Abstract
New and emerging Phytophthora-related diseases in small trees, shrubs and herbaceous plants typical of subalpine vegetation have recently been observed in Italy and Slovenia. Diseased plants showed a complex symptomatology including foliar necrosis, fruit rot, shoot blight and branch bleeding cankers. Since little information is available about the aetiology of these aerial Phytophthora diseases, from 2019 to 2022, field surveys were conducted in 54 sites to define the occurrence, distribution and impact of the Phytophthora species on mountain vegetation. A total of 360 Phytophthora isolates were obtained from 397 samples collected from 33 herbaceous and woody host species. Based on phylogenetic analysis and morphometric data, 17 Phytophthora species were identified: P. pseudosyringae (201 isolates), P. plurivora (54), P. gonapodyides (21), P. ilicis (20), P. alpina (17), P. acerina (11), P. cactorum (7), P. pseudocryptogea (6), P. cambivora (5), P. idaei (4), P. psychrophila (3), P. bilorbang (2), P. chlamydospora (2), P. hedraiandra (1), P. kelmanii (1), P. rosacearum (1) and P. syringae (1). In addition, three isolates of a new putative Phytophthora species obtained from Alnus viridis, Juniperus communis and Rhododendron ferrugineum are described here as Phytophthora pseudogregata sp. nov. Overall, the results highlighted an unexpectedly high diversity of Phytophthora species in mountain areas, with many species able to cause aerial infections due to the production of caducous sporangia.
1. Introduction
The large genus Phytophthora de Bary includes several invasive plant pathogens that represent an increasing threat to forest ecosystems and agriculture productions worldwide [1,2,3,4]. Over the last 20 years, scientific interest in this group of oomycetes has increased rapidly in forest pathology and this has led to the discovery of several new species and pathosystems [5,6,7,8].
Most of the known Phytophthora species have a soilborne or waterborne lifestyle, due to the production of persistent sporangia and the release of motile zoospores [9,10]. The majority of Phytophthora species are necrotrophic or hemibiotrophic pathogens, able to cause root rot diseases in herbaceous and woody plant hosts; whereas a few species, especially those strongly associated with water habitats, can also survive as saprophytes [11]. The main symptoms caused by pathogenic Phytophthora species with a soilborne lifestyle include fine root losses, root rot, collar necrosis and stem bleeding cankers. Plants with root and collar infections show nonspecific secondary symptoms at the canopy level, such as epicormic shoots and sudden death [1,12].
Conversely, Phytophthora species with an airborne or mixed airborne and soilborne lifestyle have the ability to produce caducous sporangia and infect fruits, leaves, shoots, twigs and branches, causing necrosis, rots and an anticipated loss of organs [1,13,14,15]. Caducous sporangia can act directly as infective propagules or release motile zoospores [1]. Aerial Phytophthora infection can occur actively via lenticels or stomata in the epigeal organs of the host [16]. The ability to produce caducous sporangia is a feature common in the species belonging to clades 1, 3, 4 and 8 [17]. Within this last clade, one of the most aggressive species is Phytophthora ramorum, known to cause leaf blight, shoot blight and bleeding cankers on forest and ornamental plant species in the temperate areas of North America and Europe [5,18,19]. Other species belonging to clade 8, such as P. foliorum and P. hibernalis have been reported as airborne pathogens on Rhododendron and Citrus spp. [20,21,22]. In agriculture and horticulture, species of clades 1 and 4, such as P. cactorum, P. infestans, P. nicotianae and P. palmivora, are well known to cause leaf, stem and fruit diseases on many herbaceous and wood crops [23,24,25,26,27,28,29].
Clade 3 includes a few cryptic species characterized by a partial aerial lifestyle with a relatively low optimum temperature for growth and a common association with native forest species [14,30,31]. In particular, Phytophthora pseudosyringae is emerging as an invasive pathogen on a broad number of hosts at global scale [14,32,33,34].
In Europe, aerial Phytophthora diseases have been studied mainly on agricultural crops [1,26,35,36,37,38] and to a much lesser extent on forest trees, especially in subalpine ecosystems [14]. Alpine and subalpine regions are important biodiversity hotspots for the flora, including a large number of plants and many endemisms in very confined environments and extreme conditions [39]. Due to the huge floristic diversity in small spatial scales, mountain forests could represent useful models to understand the ecological and evolutionary host–pathogen dynamics and to conserve pristine ecosystems [40,41].
Therefore, given the growing expansion of Phytophthora diseases in subalpine ecosystems in Italy and Slovenia and the still limited information available about these pathosystems, a study was conducted to isolate, identify and characterize the main pathogens associated with these new and emerging diseases.
2. Materials and Methods
2.1. Field Survey and Sampling Procedure
Field surveys were conducted from autumn 2019 to summer 2022 in 54 sites distributed in different mountainous areas of Northeast Italy, Sardinia and Western Slovenia (Table 1). The monitored sites are located at an altitude ranging from the valley bottom (700 m a.s.l.) to above the tree line (2100 m a.s.l.) and include forests, riparian ecosystems and heathlands typical of alpine and subalpine formations.

Table 1.
Study sites information, plant species monitored and number of samples collected.
At each site, plants were visually checked for the occurrence of typical Phytophthora symptoms, such as leaf and fruit necrosis, shoot blight, wilting twigs, branches dieback and bleeding cankers. In the most impacted formations, the disease incidence and mortality rate were estimated along 25 m long linear plots. Disease incidence was calculated as the number of symptomatic trees out of the total number of trees (DI = n/N × 100) and mortality as the number of dead trees out of the total number of trees (M = d/N × 100) [42].
In each site, a variable number of tissue samples of leaves, twigs and branches was collected from symptomatic plants (Table 1). Overall, 397 samples were collected from 33 host species, small trees, shrubs and herbaceous plants. These included Acer monspessulanum, Acer pseudoplatanus, Alnus cordata, Alnus glutinosa, Alnus incana, Alnus viridis, Betula pubescens, Calluna vulgaris, Erica carnea, Fagus sylvatica, Fragaria vesca, Fraxinus excelsior, Genista corsica, Ilex aquifolium, Juniperus communis, Laburnum alpinum, Larix decidua, Lonicera alpigena, Lycopodium clavatum, Pinus mugo, Populus tremula, Quercus pubescens, Rhododendron ferrugineum, Rhododendron hirsutum, Rubus idaeus, Salix alpina, Salix atrocinerea, Salix caprea, Sorbus aria, Sorbus aucuparia, Taxus baccata, Vaccinium myrtillus and Vaccinium vitis-idaea (Table 1). The samples were sealed in plastic bags, labelled and used for Phytophthora isolations within 24–48 h.
2.2. Phytophthora Isolation and Characterization
Phytophthora isolation was performed directly from the symptomatic tissue samples. Necrotic leaves were externally disinfected and cut in small pieces along the border of active lesions, whereas shoots and bark samples from bleeding cankers, after removing the outer bark, were cut in small fragments (along the margin of each lesion) with a sterile scalpel. In both cases, small pieces of 3–5 mm2 were placed on 90 mm diameter Petri dishes containing the selective medium PDA+ [14]. In samples that resulted negative, the procedure was repeated up to three times. After incubation at 20 °C for 3 days in the dark, hyphal tips of emerging colonies were taken and transferred into new PDA and carrot agar (CA) Petri dishes and incubated at 20 °C in the dark.
Isolates were morphologically examined and then grouped into morphotypes based on colony appearance and morpho-biometric data of sporangia, oogonia, chlamydospores and hyphal swellings. To enhance the production of sporangia, CA plugs of each isolate were placed in Petri dishes containing pond water and asymptomatic alder roots. Petri dishes were kept at 20 °C in the dark and sporangia production was assessed every 12 h for 3 days.
For the new putative species, colony morphology was determined on 7-day-old cultures incubated at 20 °C in the dark as reported in Bregant et al. [14]. Cardinal temperatures for growth were evaluated on CA plates incubated at 2, 5, 10, 15,18, 20, 23, 25, 27, 30, 32 and 34 °C (±0.5 °C) in the dark. Five replicates for each isolate were made and colony diameter was measured after 7 days. Morphology of sporangia (n. 50) and the ability to produce hyphal swellings and chlamydospores was recorded for each isolate. Breeding system was examined after 20 days on CA at 20 °C in the dark. Measurements and photos of the morphological structures (sporangia, chlamydospores, hyphal swellings, oogonia and anteridia) were recorded using the software Motic Images Plus 3.0 paired with a Moticam 10+ camera connected to a Motic BA410E microscope. The sizes are presented as mean values ± standard deviation.
