanjing Kexue 2017, 38, 3054–3060. [Google Scholar] [PubMed]
- Dai, F.W.; Luo, G.; Li, Z.; Wei, X.; Wang, Z.; Lin, S.; Tang, C. Physiological and transcriptomic analyses of mulberry (Morus atropurpurea) response to cadmium stress. Ecotoxicol. Environ. Saf. 2020, 205, 10. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, L.; Guerriero, G.; Sergeant, K.; Cai, G.; Hausman, J.F. Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: Differences in the response mechanisms. Front. Plant Sci. 2015, 6, 16. [Google Scholar] [CrossRef]
- Su, R.; Xie, T.; Yao, H.; Chen, Y.; Wang, H.; Dai, X.; Wang, Y.; Shi, L.; Luo, Y. Lead Responses and Tolerance Mechanisms of Koelreuteria paniculata: A Newly Potential Plant for Sustainable Phytoremediation of Pb-Contaminated Soil. Int. J. Environ. Res. Public Health 2022, 19, 14968. [Google Scholar] [CrossRef]
- Cheng, H.-K.; Zhang, B.; Jing, X.-X.; Yang, S.-Q.; Zhao, P.; Sun, X.-X.; Zhou, Z.-Y. Response of Maize to Lead Stress and Relevant Chemical Forms of Lead. Huan Jing Ke Xue Huanjing Kexue 2015, 36, 1468–1473. [Google Scholar]
- Staszak, A.M.; Małecka, A.; Ciereszko, I.; Ratajczak, E. Differences in stress defence mechanisms in germinating seeds of Pinus sylvestris exposed to various lead chemical forms. PLoS ONE 2020, 15, 15. [Google Scholar] [CrossRef]
- He, L.; Su, R.; Chen, Y.; Zeng, P.; Du, L.; Cai, B.; Zhang, A.; Zhu, H. Integration of manganese accumulation, subcellular distribution, chemical forms, and physiological responses to understand manganese tolerance in Macleaya cordata. Environ. Sci. Pollut. Res. 2022, 29, 39017–39026. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, H.; Ji, L.; Ru, G.; Zhao, Z.; Cai, Y.; Wen, D. Physiological and enrichment characteristics of Paulownia fortunei seedlings under zinc, cadmium and their combined stress. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2021, 37, 2463–2473. [Google Scholar]
- Shakya, K.; Chettri, M.K.; Sawidis, T. Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Arch. Environ. Contam. Toxicol. 2008, 54, 412–421. [Google Scholar] [CrossRef]
- Sharma, I.; Sharma, A.; Pati, P.; Bhardwaj, R. Brassinosteroids Reciprocates Heavy Metals Induced Oxidative Stress in Radish by Regulating the Expression of Key Antioxidant Enzyme Genes. Braz. Arch. Biol. Technol. 2018, 61, 9. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.; Huang, M.; Luo, J.; Hou, X.; Wu, P.; Ma, X. Lead tolerance mechanism in Conyza canadensis: Subcellular distribution, ultrastructure, antioxidative defense system, and phytochelatins. J. Plant Res. 2016, 129, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef]


