Genome-Wide Identification and Analysis of the DGAT Gene Family in Lindera glauca and Expression Analysis during Fruit Development Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification and Characterization of DGAT Gene Family in L. glauca
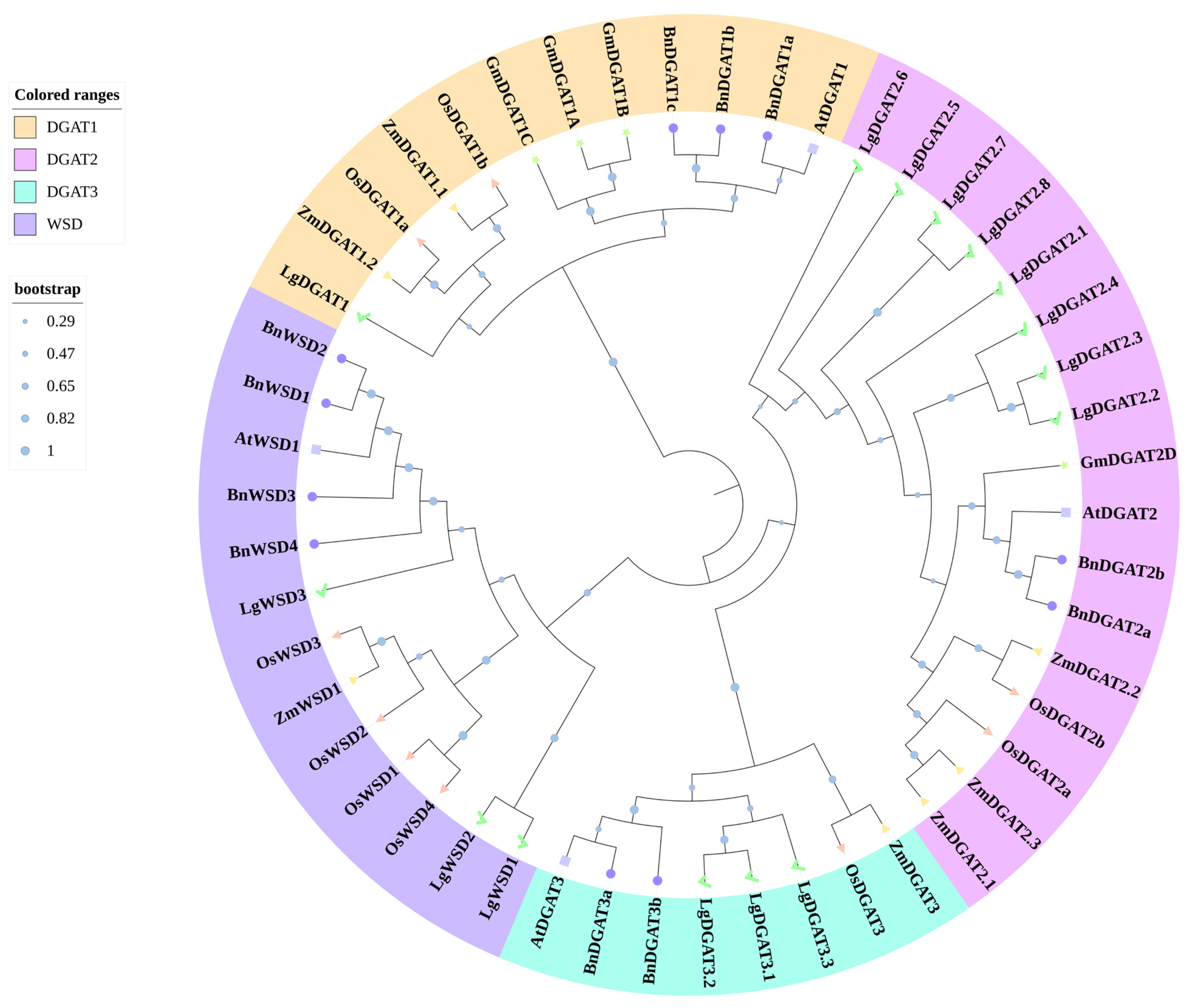
2.2. Phylogenetic Analysis of LgDGATs
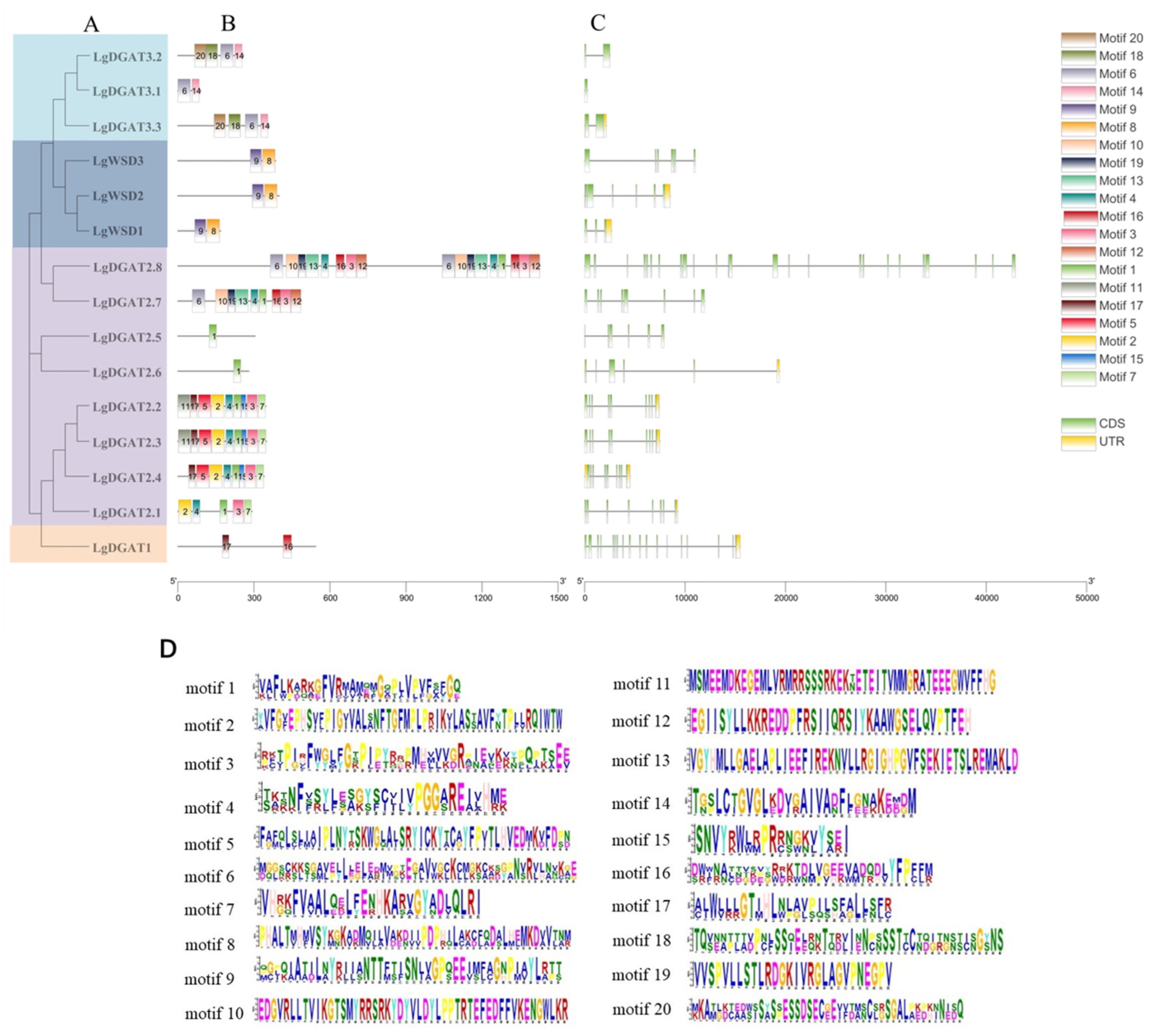
2.3. Motifs and Gene Structure Analysis
2.4. Cis-Acting Elements Analysis for LgDGAT Gene Promoters
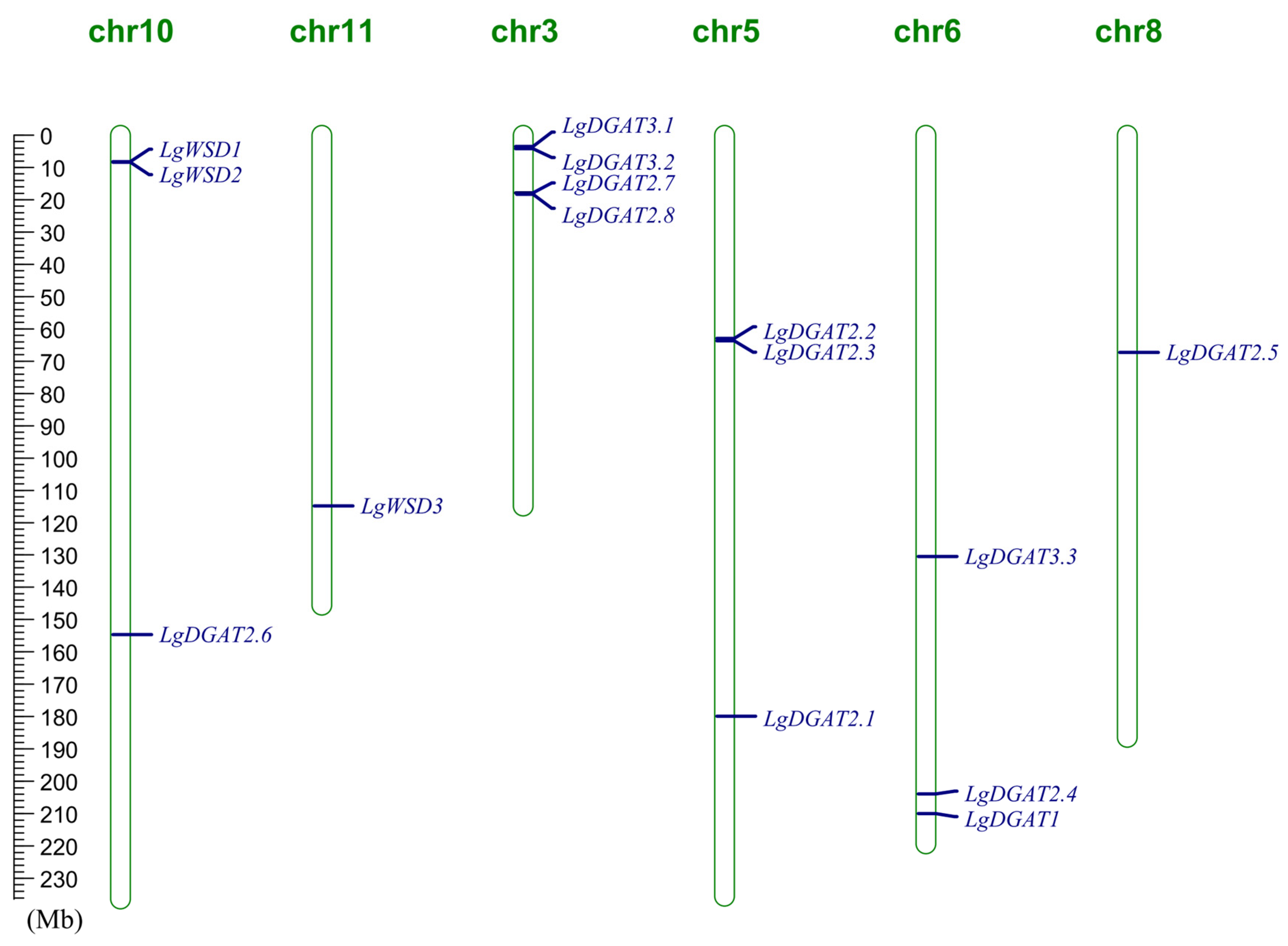
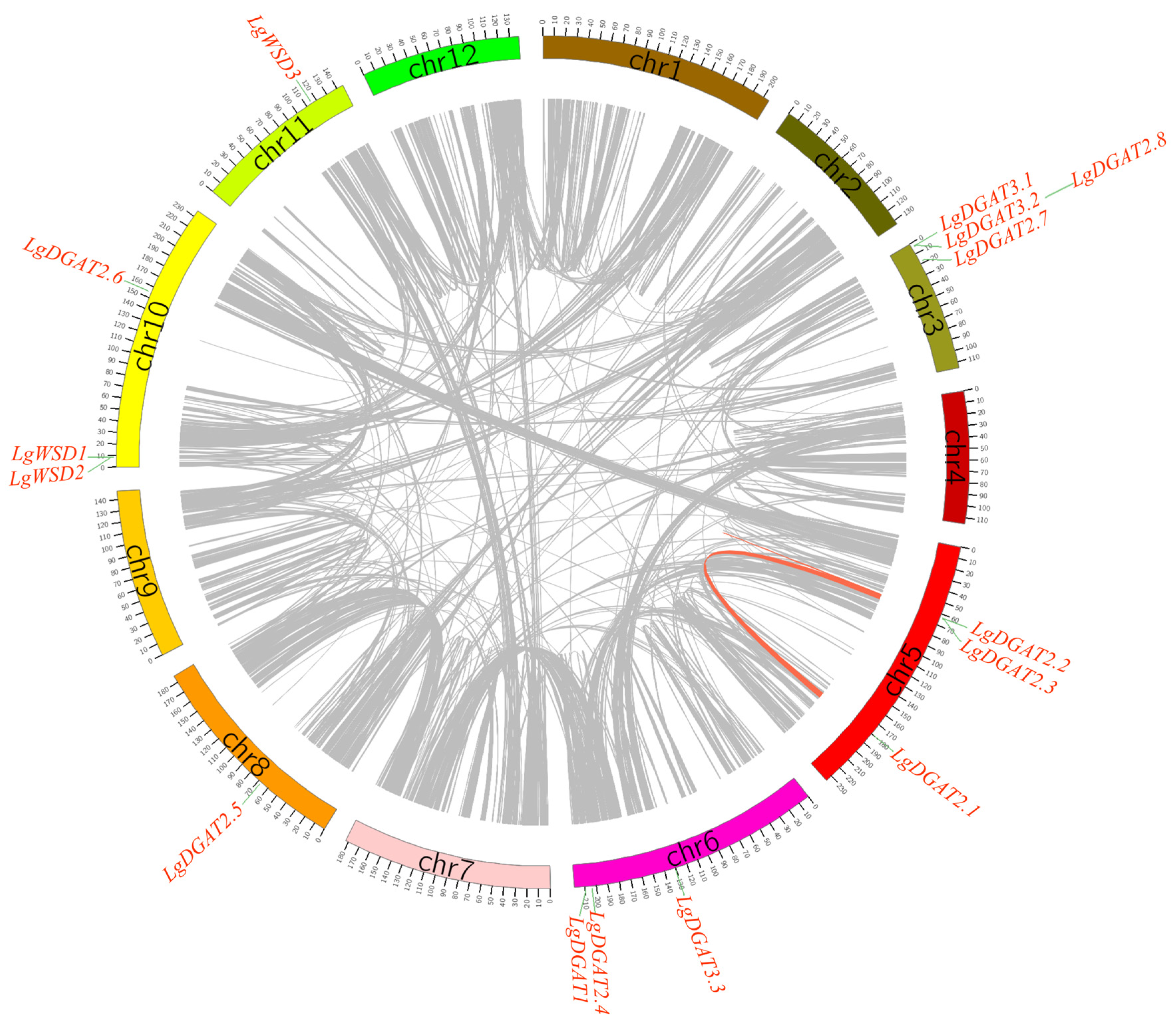
2.5. Chromosomal Distribution, Gene Duplication and Synteny Analysis of LgDGATs
2.6. Calculation of the Ka/Ks Values
2.7. Expression of the LgDGAT Genes During Fruit Development
2.8. Real-Time PCR Analysis of LgDGATs’ Gene Expression
2.9. Tissue-Specific Expression of LgDGATs
3. Results
3.1. Identification of DGAT Members in L. glauca
3.2. Phylogenetic Analysis of LgDGATs
3.3. Analysis of Conserved Motifs and Gene Structure of LgDGATs
3.4. Cis-Acting Elements in LgDGAT Promoters
3.5. Chromosomal Localization, Gene Duplication, and Genome Synteny of LgDGATs
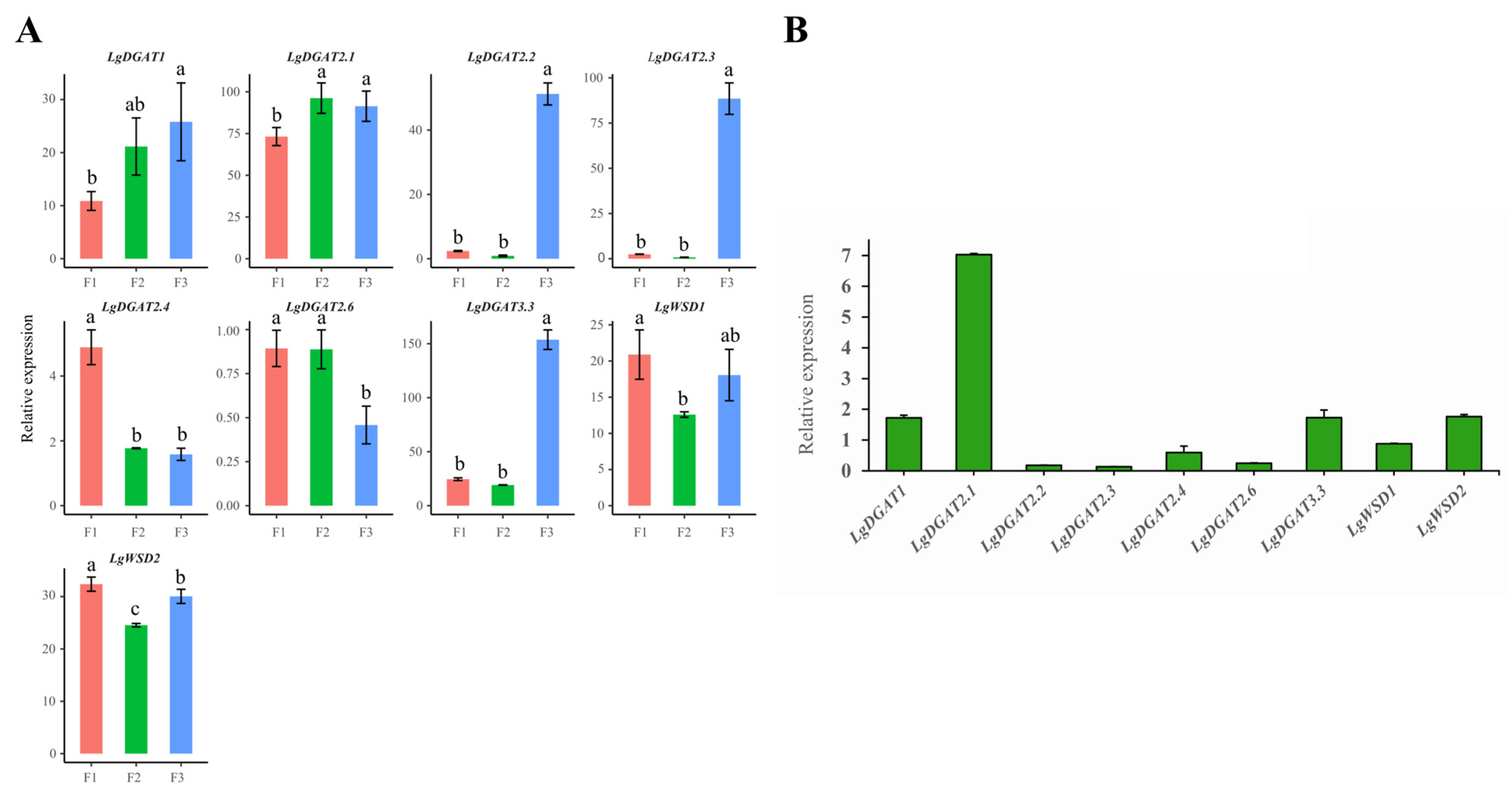
3.6. Differential Expression Levels of LgDGAT Genes in Developing Fruit
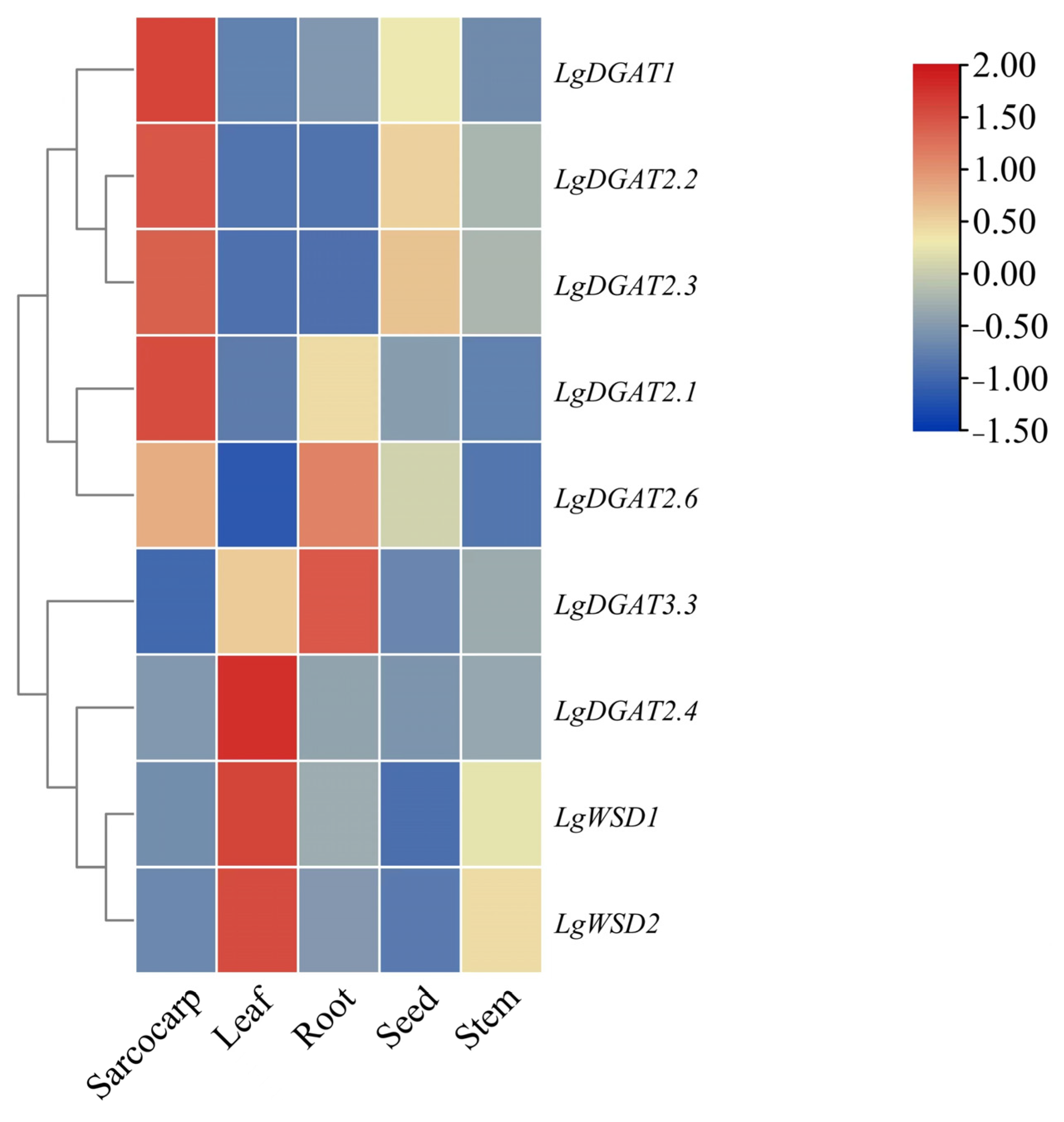
3.7. Analysis of Expression Patterns of LgDGATs in Different Plant Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lung, S.C.; Weselake, R.J. Diacylglycerol acyltransferase: A key mediator of plant triacylglycerol synthesis. Lipids 2006, 41, 1073–1088. [Google Scholar] [CrossRef]
- Liu, Q.; Siloto, R.M.P.; Lehner, R.; Stone, S.J.; Weselake, R.J. Acy1–CoA: Diacylglycerol acyltransferase: Molecular biology, biochemistry and biotechnology. Prog. Lipid Res. 2012, 51, 350–377. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.P.; Weiss, S.B. Function of cytidine coenzymes in the biosynthesis of phospholipides. J. Biol. Chem. 1956, 222, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.D.; Ohlrogge, J.B. Compartmentation of triacylglycerol accumulation in plants. J. Biol. Chem. 2012, 287, 2288–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, M.L.; Whitehead, L.; He, Z.; Gazda, V.; Gilday, A.; Kozhevnikova, E.; Vaistij, F.; Larson, T.R.; Graham, I.A. A cytosolic acyltransferase contributes to triacylglycerol synthesis in sucrose–rescued Arabidopsis seed oil catabolism mutants. Plant Physiol. 