Representative isolates of each species were stored on PDA and CA slants under oil in the culture collection of the Dipartimento Territorio e Sistemi Agro-Forestali, Università degli Studi di Padova.
The ex-type culture of the new species was deposited at the Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands, and nomenclatural data in MycoBank (www.MycoBank.org, accessed on 29 June 2023). The holotype was lodged with the herbarium of Westerdijk Fungal Biodiversity Institute as a dried culture on CA.
2.3. Molecular Identification of the Isolates
Isolation of genomic DNA was performed from the mycelium of 7-day-old Phytophthora colonies as reported in Linaldeddu et al. [28]. For all isolates, the internal transcribed spacer (ITS) region of the rDNA, including the 5.8S rRNA gene, was amplified and sequenced using the universal primers ITS1 and ITS4 [43]. ITS sequences were used to confirm the identification at species level. For three isolates of the new putative species another two DNA regions, namely ß-tubulin (Btub) and cytochrome c oxidase subunit I (cox1), were amplified and sequenced using the primer-pairs TUBUF2/TUBUR1 and FM84/FM83 [44,45], respectively. Polymerase chain reactions (PCR) were performed in 50 µl reaction mixtures using the GoTaq® Hot Start Green Master Mix (Promega, Milano, Italy) and a SimpliAmp Thermal Cycler (Thermo Fisher Scientific Inc, Waltham, MA, USA). Amplification conditions for the three DNA regions were as follows: an initial denaturation step at 94 °C for 1 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 1 min and a final elongation step of 7 min at 72 °C for ITS; an initial denaturation step at 94 °C for 2 min, followed by 35 cycles of denaturation at 9 °C for 40 s, annealing at 54 °C for 1 min, extension at 72 °C for 1 min and a final elongation step of 7 min at 72 °C for Btub; and an initial denaturation at 95 °C for 2 min followed by 38 cycles at 95 °C for 25 s, 53 °C for 50 s, 72 °C for 70 s and a final extension step of 9 min at 72 °C for cox1.
The PCR products were purified using the MonarchTM PCR & DNA Cleanup Kit according to the manufacturer’s instructions (New England Biolabs, Ipswich, MA, USA) and sequenced by BMR Genomics DNA sequencing service (www.bmr-genomics.it accessed on 23 June 2023). Sequences were edited with FinchTV v1.4.0 (Geospiza, Inc., Seattle, WA, USA, http://www.geospiza.com/finchtv, accessed on 29 June 2023) and compared with sequences of ex-type culture deposited in GenBank (http://blast.ncbi.nlm.nih.gov accessed on 23 June 2023). New sequences were deposited in GenBank (Table 2).

Table 2.
Details of Phytophthora isolates included in the phylogenetic analyses. Ex-type cultures are given in bold typeface and newly generated sequences are indicated in italics.
2.4. Phylogenetic Analysis
Molecular phylogeny based on ITS sequences was used to reconstruct evolutionary relationships among the Phytophthora species obtained in this study into the known clades of the genus [17]. Twenty ITS sequences representative of the 18 species obtained were compiled in a dataset together with 51 sequences from ex-type material of Phytophthora species representative of all phylogenetical clades (Table 2).
In addition, a multigene phylogeny based on concatenated ITS, Btub and cox1 sequences of three isolates obtained in this study and other 19 formally described Phytophthora species in the sub-clade 6b, including ex-type cultures was performed (Table 2).
Sequences were aligned with ClustalX v. 1.83 [46], using the parameters reported by Bregant et al. [14].
Phylogenetic reconstructions were performed with MEGA-X 10.1.8, including all gaps in the analyses. The best model of DNA sequence evolution was determined automatically by the software [47]. Maximum likelihood (ML) analysis was performed with a neighbour-joining (NJ) starting tree generated by the software. A bootstrap analysis (1000 replicates) was used to estimate the robustness of nodes. Alignments and trees are available in TreeBase [studies S30526 and S30527].
2.5. Pathogenicity Test
The pathogenicity of the new Phytophthora species and other seven species isolated for the first time from common juniper was tested on 5-year-old Juniperus communis seedlings grown in plastic pots (20 cm diameter, 5 L volume). Ten 2-year branches were inoculated with each isolate, and ten were used as control. Inoculated point was surface-disinfected with 70% ethanol and a small piece of outer and inner bark (3 × 3 mm) was removed with a flamed scalpel. An agar mycelium plug of the same size (3 × 3 mm) taken from the margin of an actively growing colony (4-day-old) on PDA was placed into the wound and the inoculation point was covered with moistened cotton and wrapped in aluminium foil. Control seedlings were inoculated with a sterile PDA plug applied as described above.
All inoculated seedlings were kept in a cold greenhouse at 17 to 26 °C and watered regularly for 30 days. At the end of the experimental period, seedlings were checked for the presence of external and internal disease symptoms. The length of necrotic lesion surrounding each inoculation point was measured after removing the outer bark with a scalpel.
Re-isolation was performed by transferring 10 pieces of inner bark fragments taken around the margin of the necrotic lesions onto PDA+. Growing colonies were subcultured onto CA, incubated in the dark at 20 °C for seven days and identified by morphological and molecular analysis (ITS region).
2.6. Data Analysis
The variation in Phytophthora community structure among trees, shrubs and herbaceous plant species was assessed using the Jaccard similarity index (Jc) based on presence or absence of species among different microbial communities [48], Jc = j/(a + b + j), where j = represents the number of species in common between the two groups; a = the number of species isolated from group A; b = number of species isolated from group B.
The diversity of Phytophthora species associated with the three different plant types was calculated using the Margalef richness index (d) [49], the Shannon diversity index (H) [50] and the evenness index (J) [51]. The indices were calculated using Past software, version 4.03 [52].
Similarity in terms of taxonomic richness among the communities within the three plant categories was schematized through the use of Venn diagrams [53], using GeneVenn software to generate the diagram (https://www.bioinformatics.org/gvenn/ accessed on 23 June 2023) and reconstructing it in Canva (https://www.canva.com/ accessed on 23 June 2023).
Results of the pathogenicity test were checked for normality, then subjected to analysis of variance (ANOVA). Significant differences among mean values were determined using Fisher’s least significant differences multiple range test (p = 0.05) after one-way ANOVA using XLSTAT 2008 software (Addinsoft, Paris, France).
3. Results
3.1. Symptomatology
Monitoring surveys conducted in 54 sites distributed in Italy and Slovenia allowed the occurrence of Phytophthora-related diseases to be detected in several plants typical of the alpine and subalpine climate. Disease incidence was highest in shrub vegetation, alpine heathlands and along the mountain riparian systems, ranging from 25 to 100%, with a mortality rate between 5 and 45% (Table 3). The most impacted ecosystems were heathlands dominated by common juniper and blueberry, and alder riparian systems (Figure 1). In these ecosystems, Phytophthora outbreaks showed an epidemic trend with a high mortality rate.

Table 3.
Symptoms observed on each plant host and disease incidence/mortality rate estimated.

Figure 1.
Overview of aerial Phytophthora disease symptoms observed on: Alnus viridis (1), Juniperus communis (2), Rhododendron ferrugineum (3) Vaccinium myrtillus (4); Alnus cordata (5), Alnus glutinosa (6), Alnus viridis (7,8), Juniperus communis (9,10), Ilex aquifolium (11), Lycopodium clavatum (12), Pinus mugo (13), Populus tremula (14), Salix caprea (15), Taxus baccata (16), Rhododendron spp. (17–19), Vaccinium spp. (20–22); Alnus spp. (23–25), Ilex aquifolium (26), Fagus sylvatica (27) and Salix caprea (28). On the left, starting from the top, colony morphology of: Phytophthora acerina, P. alpina, P. bilorbang, P. cactorum, P. cambivora, P. chlamydospora, P. gonapodyides, P. hedraiandra, P. idaei, P. ilicis, P. kelmanii, P. plurivora, P. pseudocryptogea, P. pseudogregata, P. pseudosyringae, P. psychrophila, P. rosacearum and P. syringae after 7 days of growth at 20 °C on CA in the dark.