| Pb Concentration (mg·L−1) | Plant Height (cm) | Total Biomass (g) | Root Length (cm) | Root Surface Area (cm)2 | Average Root Diameter (mm) | Root Tip Number (a) |
|---|---|---|---|---|---|---|
| 0 | 30.1 ± 2.9 d | 7.3 ± 1.8 d | 59.3 ± 1.5 c | 17.7 ± 1.7 d | 0.6 ± 0.4 e | 433 ± 25 d |
| 50 | 34.0 ± 1.3 c | 12.2 ± 1.1 c | 60.0 ± 4.3 c | 22.1 ± 0.9 c | 1.3 ± 0.5 d | 569 ± 44 c |
| 100 | 39.2 ± 1.1 b | 15.9 ± 1.9 c | 66.1 ± 3.8 b | 24.8 ± 1.6 c | 2.5 ± 0.2 c | 606 ± 25 c |
| 200 | 39.4 ± 1.7 b | 22.4 ± 2.7 b | 73.8 ± 1.0 a | 30.6 ± 2.2 b | 3.8 ± 0.2 b | 737 ± 28 b |
| 400 | 45.2 ± 1.4 a | 32.6 ± 2.4 a | 78.5 ± 1.7 a | 36.4 ± 1.9 a | 4.4 ± 0.3 a | 818 ± 18 a |
| 600 | 25.1 ± 1.0 e | 5.5 ± 0.4 de | 34.2 ± 1.8 d | 8.1 ± 1.3 e | 2.4 ± 0.2 c | 351 ± 36 e |
| 800 | 16.0 ± 1.3 f | 3.3 ± 0.5 e | 18.7 ± 1.7 e | 4.8 ± 0.5 f | 1.4 ± 0.2 d | 269 ± 24 f |
| Pb Treatment mg·L−1 | Pb Content (mg·kg−1) | BCF | TF | ||
|---|---|---|---|---|---|
| Roots | Stems | Leaves | |||
| 50 | 229.8 ± 15.18 f | 7.5 ± 0.85 d | 14.1 ± 1.08 f | 0.18 ± 0.01 a | 0.09 ± 0.01 e |
| 100 | 460.5 ± 9.55 e | 25.7 ± 3.98 cd | 29.8 ± 2.34 e | 0.19 ± 0.01 a | 0.12 ± 0.01 d |
| 200 | 821.0 ± 25.69 d | 55.2 ± 3.38 c | 63.7 ± 3.78 d | 0.15 ± 0.01 b | 0.15 ± 0.01 d |
| 400 | 1108 ± 41.56 c | 130.1 ± 15.33 b | 175.5 ± 10.58 c | 0.12 ± 0.01 c | 0.28 ± 0.02 c |
| 600 | 1349.4 ± 17.46 b | 177.0 ± 7.46 a | 386.1 ± 12.60 a | 0.09 ± 0.003 d | 0.42 ± 0.01 a |
| 800 | 1587.0 ± 23.41 a | 186.9 ± 7.58 a | 339.2 ± 38.00 b | 0.06 ± 0.006 e | 0.33 ± 0.02 b |
| Pb Treatment mg·L−1 | Pb Content (mg·kg−1) Percentage (%) | |||||
|---|---|---|---|---|---|---|
| Root Stem Leaves | Root Stem Leaves | |||||
| F1 | 159.9 ± 3.4 a | 4.99 ± 0.2 a | 8.3 ± 0.2 a | 75.7 | 71.1 | 60.1 |
| 50 F2 | 22.1 ± 0.3 b | 1.0 ± 0.02 b | 2.4 ± 0.1 c | 10.5 | 9.5 | 17.4 |
| F3 | 26.5 ± 2.2 b | 1.4 ± 0.04 b | 3.1 ± 0.1 b | 13.8 | 19.4 | 22.5 |
| F1 | 300.7 ± 7.8 a | 13.9 ± 0.8 a | 13.4 ± 1.8 a | 71.8 | 63.5 | 49.6 |
| 100 F2 | 43.0 ± 2.5 c | 3.2 ± 0.2 c | 6.6 ± 0.1 c | 10.8 | 10.5 | 20.8 |
| F3 | 72.7 ± 2.4 b | 5.7 ± 0.3 b | 8.0 ± 0.3 b | 17.4 | 26.0 | 29.6 |