2012, 160, 215–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalscheuer, R.; Steinbüchel, A. A novel bifunctional wax ester synthase/acyl–CoA: Diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J. Biol. Chem. 2003, 278, 8075–8082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nykiforuk, C.L.; Furukawa-Stoffer, T.L.; Huff, P.W.; Sarna, M.; Laroche, A.; Moloney, M.M.; Weselake, R.J. Characterization of cDNAs encoding diacylglycerol acyltransferase from cultures of Brassica napus and sucrose–mediated induction of enzyme biosynthesis. Biochim. Biophys. Acta 2002, 1580, 95–109. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Yang, M.F.; Xu, Y.N. Silencing of DGAT1 intobacco causes a reduction in seed oil content. Plant Sci. 2005, 169, 689–694. [Google Scholar] [CrossRef]
- Lock, Y.Y.; Snyder, C.L.; Zhu, W.; Siloto, R.M.; Weselake, R.J.; Shah, S. Antisense suppression of type 1 diacylglycerol acyltransferase adversely affects plant development in Brassica napus. Physiol. Plant 2009, 137, 61–71. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, J.; Taylor, D.C.; Ohlrogge, J.B. DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell. 2009, 21, 3885–3901. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Wei, Y.; Jako, C.; Kumar, A.; Selvaraj, G.; Taylor, D.C. The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J. Cell Mol. Biol. 1999, 19, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirier, Y.; Ventre, G.; Caldelari, D. Increased flow of fatty acids toward beta– oxidation in developing seeds of Arabidopsis deficient in diacylglycerol acyltransferase activity or synthesizing medium–chain–length fatty acids. Plant Physiol. 1999, 121, 1359–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrianov, V.; Borisjuk, N.; Pogrebnyak, N.; Brinker, A.; Dixon, J.; Spitsin, S.; Flynn, J.; Matyszczuk, P.; Andryszak, K.; Laurelli, M.; et al. Tobacco as a production platform for biofuel: Overexpression of Arabidopsis DGAT and LEC2 genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnol. J. 2010, 8, 277–287. [Google Scholar] [CrossRef]
- Lu, G.; Li, D.; Zhang, T.; Tang, X.; Lu, J.X.; Hu, Q.M.; Hu, T.M. Cloning and function analysis of a type 2 diacylglycerol acyltransferase (DGAT2) from Perilla frutescens. Acta Agron. Sin. 2020, 46, 1283–1290. [Google Scholar] [CrossRef]