Many of the aerial Phytophthora symptoms observed were new and involved various plant organs such as leaves (moist necrotic lesions), fruit (rot), twigs (wilting and shoot blights). Moreover, on tree and shrub species stem and branches extensive bleeding cankers were observed (Figure 1). Cankers and necrosis progressively girdled the circumference of the branch, causing partial or total death of the crown.
On shrubs and heath formations, the disease was initially observed in small areas and progressively spread in a concentric manner affecting more plant species (Figure 1).
3.2. Aetiology
Isolations performed on 397 samples yielded a total of 360 Phytophthora isolates. Based on morphological features and ITS sequence data, 17 known Phytophthora species were identified, namely: P. pseudosyringae (201 isolates), P. plurivora (54), P. gonapodyides (21), P. ilicis (20), P. alpina (17), P. acerina (11), P. cactorum (7), P. pseudocryptogea (6), P. cambivora (5), P. idaei (4), P. psychrophila (3), P. bilorbang (2), P. chlamydospora (2), P. hedraiandra (1), P. kelmanii (1), P. rosacearum (1) and P. syringae (1).
In addition, three isolates obtained from necrotic tissues of Alnus viridis, Juniperus communis and Rhododendron ferrugineum could not be assigned to any known Phytophthora species and are therefore described here as Phytophthora pseudogregata sp. nov.
The assemblage and distribution of Phytophthora species was very variable among hosts and geographic areas. The 33 plant species monitored were divided into three main categories: small trees (Table 4), shrubs/heathland species (Table 5) and herbaceous/perennial plant species (Table 6).

Table 4.
Number of Phytophthora isolates obtained from the different plant hosts. In brackets the number of sites for each Phytophthora species: Phytophthora acerina (ACE), P. cactorum (CAC), P. cambivora (CAM), P. gonapodyides (GON), P. idaei (IDA), P. ilicis (ILI), P. kelmanii (KEL), P. plurivora (PLU), P. pseudosyringae (PSS) and P. psychrophila (PSY).

Table 5.
Number of Phytophthora isolates obtained from the different plant hosts. In brackets the number of sites for each Phytophthora species: Phytophthora acerina (ACE), P. alpina (ALP), P. cactorum (CAC), P. chlamydospora (CHL), P. hedraiandra (HED), P. plurivora (PLU), P. pseudocryptogea (PSC), P. pseudogregata (PSG), P. pseudosyringae (PSS), P. rosacearum (ROS) and P. syringae (SYR).

Table 6.
Number of Phytophthora isolates obtained from the herbaceous plant hosts. In brackets the number of sites for each Phytophthora species: Phytophthora cactorum (CAC), P. idaei (IDA) and P. pseudosyringae (PSS).
The most common and widespread Phytophthora species detected in this study was P. pseudosyringae. This species was isolated from 25 out of the 33 hosts, in 36 sites distributed in all monitored geographic regions. Together with P. cactorum, it is the only species detected in all three types of hosts, while the other Phytophthora species were isolated from only one or two types (Figure 2). Phytophthora plurivora was the second most-isolated species, obtained from 12 hosts in 24 sites.
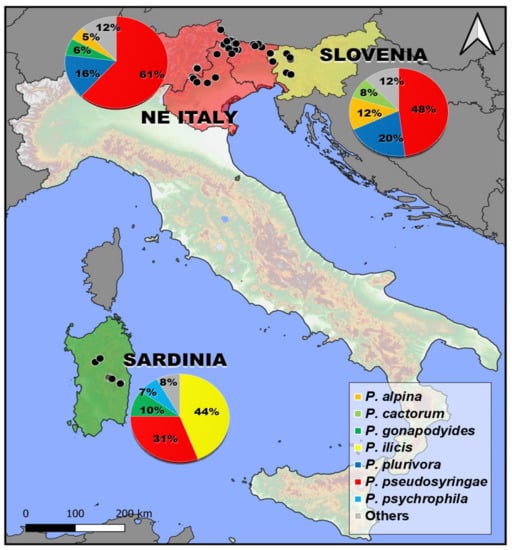
Figure 2.
Isolation frequency and distribution of the 7 most common Phytophthora species isolated in this study.
Phytophthora pseudosyringae and P. plurivora were the most frequently isolated species in NE Italy and Slovenia (Figure 2). In addition to these two species, some species belonging to clade 1, such as P. alpina and P. cactorum, were frequently isolated from different hosts in the NE Alps. In the mountainous areas of Sardinia, in addition to P. pseudosyringae, other two species P. ilicis and P. psychrophila belonging to clade 3 were constantly isolated (Figure 2).
As regards the distribution within Phytophthora clades, clade 6 is the most represented in terms of species (five species) followed by clade 1 (4), clade 3 (3) and clade 8 (3). Only one or two species were obtained for clades 2 and 7. Overall, 56 new host–pathogen associations were detected (Table 4, Table 5 and Table 6).
3.3. Structure and Diversity of Phytophthora Communities
The diversity indices of the Phytophthora assemblages detected in the subalpine vegetation varied among the three categories of hosts, but in general they displayed high diversity and richness and moderate evenness, with the exception of the shrub Phytophthora community dominated by P. pseudosyringae (Table 7).

Table 7.
Values of the diversity indices, Shannon diversity index (S), Margalef index (d) and Pielou Evenness (J) of Phytophthora populations from three different plant communities.
Tree and shrub species displayed the highest number of taxa and Shannon index (H) values. As regards the degree of similarity between the three Phytophthora communities, the Jaccard similarity index (Jc) was variable between 0.11 and 0.20. Only two Phytophthora species, P. pseudosyringae and P. cactorum, were isolated from all host groups. Relationships among the three categories of hosts are shown in Figure 3.
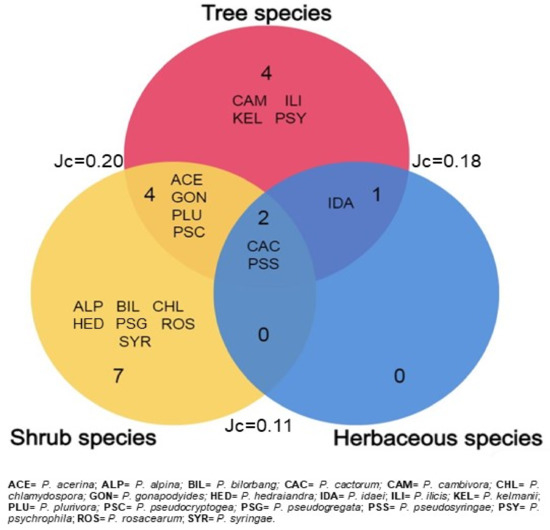
Figure 3.
Venn diagrams illustrating the number of unique and shared Phytophthora species among the three categories of plant species. The outer numbers indicate values of the Jaccard similarity coefficient.
3.4. DNA Phylogeny
Phylogenetic relationships among the Phytophthora isolates obtained in this study were elucidated using ITS sequences (Figure 4). In particular, the 20 isolates included in the phylogenetic analysis were distributed in 18 terminal clades, 17 of which belong to formally described species (Figure 4). Instead, three isolates clustered together in a separate and well-supported terminal clade (ML bootstrap = 100%) representing a previously unrecognized species closely related to P. gregata, which is described here as Phytophthora pseudogregata sp. nov. (Figure 4).
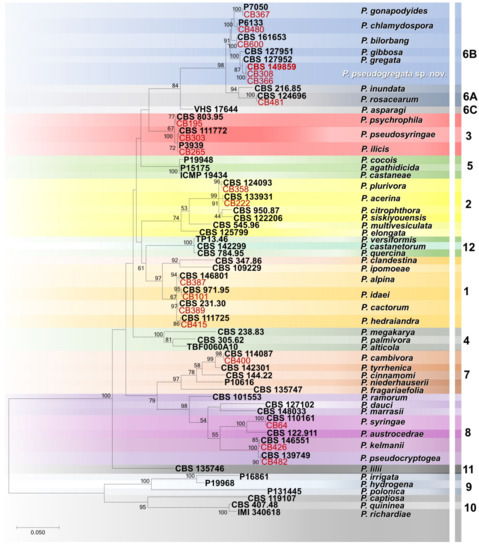
Figure 4.