| F1 | 549.2 ± 5.9 a | 29.4 ± 1.7 a | 30.4 ± 1.4 a | 68.3 | 60.4 | 47.6 |
| 200 F2 | 96.3 ± 2.2 c | 5.8 ± 0.1 c | 14.2 ± 1.3 c | 12.0 | 11.9 | 21.1 |
| F3 | 157.8 ± 2.1 b | 13.4 ± 2.0 b | 20.0 ± 3.7 b | 19.7 | 27.7 | 31.3 |
| F1 | 689.4 ± 6.0 a | 68.0 ± 3.3 a | 74.9 ± 1.8 a | 65.2 | 56.9 | 45.5 |
| 400 F2 | 112.7 ± 5.7 c | 13.5 ± 3.6 c | 34.5 ± 1.8 c | 11.3 | 11.3 | 21.0 |
| F3 | 248.6 ± 2.9 b | 36.0 ± 2.9 b | 55.6 ± 3.2 b | 23.5 | 31.8 | 33.5 |
| F1 | 830.8 ± 5.2 a | 86.0 ± 5.4 a | 155.6 ± 4.7 a | 61.7 | 49.3 | 41.3 |
| 600 F2 | 192.7 ± 4.1 c | 32.5 ± 2.2 c | 95.9 ± 2.9 c | 14.3 | 18.6 | 25.4 |
| F3 | 325.9 ± 15.8 b | 59.4 ± 1.0 b | 126.1 ± 5.4 b | 24.0 | 32.1 | 33.3 |
| F1 | 837.0 ± 8.9 a | 84.4 ± 9.0 a | 123.6 ± 7.8 a | 54.8 | 46.0 | 38.0 |
| 800 F2 | 261.6 ± 3.4 c | 40.5 ± 2.6 c | 87.4 ± 2.8 b | 17.1 | 20.8 | 26.8 |
| F3 | 430.0 ± 3.4 b | 61.0 ± 0.8 b | 114.2 ± 3.3 a | 28.1 | 33.2 | 35.2 |
| Pb Concentration | Chlorophyll a (mg·g−1) | Chlorophyll b (mg·g−1) | Carotenoids (mg·g−1) | Total Chlorophyll (mg·g−1) | Chlorophyll a/b |
|---|---|---|---|---|---|
| 0 | 9.3 ± 1.63 c | 5.4 ± 0.28 c | 2.0 ± 0.27 b | 1.9 ± 0.35 c | 1.8 ± 0.74 c |
| 50 | 9.3 ± 1.03 c | 5.5 ± 0.40 bc | 2.0 ± 0.11 b | 2.0 ± 0.04 c | 1.7 ± 0.10 c |
| 100 | 11.8 ± 0.80 b | 6.0 ± 0.21 a | 2.1 ± 0.09 b | 2.3 ± 0.05 b | 2.0 ± 0.07 b |
| 200 | 12.7 ± 1.59 b | 6.1 ± 0.20 a | 2.3 ± 0.08 ab | 2.5 ± 0.06 b | 2.1 ± 0.21 b |
| 400 | 14.9 ± 0.73 a | 5.9 ± 0.19 ab | 2.5 ± 0.19 a | 2.8 ± 0.11 a | 2.5 ± 0.08 a |
| 600 | 7.4 ± 0.44 c | 5.3 ± 0.14 c | 1.7 ± 0.16 c | 1.6 ± 0.04 d | 1.4 ± 0.05 d |
| 800 | 5.0 ± 0.17 d | 4.4 ± 0.04 d | 1.2 ± 0.12 d | 1.2 ± 0.11 e | 1.1 ± 0.03 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.; Yang, H.; Xie, J.; Han, L.; Liu, Z.; Liu, Z.; Chen, Y.; Su, R. The Potential of Paulownia fortunei L. for the Phytoremediation of Pb. Forests 2023, 14, 1245. https://doi.org/10.3390/f14061245
Du L, Yang H, Xie J, Han L, Liu Z, Liu Z, Chen Y, Su R. The Potential of Paulownia fortunei L. for the Phytoremediation of Pb. Forests. 2023; 14(6):1245. https://doi.org/10.3390/f14061245
Chicago/Turabian StyleDu, Lu, Hang Yang, Juan Xie, Liangze Han, Zhiyi Liu, Zhiming Liu, Yonghua Chen, and Rongkui Su. 2023. "The Potential of Paulownia fortunei L. for the Phytoremediation of Pb" Forests 14, no. 6: 1245. https://doi.org/10.3390/f14061245