- Zhang, T.; He, H.; Xu, C.; Fu, Q.; Tao, Y.; Xu, R.; Xu, Z. Overexpression of Type 1 and 2 Diacylglycerol Acyltransferase Genes (JcDGAT1 and JcDGAT2) Enhances Oil Production in the Woody Perennial Biofuel Plant Jatropha curcas. Plants 2021, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sun, Y.; Gao, H.L.; Xue, J.A.; Jia, X.Y.; Li, R.Z. Functional analysis of CEDGAT2–2 gene in Cyperus esculentus L. and cultivation of oil fuel–type tobacco. In Proceedings of the 19th Annual Conference of Crop Science Society of China, Wuhan, China, 8–9 November 2020; Crop Science Society of China: Wuhan, China, 2020; Volume 1. [Google Scholar]
- Ayme, L.; Arragain, S.; Canonge, M.; Baud, S.; Touati, N.; Bimai, O.; Jagic, F.; Louis-Mondesir, C.; Briozzo, P.; Fontecave, M.; et al. Arabidopsis thaliana DGAT3 is a [2Fe–2S] protein involved in TAG biosynthesis. Sci. Rep. 2018, 8, 17254. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.L.; Gao, Y.; Zhang, F.; Liu, B.L.; Ji, C.L.; Xue, J.A.; Yuan, L.X.; Li, R.Z.; Sativa, C. Functional characterization of an novel acyl–CoA: Diacylglycerol acyltransferase 3–3 (CsDGAT3–3) gene from Camelina sativa. Plant Sci. 2021, 303, 110752. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.; Lam, P.; Bird, D.; Zheng, H.; Samuels, L.; Jetter, R.; Kunst, L. Identification of the wax ester synthase/acyl–coenzyme A: Diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis. Plant Physiol. 2008, 148, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Patwari, P.; Salewski, V.; Gutbrod, K.; Kreszies, T.; Dresen-Scholz, B.; Peisker, H.; Steiner, U.; Meyer, A.J.; Schreiber, L.; Dormann, P. Surface wax esters contribute to drought tolerance in Arabidopsis. Plant J. 2019, 98, 727–744. [Google Scholar] [CrossRef]
- Wang, Y.L.; Gao, X.M.; Yu, X.P.; Cheng, S.L.; Kong, L.H. Study on the resource and its utilizations of Lindera glauca in China. Henan Sci. 1994, 12, 331–334. [Google Scholar]
- Seki, K.; Sasaki, T.; Haga, K.; Kaneko, R. Two methoxybutanolides from Lindera glauca. Phytochemistry 1994, 36, 949–951. [Google Scholar] [CrossRef]
- Huh, G.W.; Park, J.H.; Kang, J.H.; Jeong, T.S.; Kang, H.C.; Baek, N.I. Flavonoids from Lindera glauca Blume as low–density lipoprotein oxidation inhibitors. Nat. Prod. Res. 2014, 28, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Dupont, Y.L. Evolution of apomixis as a strategy of colonization in the dioecious species Lindera glauca (Lauraceae). Popul. Ecol. 2002, 44, 293–297. [Google Scholar] [CrossRef]