Maximum likelihood tree obtained from internal transcribed spacer (ITS) sequences of Phytophthora species representative of the 12 clades. Data are based on the General Time Reversible model. A discrete Gamma distribution was used to model evolutionary rate differences among sites. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. Bootstrap support values in percentage (1000 replicates) are given at the nodes. Ex-type cultures are in bold, and isolates obtained in this study in red.
To resolve the phylogenetic position of P. pseudogregata within subclade 6b, a concatenated nuclear and mitochondrial dataset (the length of the final alignment was 2129 bp) was analysed. Individual gene phylogenies revealed no major conflicts, thus indicating that the three loci (ITS, Btub and cox1) could be combined. The ML analysis resolved the positions of all formally described Phytophthora species in subclade 6b, accommodating the isolates P. pseudogregata in a terminal clade sister to P. gibbosa (Figure 5). Phytophthora pseudogregata is separated by the two closely related species, P. gregata and P. gibbose, by three, two, and 18 bp and by eight, three, and 17 bp in ITS, Btub, and cox1 loci, respectively.
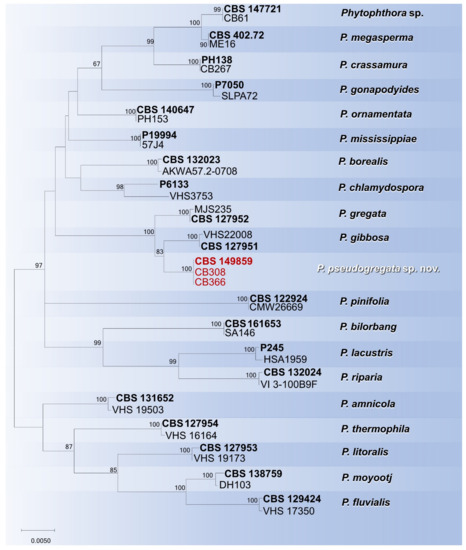
Figure 5.
Maximum likelihood tree obtained from concatenated ITS, Btub and cox1 sequences of the Phytophthora species belonging to subclade 6b. Data are based on the General Time Reversible model. A discrete Gamma distribution was used to model evolutionary rate differences among sites. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. Bootstrap support values in percentage (1000 replicates) are given at the nodes. Ex-type cultures are in bold and isolates of the study in red.
3.5. Taxonomy
Phytophthora pseudogregata Bregant, Ogris, Meli and Linaldeddu sp. nov.
MycoBank: MB849354
Etymology: the name refers to the morphological similarity to Phytophthora gregata.
Holotype: CBS H-25226
Host/distribution: Alnus viridis, Juniperus communis and Rhododendron ferrugineum with foliar necrosis and shoot blight symptoms in Italy and Slovenia.
Description: Sporangia were produced on CA plugs flooded in unsterile pond water after 36–72 h of incubation at 25 °C on simple sporangiophores. Sporangia were persistent, mostly nonpapillate (80%), rarely semipapillate (20%), from ovoid to obpyriform, sometimes ellipsoid, borne terminally on unbranched sporangiophores, average 50.3 ± 6.5 × 29.9 ± 3.8 µm (total range 32.1–65.1 × 22.1–38.4 µm), with a length/breadth ratio of 1.7 ± 0.2 (n = 50) (Figure 6d–g). Zoospores were abundantly produced in liquid cultures after 24–36 h at 25 °C in the dark (Figure 6h). Sporangia proliferated, usually externally and rarely internally, in both a nested and extended way (Figure 6i–k). Hyphal swellings were not formed on solid agar and rarely in pond water, they were globose to subglobose, mostly intercalary catenulate, rarely terminal (Figure 6l–m). Chlamydospores were not observed. All isolates produced gametangia in single culture on carrot agar after 7–10 days at 20 °C in the dark. Oogonia were smooth-walled, borne mainly terminally, with an average diameter of 33.2 ± 3.4. Oospores were spherical and usually aplerotic and 29.0 ± 3.7 µm in diameter. Antheridia were mostly amphigynous (58%), less frequently paragynous (42%) hyaline, rounded, club-shaped, or irregular: average 16.2 ± 2.7 × 12.5 ± 2.2 µm (Figure 6o–r).
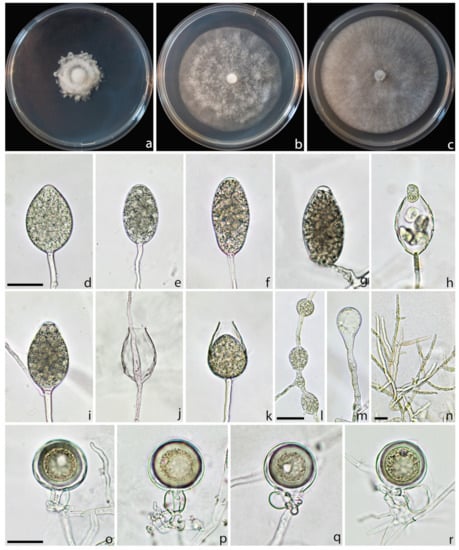
Figure 6.
Colony morphology of Phytophthora pseudogregata after 7 days growth at 20 °C on PDA (a), MEA (b), and CA (c). Persistent sporangia, nonpapillate (d,e) semipapillate (f,g), releasing of zoospores (h), external (i) and internal (j,k) proliferations; intercalary (l) and terminal hyphal swellings (m); mycelia (n). Oogonia with amphygynous (o,p) and paragynous antheridia (q,r). Scale bars = 20 µm.
Cultural characteristics: colony growth pattern cottony on PDA with an irregular border, with an indistinct pattern on MEA and CA. On PDA, growth was slow, whereas on MEA and CA, colonies reached a diameter of 55 and 70 mm in 7 days at 23 °C, respectively.
Cardinal temperatures for growth: minimum <2 °C, maximum 32 °C, and optimum 23 °C. Isolates failed to grow at 34 °C, and mycelium did not resume growth when plates were moved to 20 °C.
Material examined: ITALY: Borso del Grappa, isolated from a necrotic shoot of Juniperus communis, 13 June 2022, collected by Letizia Meli, isolated by Carlo Bregant, HOLOTYPE CBS H-25226, a dried culture on CA, culture ex-holotype CB234 = CBS 149859. ITALY: San Nicolò di Comelico, isolated from necrotic leaves of Rhododendron ferrugineum, 3 July 2021, collected and isolated by C. Bregant (isolate CB308). SLOVENIA: Bohinj, isolated from a necrotic branch of Alnus viridis, 7 October 2021, collected by C. Bregant and Nikica Ogris and isolated by C. Bregant (isolate CB366).
Notes: Phytophthora pseudogregata belongs to subclade 6b. The closest species are P. gregata and P. gibbosa, from which it differs through a combination of unique morphological features (Table 8) and sequence data such as sporangia size and proliferation, oogonia and antheridia shapes and cardinal temperature values, as well as a total of 23 (P. gregata) and 28 (P. gibbosa) fixed nucleotide differences in the ITS, Btub, and cox1 sequences.

Table 8.
Morphological features, morphometric data and temperature–growth relationship of Phytophthora pseudogregata and closely related species in subclade 6b.
3.6. Pathogenicity
All Phytophthora species proved to be pathogenic on Juniperus communis. At the end of the experimental period, inoculated seedlings showed dark brown inner bark lesions that spread up and down from the inoculation point (Figure 7).

Figure 7.
Symptoms observed on common juniper twigs 30 days after inoculation with Phytophthora acerina (a,b), P. bilorbang (c,d), P. gonapodyides (e,f), P. plurivora (g,h), P. pseudocryptogea (i,j), P. pseudogregata (k,l), P. pseudosyringae (m,n) and P. rosacearum (o,p). Control (q,r).
Among the different species assayed, the length of the necrotic lesion differed significantly (Table 9). The lesions caused by P. pseudosyringae were significantly larger than those caused by other species (Table 9). Lesions caused by P. pseudosyringae, P. plurivora and P. acerina progressively girdled the twigs causing shoot blight, browned foliage and wilting symptoms.