- Tsui, H.P.; Xia, Z.D.; Li, J.L. Lauraceae and Hernandiaceae; Li, H.W., Ed.; Flora of China Science Press: Beijing, China, 1982; Volume 31, pp. 393–394. [Google Scholar]
- Tsui, H.P.; Werff, H. Lauraceae and Hernandiaceae; Li, H.W., Ed.; Flora of China Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2008; Volume 7, pp. 146–147. [Google Scholar]
- Huh, G.W.; Park, J.H.; Shrestha, S.; Lee, Y.H.; Ahn, E.M.; Kang, H.C.; Baek, N.I. Sterols from Lindera glauca Blume stem wood. J. Appl. Biol. Chem. 2011, 54, 309–312. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, L.; Dong, S.; Zhang, Z. Population genetic structure and variability in Lindera glauca (Lauraceae) indicates low levels of genetic diversity and skewed sex ratios in natural populations in mainland China. PeerJ 2020, 8, 8304. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Xiong, B.; Ju, Y.X.; Hao, Q.; Zhang, Z.X. Study on fruit growth regularity and lipid accumulation of Lindera glauca. Chinese Agri Sci Bull. 2015, 31, 29–33. [Google Scholar]
- Lin, Z.; An, J.; Wang, J.; Niu, J.; Ma, C.; Wang, L.; Yuan, G.; Shi, L.; Liu, L.; Zhang, J.; et al. Integrated analysis of 454 and Illumina transcriptomic sequencing characterizes carbon flux and energy source for fatty acid synthesis in developing Lindera glauca fruits for woody biodiesel. Biotechnol. Biofuels 2017, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Hou, X.; Niu, J.; Li, P.; Fang, C.; Qiu, L.; Ha, D.; Zhang, Z.; Sun, J.; Li, Y.; et al. Volatile constituents from the fruits of Lindera glauca (Sieb. et Zucc.) with different maturities. J. Essent. Oil Bear. Plants 2016, 19, 926–935. [Google Scholar] [CrossRef]
- Sun, H.L.; Wang, J.X.; Gu, X.Z.; Kang, W.Y. Analysis of volatile compounds from leaves and fruits of Lindera glauca. Chin. J. Exp. Tradit. Med. 2011, 7, 033. [Google Scholar]
- Kim, K.H.; Moon, E.; Ha, K.; Suh, W.S.; Kim, H.H.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Bioactive lignan constituents from the twigs of Lindera glauca. Chem. Pharm. Bull. 2014, 62, 1136–1140. [Google Scholar] [CrossRef] [Green Version]