Table 9.
Mean lesion length ± standard deviation caused by each Phytophthora species on common juniper twigs.
Control seedlings, inoculated with sterile PDA plugs, remained symptomless; in only two twigs, a small light brown discoloration was observed restricted to the inoculation point.
All eight Phytophthora species were successfully re-isolated from the necrotic inner bark lesions of all seedlings, thus fulfilling Koch’s postulates. No Phytophthora or other fungal isolates were obtained from control plants.
4. Discussion
This study represents the most comprehensive investigation to date on aerial diseases caused by Phytophthora species on mountain vegetation in Italy and Slovenia. The results obtained have allowed us to clarify both symptomatology and aetiology of the emerging pathosystems affecting mountain and subalpine formations. The progressive spread of several airborne Phytophthora species is causing the destruction of vast ecosystems and compromising the biodiversity of these ecologically fragile habitats.
Based on combined sequence data and micromorphological features, 18 Phytophthora species belonging to six out the 12 major Phytophthora phylogenetic clades were identified from a collection of 397 symptomatic samples collected from 33 herbaceous and woody hosts. These included: P. acerina, P. alpina, P. bilorbang, P. cactorum, P. cambivora, P. chlamydospora, P. gonapodyides, P. hedraiandra, P. idaei, P. ilicis, P. plurivora, P. pseudocryptogea, P. pseudosyringae, P. psychrophila, P. rosacearum and P. syringae. In addition, three isolates described here as Phytophthora pseudogregata sp. nov. were isolated and characterized.
The most frequently isolated Phytophthora species belong mainly to clades 1 and 3. These species are characterized by the ability to produce caducous sporangia useful for aerial infections [1]. Furthermore, the relatively low cardinal temperatures for growth suggest that these species have a great potential to threaten mountain vegetation [14,31,55].
In particular, in Northeast Italy a higher number of species belonging to the ITS clade 1 was isolated (P. alpina, P. cactorum, P. hedraiandra and P. idaei), while in Sardinia, clade 3 was dominant, with three species, P. pseudosyringae, P. ilicis and P. psychrophila. Overall, P. pseudosyringae (clade 3) was the most frequent species in terms of number of hosts infected and distribution among sites. Two hundred and one out of the 360 isolates obtained in this study belonged to this species. In particular, P. pseudosyringae have been detected in 36 sites and 25 hosts of all three plant categories investigated: trees, shrubs and herbaceous plants (17 new host–pathogen associations). The wide spread of P. pseudosyringae in different mountain and subalpine formations and its involvement in several new diseases highlight the polyphagous nature of this invasive pathogen and its aerial lifestyle. This agrees with previous studies conducted in mountain environments in Asia, Europe, North and South America [14,32,34,55,56,57,58,59,60,61]. Phytophthora pseudosyringae is the key species in the aetiology of aerial infections detected in high-altitude shrubs and heaths such as blueberry, dwarf pine, juniper, rhododendron and alpine willow formations; these shrubs are characterized by creeping behaviour with very limited heights above the ground, this habitus could favour the attack of Phytophthora sporangia and zoospores. The attacks of P. pseudosyringae on Vaccinium myrtillus (leaf necrosis and shoot blight) were particularly severe, confirming the susceptibility of this small shrub as previously reported in Ireland [33,62]. Many aspects regarding the infectivity and survival of P. pseudosyringae sporangia in the infected tissues fallen to the ground in subalpine areas remain to be clarified. At the same time the ability of oospores to persist for years and their infectivity in environments where the pathogen is subjected to extreme low temperatures need further investigations. Probably the survival of this species in cold habitats is guaranteed by the production of very large and thick wall chlamydospores. In fact, unlike what was reported in previous studies and in the original description, all isolates of P. pseudosyringae examined produced a large amount of globose chlamydospores on CA both in solid and liquid culture. The chlamydospores were mainly terminal and 76.6 ± 22.02 (range 39.9–102.3, n = 25) μm in diameter. Based on the wide variation in morphological characters found in this study, the description of P. pseudosyringae needs to be redefined. Undoubtedly, increased inoculum in the litter due to the diseased fallen leaves not only could represent an increased risk of outbreaks but also a faster disease progression in these habitats [Bregant & Linaldeddu, unpublished]. In the pathogenicity test, P. pseudosyringae shows high aggressivity on common juniper, producing wood necrosis and shoot blight after four weeks from the inoculation.
The other two species in clade 3 were isolated only in the mountain area of Sardinia. Phytophthora psychrophila have been isolated from bleeding cankers of Quercus pubescens, confirming the affinity of this pathogen towards oak species [63]; the geographic distribution and impact of this species is still unknown; there have been a few reports of it in European and American natural forests and nurseries [4,31,64]. Phytophthora ilicis has been known for a long time as a specific pathogen of Ilex aquifolium in the mountains of the Mediterranean basin and a few other areas of Europe and North America [55,65,66,67].
Four species belonging to subclade 1a have been isolated in the northeastern Alps. Phytopththora alpina shows the highest ability to survive in extremely cold conditions due to the low temperature values for growth and the high production of caducous sporangia and chlamydospores [14]; in addition to Alnus viridis, its discovery on three new hosts in Italy and Slovenia suggest that this recently described species is well adapted to affect typical alpine and subalpine shrubs. The second most common species in subclade 1a was P. cactorum, an invasive and polyphagous pathogen widespread from tropical to temperate climates where it is responsible for severe diseases on agriculture crops and forest trees [1,29,68]. The occurrence of P. cactorum in cold areas has recently been reported in Europe and Australia [4,14,60,69]. Together, P. pseudosyringae and P. cactorum are the two species obtained from all three plant types.
In addition to the numerous new host–pathogen associations (Table 4, Table 5 and Table 6), some species detected such as P. hedraiandra and P. idaei are reported for the first time in natural ecosystems in Europe. Previous studies have ascertained the involvement of these two pathogens in root and foliar disease in agriculture and ornamental nurseries; P. idaei appears restricted to the genus Rubus [70], while P. hedraiandra has a wider range of ornamental hosts [71,72,73,74,75]. Although, in the original description P. idaei is reported to have persistent-sporangia, the Italian isolates obtained in this study showed a moderate production of caducous sporangia.
The second most common species obtained in this study was P. plurivora. Isolates of this species were obtained from 54 symptomatic samples of 12 plant species including eight new hosts. Phytophthora plurivora resides in clade 2 and is common in forest ecosystems of Central Europe; from a recent population study it is considered to be originally of this continent and spread to others by human activities [76]. This agrees with the results of this and previous studies [8,14] given the wide distribution of this pathogen in various extreme and nonhumanized natural environments. While the distribution and impact of P. plurivora is well studied, little is known about its closely related species, P. acerina. To date, this species appears widespread in agricultural systems, nurseries, forests and ornamental trees in northern Italy and Sardinia, and much rarer worldwide [4,77,78,79]. Both P. acerina and P. plurivora were already known as primary pathogens involved in common and grey alder decline in Italy [14]. Isolates of Phytophthora acerina obtained in this study confirm a single polymorphism in the ITS region between northern Italy and Sardinia populations [14].
Among the other Phytophthora species isolated in this study, five belong to clade 6, including the newly described species P. pseudogregata. Clade 6 encompasses species very common in European forests, such as P. bilorbang and P. gonapodyides and species with more limited or still unknown distribution, such as P. amnicola and P. rosacearum [8]. Some species in this clade are reported as saprophyte or occasionally weak opportunistic pathogens [11,54,80,81]; the involvement of five species of this clade in the aetiology of aerial infections on mountain vegetation highlight the ecological versatility of these organisms. The ability of P. bilorbang, P. gonapodyides and P. pseudogregata to reproduce the symptoms observed in nature on common juniper suggest their active role in the aetiology of the emerging disease affecting woody trees in mountain areas.
Phytophthora pseudogregata resides in subclade 6b; it is closely related to P. gregata and P. gibbosa, from which it can be distinguished by unique morphological features and sequence data. Phytophthora gregata was originally described in 2011 in Australia in wet native forests and in Tasmania associated with dying alpine heathland vegetation [54,69] and then recently reported in the Czech Republic and Finland [60,82], whereas P. gibbosa is known to occur only in Australia associated with dying native vegetation on seasonally wet sites [54].
Sub-clade 6b is larger and contains several described species (P. amnicola, P. borealis, P. chlamydospora, P. bilorbang, P. crassamura, P. fluvialis, P. gibbosa, P. gonapodyides, P. gregata, P. lacustris, P. litoralis, P. megasperma, P. mississippiae, P. moyootj, P. ornamentata, P. pinifolia, P. pseudogregata, P. riparia and P. thermophila), some not formally described species and a few hybrids [83]. Most of the species in this sub-clade have been described in the last 12 years, the only species known until 2011 were P. gonapodyides and P. megasperma [1]. The majority of species in sub-clade 6b, including P. pseudogregata, have been described in forest ecosystems, underlining the key role played by natural areas in exploring the biodiversity of the Phytophthora genus, which currently includes 220 species (Figure 8).
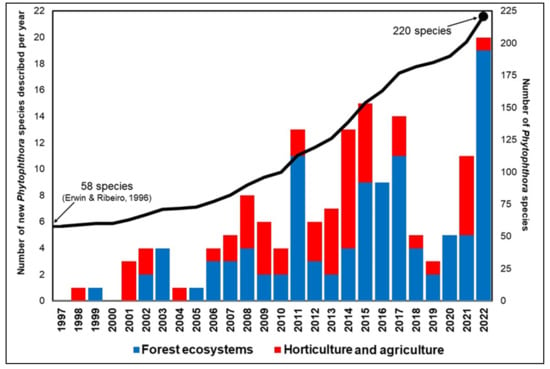
Figure 8.
Number of Phytophthora species described per year since 1997, divided into species isolated from nurseries and agriculture and forest ecosystems. The graph also reports the progression of the described species over time (black line). (Source: Scopus, May 2023 and IndexFungorum May 2023, [1]).
Finally, three species of clade 8 (P. kelmanii, P. pseudocryptogea and P. syringae) and one from clade 7 (P. cambivora) have been isolated, mainly from stem bleeding cankers of small trees and shrubs. While P. kelmanii and P. syringae have a very limited distribution, P. pseudocryptogea is widespread along the Alps. The large range of growth temperatures and polyphagous nature explain it being widespread in Italian ecosystems spanning from Mediterranean areas to the tree line in the Dolomites [4,14,78]. Both mating types of P. cambivora occurred in the NE Alps (A2 on Alnus incana in Slovenia and A1 on Laburnum alpinum and Sorbus aucuparia in Italy).
5. Conclusions
In conclusion, the discovery of several emerging Phytophthora diseases in the canopy of subalpine vegetation in Europe is of particular concern and underlines the need to further extend research into these environments to assess the full diversity of Phytophthora clades and species and the factors driving the emergence and diffusion of these invasive pathogens. Studying Phytophthora communities on necrotic leaves naturally fallen, would be useful to evaluate host specificity, geographic distribution and survival strategies of the main Phytophthora species detected in this study.
A survey is currently in progress to map the distribution of P. pseudogregata in Alpine habitats and to establish the natural host range of this new taxon.
Author Contributions
B.T.L., C.B. and N.O. conceptualization; B.T.L., C.B., G.R., L.M. (Letizia Meli), N.O., N.S. and A.B. field survey, sample collection and assay; B.T.L., C.B., G.R. and L.M. (Letizia Meli) data analysis; B.T.L., L.M. (Lucio Montecchio), L.M. (Lucia Maddau), B.P. and N.O. funding acquisition; C.B. draft writing; B.T.L., L.M. (Lucio Montecchio), L.M. (Lucia Maddau), A.B., B.P. and N.O. review and manuscript editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by grant number DOR2305524/2023 “Monitoraggio dei marciumi radicali da Phytophthora negli ecosistemi forestali Italiani”, by the Land Environment Resources and Health (L.E.R.H.) doctoral course (University of Padova), by Fondo di Ateneo 2020, an internal funding by the University of Sassari and by Slovenian Research Agency (Research Program P4-0107 Forest Biology, Ecology and Technology), Slovenian Ministry of Agriculture, Forestry and Food (Public Forestry Service).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Erwin, C.D.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: Saint Paul, MN, USA, 1996. [Google Scholar]
- Hansen, E.M.; Reeser, P.W.; Sutton, W. Phytophthora beyond agriculture. Annu. Rev. Phytopathol. 2012, 50, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Bader, M.K.F.; Burgess, T.I.; Hardy, G.; Williams, N. Global biogeography and invasion risk of the plant pathogen genus Phytophthora. Environ. Sci. Policy 2019, 101, 175–182. [Google Scholar] [CrossRef]
- Bregant, C.; Mulas, A.A.; Rossetto, G.; Deidda, A.; Maddau, L.; Piras, G.; Linaldeddu, B.T. Phytophthora mediterranea sp. nov., a New Species Closely Related to Phytophthora cinnamomi from Nursery Plants of Myrtus communis in Italy. Forests 2021, 12, 682. [Google Scholar] [CrossRef]
- Rizzo, D.M.; Garbelotto, M.; Davidson, J.M.; Slaughter, G.W.; Koike, S.T. Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Dis. 2002, 86, 205–214. [Google Scholar] [CrossRef]
- Hansen, E.M. Phytophthora species emerging as pathogens of forest trees. Curr. For. Rep. 2015, 1, 16–24. [Google Scholar] [CrossRef]
- Burgess, T.I.; Simamora, A.V.; White, D.; Wiliams, B.; Schwager, M.; Stukely, M.J.C.; Hardy, G.E.S.J. New species from Phytophthora Clade 6a: Evidence for recent radiation. Pers. Mol. Phylogeny Evol. Fungi 2018, 41, 1–17. [Google Scholar] [CrossRef]
- Bregant, C.; Batista, E.; Hilário, S.; Linaldeddu, B.T.; Alves, A. Phytophthora species involved in Alnus glutinosa decline in Portugal. Pathogens 2023, 12, 276. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.A.; van West, P. Zoospore development in the oomycetes. Fungal Biol. Rew. 2007, 21, 10–18. [Google Scholar] [CrossRef]
- Bassani, I.; Larousse, M.; Tran, Q.D.; Attard, A.; Galiana, E. Phytophthora zoospores: From perception of environmental signals to inoculum formation on the host-root surface. Comput. Struct. Biotechnol. J. 2020, 18, 3766–3773. [Google Scholar] [CrossRef]
- Marano, A.V.; Jesus, A.L.; De Souza, J.I.; Jerônimo, G.H.; Gonçalves, D.R.; Boro, M.C.; Rocha, S.C.O.; Pires-Zottarelli, C.L.A. Ecological roles of saprotrophic Peronosporales (Oomycetes, Straminipila) in natural environments. Fungal Ecol. 2016, 19, 77–88. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Scanu, B.; Maddau, L.; Franceschini, A. Diplodia corticola and Phytophthora cinnamomi: The main pathogens involved in holm oak decline on Caprera Island (Italy). For. Pathol. 2014, 44, 191–200. [Google Scholar] [CrossRef]
- Hao, W.; Richardson, P.A.; Hong, C.X. Foliar blight of annual vinca (Catharanthus roseus) caused by Phytophthora tropicalis in Virginia. Plant Dis. 2010, 94, 274. [Google Scholar] [CrossRef] [PubMed]
- Bregant, C.; Sanna, G.P.; Bottos, A.; Maddau, L.; Montecchio, L.; Linaldeddu, B.T. Diversity and pathogenicity of Phytophthora species associated with declining alder trees in Italy and description of Phytophthora alpina sp. nov. Forests 2020, 11, 848. [Google Scholar] [CrossRef]
- Perrine-Walker, F. Phytophthora palmivora–cocoa interaction. J. Fungi 2020, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Oßwald, W.; Fleischmann, F.; Rigling, D.; Coelho, A.C.; Cravador, A.; Diez, J.; Dalio, R.J.; Horta Jung, M.; Pfanz, H.; Robin, C.; et al. Strategies of attack and defence in woody plant—Phytophthora interactions. For. Pathol. 2014, 44, 169–190. [Google Scholar] [CrossRef]