- Suh, W.S.; Kim, K.H.; Kim, H.K.; Choi, S.U.; Lee, K.R. Three new lignan derivatives from Lindera glauca (Siebold et Zucc.) Blume. Helv. Chim. Acta 2015, 98, 1087–1094. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Johnson, L.S.; Eddy, S.R.; Portugaly, E. Hidden Markov model speed heuristic and iterative HMM search procedure. BMC Bioinform. 2010, 11, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchler-Bauer, A.; Bryant, S.H. CD–Search: Protein domain annotations on the fly. Nucleic Acids Res. 2004, 32 (Suppl. S2), W327–W331. [Google Scholar] [CrossRef]
- Garg, V.K.; Avashthi, H.; Tiwari, A.; Jain, P.A.; Ramkete, P.W.; Kayastha, A.M.; Singh, V.K. MFPPI–multi FASTA ProtParam interface. Bio. Inf. 2016, 12, 74–77. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letunic, I.; Bork, P. Interactive tree of life (ITOL) v5: An online tool for phylogenetic tree displays and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Wall, P.K.; Leebens-Mack, J.H.; Lindsay, B.G.; Soltis, D.E.; Doyle, J.J.; Soltis, P.S.; Carlson, J.E.; Arumuganathan, K.; Barakat, A.; et al. Widespread genome duplications throughout the history of flowering plants. Genome Res. 2006, 16, 738–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage Publications Ltd.: Thousand Oaks, CA, USA, 2013. [Google Scholar]
- Wang, R.; Hanna, M.A.; Zhou, W.W.; Bhadury, P.S.; Chen, Q.; Song, B.A.; Yang, S. Production and selected fuel properties of biodiesel from promising non–edible oils: Euphorbia lathyris L., Sapium sebiferum L. and Jatropha curcas L. Bioresour. Technol. 2011, 102, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.S.; Xiao, Z.C. Study and utilization of Lindera glauca in China. Wild Plant Resour. 1985, 2, 2–6. [Google Scholar]
- Wang, J.P.; Meng, S.J.; Li, J.M. Fatty acid of oils of Lauraceae. Acta Bot. Sin. 1984, 27, 175–185. [Google Scholar]
- Baud, S.; Lepiniec, L. Physiological and developmental regulation of seed oil production. Prog. Lipid Res. 2010, 49, 235–249. [Google Scholar] [CrossRef]
- Barthole, G.; Lepiniec, L.; Rogowsky, P.M.; Baud, S. Controlling lipid accumulation in cereal grains. Plant Sci. 2012, 185, 33–39. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Zhang, G.; Liu, J.; Manan, S.; Hu, H.; Zhao, J. Two types of soybean diacylglycerol acyltransferases are differentially involved in triacylglycerol biosynthesis and response to environmental stresses and hormones. Sci. Rep. 2016, 6, 28541. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Hatanaka, T.; Yu, K.; Wu, Y.; Fukushige, H.; Hildebrand, D. Soybean oil biosynthesis: Role of diacylglycerol acyltransferases. Funct. Integr. Genom. 2013, 13, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Shockey, J.M.; Gidda, S.K.; Chapital, D.C.; Kuan, J.C.; Dhanoa, P.K.; Bland, J.M.; Rothstein, S.J.; Mullen, R.T.; Dyer, J.M. Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 2006, 18, 2294–2313. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, J.; Gai, J.; Chen, S. Cloning and comparative analysis of the gene encoding diacylglycerol acyltransferase from wild type and cultivated soybean. Theor. Appl. Genet. 2006, 112, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Allen, W.B.; Roesler, K.; Williams, M.E.; Zhang, S.; Li, J.; Glassman, K.; Ranch, J.; Nubel, D.; Solawetz, W.; et al. A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat. Genet. 2008, 40, 367–372. [Google Scholar] [CrossRef]
- Xu, J.; Francis, T.; Mietkiewska, E.; Giblin, E.M.; Barton, D.L.; Zhang, Y.; Zhang, M.; Taylor, D.C. Cloning and characterization of an acyl–CoA–dependent diacylglycerol acyltransferase 1 (DGAT1) gene from Tropaeolum majus, and a study of the functional motifs of the DGAT protein using site–directed mutagenesis to modify enzyme activity and oil content. Plant Biotechnol. J. 2008, 6, 799–818. [Google Scholar] [CrossRef]
- Zhao, J.; Bi, R.; Li, S.; Zhou, D.; Bai, Y.; Jing, G.; Zhang, K.; Zhang, W. Genome–wide analysis and functional characterization of Acyl–CoA: Diacylglycerol acyltransferase from soybean identify GmDGAT1A and 1B roles in oil synthesis in Arabidopsis seeds. J. Plant Physiol. 2019, 242, 153019. [Google Scholar] [CrossRef]
- Yan, B.; Xu, X.; Gu, Y.; Zhao, Y.; Zhao, X.; He, L.; Zhao, C.; Li, Z.; Xu, J. Genome–wide characterization and expression profiling ofdiacylglycerol acyltransferase genes from maize. Genome 2018, 61, 735–743. [Google Scholar] [CrossRef]
- Sharpton, T.J.; Neafsey, D.E.; Galagan, J.E.; Taylor, J.W. Mechanisms of intron gain and loss in Cryptococcus. Genome Biol. 2008, 9, R24. [Google Scholar] [CrossRef] [Green Version]
- Lynch, M.; Richardson, A.O. The evolution of spliceosomal introns. Curr. Opin. Genet. Dev. 2002, 12, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.W.; Gilbert, W. The evolution of spliceosomal introns: Patterns, puzzles and progress. Nat. Rev. Genet. 2006, 7, 211–221. [Google Scholar]