- Yang, X.; Tyler, B.M.; Hong, C. An expanded phylogeny for the genus Phytophthora. IMA Fungus 2017, 8, 355–384. [Google Scholar] [CrossRef]
- Brasier, C.; Webber, J. Sudden larch death. Nature 2010, 466, 824–825. [Google Scholar] [CrossRef]
- Harris, A.R.; Webber, J.F. Sporulation potential, symptom expression and detection of Phytophthora ramorum on larch needles and other foliar hosts. Plant Pathol. 2016, 65, 1441–1451. [Google Scholar] [CrossRef]
- Cutuli, G. Field and laboratory observations on brown rot of citrus fruit caused by Phytophthora hibernalis. Ann. Dell’ Ist. Sper. Per L’ Agrumic. 1975, 5, 99–105. [Google Scholar]
- Donahoo, R.; Blomquist, C.L.; Thomas, S.L.; Moulton, J.K.; Cooke, D.E.; Lamour, K.H. Phytophthora foliorum sp. nov., a new species causing leaf blight of azalea. Mycol. Res. 2006, 110, 1309–1322. [Google Scholar] [CrossRef]
- Álvarez, L.A.; Pérez-Sierra, A.; García-Jiménez, J.; Abad-Campos, P.; Landeras, E.; Alzugaray, R. First report of leaf spot and twig blight of Rhododendron spp. caused by Phytophthora hibernalis in Spain. Plant Dis. 2007, 91, 909. [Google Scholar] [CrossRef]
- Roberts, P.D.; Trujillo, E. First report of Phytophthora nicotianae causing leaf blight, fruit rot, and root rot of papaya in American Samoa. Plant Dis. 1998, 82, 712. [Google Scholar] [CrossRef]
- Hantula, J.; Lilja, A.; Nuorteva, H.; Parikka, P.; Werres, S. Pathogenicity, morphology and genetic variation of Phytophthora cactorum from strawberry, apple, rhododendron, and silver birch. Mycol. Res. 2000, 104, 1062–1068. [Google Scholar] [CrossRef]
- Nowicki, M.; Foolad, M.R.; Nowakowska, M.; Kozik, E.U. Potato and tomato late blight caused by Phytophthora infestans: An overview of pathology and resistance breeding. Plant Dis. 2012, 96, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Drenth, A.; Guest, D. Phytophthora palmivora in tropical tree crops. In Phytophthora: A Global Perspective; CABI: Wallingford UK, 2013; pp. 187–196. [Google Scholar]
- Marin, M.V.; Seijo, T.; Oliveira, M.S.; Zuchelli, E.; Mertely, J.; Peres, N.A. First report of Phytophthora nicotianae causing crown rot of strawberry in the United States. Plant Dis. 2018, 102, 1463. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Bregant, C.; Ruzzon, B.; Montecchio, L. Coniella granati and Phytophthora palmivora the main pathogens involved in pomegranate dieback and mortality in north-eastern Italy. Ital. J. Mycol. 2020, 49, 92–100. [Google Scholar]
- Chen, X.R.; Wen, K.; Zhou, X.; Zhu, M.Y.; Liu, Y.; Jin, J.H.; Nellist, C.F. The devastating oomycete phytopathogen Phytophthora cactorum: Insights into its biology and molecular features. Mol. Plant Pathol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Wickland, A.C.; Jensen, C.E.; Rizzo, D.M. Geographic distribution, disease symptoms and pathogenicity of Phytophthora nemorosa and Phytophthora pseudosyringae in California, USA. Forest Pathol. 2008, 38, 288–298. [Google Scholar] [CrossRef]
- Hansen, E.M.; Reeser, P.W.; Sutton, W. Ecology and pathology of Phytophthora ITS clade 3 species in forests in western Oregon, USA. Mycologia 2017, 109, 100–114. [Google Scholar] [CrossRef]
- Jung, T.; Nechwatal, J.; Cooke, D.E.; Hartmann, G.; Blaschke, M.; Oßwald, W.F.; Duncan, G.M.; Delatour, C. Phytophthora pseudosyringae sp. nov., a new species causing root and collar rot of deciduous tree species in Europe. Mycol. Res. 2003, 107, 772–789. [Google Scholar] [CrossRef]
- Beales, P.A.; Giltrap, P.M.; Webb, K.M.; Ozolina, A. A further threat to UK heathland bilberry (Vaccinium myrtillus) by Phytophthora pseudosyringae. Plant Pathol. 2010, 59, 406. [Google Scholar] [CrossRef]
- Fajardo, S.N.; Valenzuela, S.; Dos Santos, A.F.; Gonzßlez, M.P.; Sanfuentes, E.A. Phytophthora pseudosyringae associated with the mortality of Nothofagus obliqua in a pure stand in central-southern Chile. For. Pathol. 2017, 47, e12361. [Google Scholar] [CrossRef]
- López-Herrera, C.J.; Pérez-Jiménez, R.M.; Zea-Bonilla, T. First report of Phytophthora cactorum causing fruit rot on avocado in Spain. Plant Dis. 2005, 89, 1363. [Google Scholar] [CrossRef] [PubMed]
- Markakis, E.A.; Tzima, A.K.; Palavouzis, S.C.; Antoniou, P.P.; Paplomatas, E.J.; Tjamos, E.C. First report of Phytophthora palmivora causing fruit rot on pomegranate in Greece. Plant Dis. 2017, 101, 1060. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Schena, L.; Agosteo, G.E.; Magnano di San Lio, G.; Cacciola, S.O. Phytophthora oleae sp. nov. causing fruit rot of olive in southern Italy. Plant Pathol. 2018, 67, 1362–1373. [Google Scholar] [CrossRef]
- Pánek, M.; Maňasová, M.; Wenzlová, J.; Zouhar, M.; Mazáková, J. Peronosporales Species Associated with Strawberry Crown Rot in the Czech Republic. J. Fungi 2022, 8, 346. [Google Scholar] [CrossRef]
- Körner, C.; Paulsen, J.; Spehn, E.M. A definition of mountains and their bioclimatic belts for global comparisons of biodiversity data. Alp. Bot. 2011, 121, 73–78. [Google Scholar] [CrossRef]
- Pauchard, A.; Milbau, A.; Albihn, A.; Alexander, J.; Burgess, T.; Daehler, C.; Englund, G.; Essl, F.; Evengard, B.; Greewood, G.B.; et al. Non-native and native organisms moving into high elevation and high latitude ecosystems in an era of climate change: New challenges for ecology and conservation. Biol. Invasions 2016, 18, 345–353. [Google Scholar] [CrossRef]
- Khaliq, I.; Burgess, T.I.; Hardy, G.E.S.J.; White, D.; McDougall, K.L. Phytophthora and vascular plant species distributions along a steep elevation gradient. Biol. Invasions 2021, 23, 1443–1459. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Bottecchia, F.; Bregant, C.; Maddau, L.; Montecchio, L. Diplodia fraxini and Diplodia subglobosa: The Main Species Associated with Cankers and Dieback of Fraxinus excelsior in North-Eastern Italy. Forests 2020, 11, 883. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Martin, F.N.; Tooley, P.W. Phylogenetic relationships among Phytophthora species inferred from sequence analysis of mitochondrially encoded cytochrome oxidase I and II genes. Mycologia 2003, 95, 269–284. [Google Scholar] [CrossRef]
- Kroon, L.P.N.M.; Bakker, F.T.; Van Den Bosch, G.B.M.; Bonants, P.J.M.; Flier, W.G. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genet. Biol. 2004, 41, 766–782. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Jaccard, P. Distribution comparée de la flore alpine dans quelques régions des Alpes occidentales et orientales. Bull. Soc. Vaudoise Sci. Nat. 1901, 37, 241–272. [Google Scholar]
- Margalef, R. Temporal succession and spatial heterogeneity in phytoplankton. In Perspectives in Marine Biology; Buzzati-Traverso, A.A., Ed.; University of California Press: Berkeley, CA, USA, 1958; pp. 323–347. [Google Scholar]
- Shannon, C.E.; Wiener, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; 177p. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theoret. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron 2001, 4, 9. [Google Scholar]