- Turchetto-Zolet, A.C.; Maraschin, F.S.; de Morais, G.L.; Cagliari, A.; Andrade, C.M.; Margis-Pinheiro, M.; Margis, R. Evolutionaryview of acyl–CoA diacylglycerol acyltransferase (DGAT), a key enzyme in neutral lipid biosynthesis. BMC Evol. Biol. 2011, 11, 263. [Google Scholar] [CrossRef] [Green Version]
- Turchetto-Zolet, A.C.; Christoff, A.P.; Kulcheski, F.R.; Loss-Morais, G.; Margis, R.; Margis-Pinheiro, M. Diversity and evolution of plant diacylglycerol acyltransferase (DGATs) unveiled by phylogenetic, gene structure and expression analyses. Genet. Mol. Biol. 2016, 39, 524–538. [Google Scholar] [CrossRef] [Green Version]
- Bhatt-Wessel, B.; Jordan, T.W.; Miller, J.H.; Peng, L. Role of DGAT enzymes in triacylglycerol metabolism. Arch. Biochem. Biophys. 2018, 655, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ganko, E.W.; Meyers, B.C.; Vision, T.J. Divergence in expression between duplicated genes in Arabidopsis. Mol. Biol. Evol. 2007, 24, 2298–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, S.D.; Zou, J.; Weselake, R.J. Abiotic factors influence plant storage lipid accumulation and composition. Plant Sci. 2016, 243, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Young, D.Y.; Shachar-Hill, Y. Large fluxes of fatty acids from membranes to triacylglycerol and back during N–deprivation and recovery in Chlamydomonas. Plant Physiol. 2020, 185, 796–814. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr.Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Ji, H.; Yang, Z. Functional characterization of three novel genes encoding Diacylglycerol Acyltransferase (DGAT) from oil–rich tubers of Cyperus esculentus. Plant Cell Physiol. 2020, 61, 118–129. [Google Scholar] [CrossRef]
- Kaup, M.T.; Froese, C.D.; Thompson, J.E. A role for diacylglycerol acyltransferase during leaf senescence. Plant Physiol. 2002, 129, 1616–1626. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.L.; de Noyer, S.B.; Hobbs, D.H.; Kang, J.; Wen, Y.; Krachtus, D.; Hills, M.J. Expression pattern of diacylglycerol acyltransferase–1, an enzyme involved in triacylglycerol biosynthesis, in Arabidopsis thaliana. Plant Mol. Biol. 2003, 52, 31–41. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Yang, J.B.; Meng, W.Q.; Zeng, L.L.; Sun, L. Genome–wide identification and relative expression analysis of DGATs gene family in sunflower. Acta Agron. Sin. 2023, 49, 73–85. [Google Scholar]