- Venn, J.I. On the diagrammatic and mechanical representation of propositions and reasonings. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1880, 10, 1–18. [Google Scholar] [CrossRef]
- Jung, T.; Stukely, M.J.C.; Hardy, G.E.S.J.; White, D.; Paap, T.; Dunstan, W.A.; Burgess, T.I. Multiple new Phytophthora species from ITS Clade 6 associated with natural ecosystems in Australia: Evolutionary and ecological implications. Pers. Mol. Phylogeny Evol. Fungi 2011, 26, 13–39. [Google Scholar] [CrossRef]
- Scanu, B.; Linaldeddu, B.T.; Perez-Sierra, A.; Deidda, A.; Franceschini, A. Phytophthora ilicis as a leaf and stem pathogen of Ilex aquifolium in Mediterranean islands. Phytopathol. Mediterr. 2014, 53, 480–490. [Google Scholar]
- Jung, T.; Duràn, A.; Sanfuentes von Stowasser, E.; Schena, L.; Mosca, S.; Fajardo, S.; Gonzalez, M.; Ortega, A.D.N.; Bakonyi, J.; Seress, D.; et al. Diversity of Phytophthora species in Valdivian rainforests and association with severe dieback symptoms. For. Pathol. 2018, 48, e12443. [Google Scholar] [CrossRef]
- Hwang, J.; Oak, S.W.; Jeffers, S.N. Recovery of Phytophthora species from drainage points and tributaries within two forest stream networks: A preliminary report. N. Z. J. For. Sci. 2007, 41, S83–S87. [Google Scholar]
- Varela, C.P.; Vázquez, J.M.; Casal, O.A.; Martínez, C.R. First report of Phytophthora pseudosyringae on chestnut nursery stock in Spain. Plant Dis. 2007, 91, 1517. [Google Scholar] [CrossRef]
- Redondo, M.Á.; Oliva, J. First report of Phytophthora pseudosyringae causing stem canker on Fagus sylvatica in Spain. Plant Dis. 2016, 100, 1508. [Google Scholar] [CrossRef]
- Redondo, M.A.; Boberg, J.; Stenlid, J.; Oliva, J. Functional traits associated with the establishment of introduced Phytophthora spp. in Swedish forests. J. Appl. Ecol. 2018, 55, 1538–1552. [Google Scholar] [CrossRef]
- Christova, P.K.; Lyubenova, A.B.; Kostov, K.V.; Slavov, S.B. First report of Phytophthora pseudosyringae recovered from aquatic ecosystems in Bulgaria. For. Pathol. 2019, 49, e12505. [Google Scholar] [CrossRef]
- O’Hanlon, R.; Choiseul, J.; Corrigan, M.; Catarame, T.; Destefanis, M. Diversity and detections of Phytophthora species from trade and non-trade environments in Ireland. EPPO Bull. 2016, 46, 594–602. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.; López-García, C.; León, M.; García-Jiménez, J.; Abad-Campos, P.; Jung, T. Previously unrecorded low temperature Phytophthora species associated with Quercus decline in a Mediterranean forest in Eastern Spain. For. Pathol. 2013, 43, 331–339. [Google Scholar] [CrossRef]
- Jung, T.; Hansen, E.M.; Winton, L.; Oswald, W.; Delatour, C. Three new species of Phytophthora from European oak forests. Mycol. Res. 2002, 106, 397–411. [Google Scholar] [CrossRef]
- Buddenhagen, I.W.; Young, R.A. A leaf and twig disease of English Holly caused by Phytophthora ilicis n. sp. Phytopathology 1957, 47, 95–101. [Google Scholar]
- Strouts, R.G.; Rose, D.R.; Reffold, T.C. Advisory services. Wales and southern England. In Report on Forest Research; HMSO: London, UK, 1988; pp. 42–43. [Google Scholar]
- Pintos, C.; Rial, C.; Aguín, O.; Mansilla, J.P. First report of Phytophthora ilicis causing twig blight on holly in Spain. New Dis. Rep. 2012, 26, 16. [Google Scholar] [CrossRef]
- Pánek, M.; Fér, T.; Mráček, J.; Tomšovský, M. Evolutionary relationships within the Phytophthora cactorum species complex in Europe. Fungal Biol. 2016, 120, 836–851. [Google Scholar] [CrossRef]
- Khaliq, I.; Hardy, G.E.S.J.; Mc Dougall, K.L.; Burgess, T.I. Phytophthora species isolated from alpine and sub-alpine regions of Australia, including the description of two new species; Phytophthora cacuminis sp. nov. and Phytophthora oreophila sp. nov. Fungal Biol. 2018, 123, 29–41. [Google Scholar] [CrossRef]
- Kennedy, D.M.; Duncan, J.M. A papillate Phytophthora species with specificity to Rubus. Mycol. Res. 1995, 99, 57–68. [Google Scholar] [CrossRef]
- De Cock, A.W.A.M.; Lévesque, C.A. New species of Pythium and Phytophthora. Stud. Mycol. 2004, 50, 481–487. [Google Scholar]
- Belisario, A.; Gilli, G.; Maccaroni, M. First report of Phytophthora hedraiandra on Viburnum tinus in Italy. Plant Pathol. 2006, 55, 573. [Google Scholar] [CrossRef]
- Moralejo, E.; Belbahri, L.; Calmin, G.; Lefort, F.; García, J.A.; Descals, E. First report of Phytophthora hedraiandra on Viburnum tinus in Spain. Plant Pathol. 2006, 55, 574. [Google Scholar] [CrossRef]
- Munda, A.; Žerjav, M.; Schroers, H.J. Phytophthora hedraiandra on rhododendron in Slovenia. Plant Pathol. 2007, 56, 355. [Google Scholar] [CrossRef]
- Yosilia, R.; Morishima, M.; Hieno, A.; Suga, H.; Kageyama, K. First report of stem rot on hydrangea caused by Phytophthora hedraiandra in Japan. J. Gen. Plant Pathol. 2020, 86, 507–512. [Google Scholar] [CrossRef]
- Schoebel, C.N.; Stewart, J.; Gruenwald, N.J.; Rigling, D.; Prospero, S. Population history and pathways of spread of the plant pathogen Phytophthora plurivora. PLoS ONE 2014, 9, e85368. [Google Scholar] [CrossRef] [PubMed]
- Ginetti, B.; Moricca, S.; Squires, J.N.; Cooke, D.E.L.; Ragazzi, A.; Jung, T. Phytophthora acerina sp. nov., a new species causing bleeding cankers and dieback of Acer pseudoplatanus trees in planted forests in northern Italy. Plant Pathol. 2014, 63, 858–876. [Google Scholar] [CrossRef]
- Seddaiu, S.; Linaldeddu, B.T. First Report of Phytophthora acerina, P. plurivora, and P. pseudocryptogea associated with declining common alder trees in Italy. Plant Dis. 2020, 104, 1874. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Bregant, C.; Montecchio, L.; Favaron, F.; Sella, L. First report of Phytophthora acerina, P. pini and P. plurivora causing root rot and sudden death on olive trees in Italy. Plant Dis. 2020, 104, 996. [Google Scholar] [CrossRef]
- Brasier, C.M.; Cooke, D.E.; Duncan, J.M.; Hansen, E.M. Multiple new phenotypic taxa from trees and riparian ecosystems in Phytophthora gonapodyides–P. megasperma ITS Clade 6, which tend to be high-temperature tolerant and either inbreeding or sterile. Mycol. Res. 2003, 107, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Nechwatal, J.; Bakonyi, J.; Cacciola, S.O.; Cooke, D.E.L.; Jung, T.; Nagy, Z.A.; Vannini, A.; Brasier, C.M. (2013). The morphology, behaviour and molecular phylogeny of Phytophthora taxon Salixsoil and its redesignation as Phytophthora lacustris sp. nov. Plant Pathol. 2013, 62, 355–369. [Google Scholar] [CrossRef]
- Grígel, J.; Černý, K.; Mrázková, M.; Havrdová, L.; Zahradník, D.; Jílková, B.; Hrabětová, M. Phytophthora root and collar rots in fruit orchards in the Czech Republic. Phytopathol. Medit. 2019, 58, 261–276. [Google Scholar]
- Abad, Z.G.; Burgess, T.I.; Redford, A.J.; Bienapfl, J.C.; Srivastava, S.; Mathew, R.; Jennings, K. IDphy: An international online resource for molecular and morphological identification of Phytophthora. Plant Dis. 2023, 107, 987–998. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).