- Zhao, Y.P.; Wu, N.; Li, W.J.; Shen, J.L.; Chen, C.; Li, F.G.; Hou, Y.X. Evolution and Characterization of Acetyl Coenzyme A: Diacylglycerol Acyltransferase Genes in Cotton Identify the Roles of GhDGAT3D in Oil Biosynthesis and Fatty Acid Composition. Genes 2021, 12, 1045. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Iwasaki, A.; Matsumoto, N.; Uemura, T.; Tatematsu, K.; Okada, K. Physical interaction of floral organs controls petal morphogenesis in Arabidopsis. Plant Physiol. 2013, 161, 1242–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene ID | Forward Primer | Reverse Primer |
|---|---|---|
| LgDGAT1 | CGACTCCTCCTCCAAGACCTG | ACCGACGGATTCCTCTGTTCTC |
| LgDGAT2.1 | TCAGTGAGGTTATCTGTTGC | GACCATAGCAGAAAACAGGA |
| LgDGAT2.2 | CACACCACTACTAAGGCAAA | ATATGAACAATCTCCCGAGC |
| LgDGAT2.3 | GAGGTCATCCTCCAGAAAAG | AAGTTGAGATGAATGGTCCC |
| LgDGAT2.4 | GTGTTTGGGATGCTGTTATG | ACGTGAAGTGTAATGGGAAA |
| LgDGAT2.6 | CTCTGTCAACGCAACCATACTCAC | TGTGGACTGTGGTGTGGATGG |
| LgDGAT3.3 | ATAGACCAACCACAACCCATTCAG | AAGAGAAGCAAGGAACAGCAGTAG |
| LgWSD1 | TCCCAAGCCAGTCCAGTGTC | TTGAGATTGTGAAGGTTGTGTTAGC |
| LgWSD2 | AAGTTTCGACATTCAGGACA | GTAGCCCTTCTAATTCTCGG |
| UBC | CTGGGATACCATCCAGAACATC | CTCAAGTGTCCTTCCAGCATAG |
| RPL32 | CCGCCACCTCTCTCTTTATTT | GCGCTTCTTGACAATCTTCTTG |
| Gene ID | Accession Number | AA | Molecular Weight | PI | Aliphatic Index | GRAVY | Predicted Location(s) | Transmembrane Domain |
|---|---|---|---|---|---|---|---|---|
| LgDGAT1 | Lg06G6989 | 543 | 61,266.01 | 7.14 | 91.84 | 0.129 | Endoplasmic reticulum | YES |
| LgDGAT2.1 | Lg05G6132 | 292 | 32,678.24 | 9.28 | 95.72 | 0.189 | Endoplasmic reticulum | NO |
| LgDGAT2.2 | Lg05G3126 | 347 | 39,417.38 | 9.33 | 96.89 | 0.165 | Endoplasmic reticulum | YES |
| LgDGAT2.3 | Lg05G3163 | 349 | 39,622.65 | 9.33 | 96.33 | 0.175 | Endoplasmic reticulum | YES |
| LgDGAT2.4 | Lg06G6636 | 340 | 38,598.36 | 9.26 | 101.79 | 0.293 | Endoplasmic reticulum | YES |
| LgDGAT2.5 | Lg08G2897 | 304 | 33,914.54 | 5.23 | 85.89 | −0.221 | Endoplasmic reticulum | NO |
| LgDGAT2.6 | Lg10G4262 | 279 | 30,661.56 | 8.86 | 82.15 | 0.146 | Cell membrane | YES |
| LgDGAT2.7 | Lg03G1073 | 486 | 55,057.85 | 8.64 | 98.27 | −0.134 | Endoplasmic reticulum | NO |
| LgDGAT2.8 | Lg03G1089 | 1428 | 160,137.89 | 7.06 | 93.77 | −0.132 | Endoplasmic reticulum | NO |
| LgDGAT3.1 | Lg03G163 | 90 | 9049.4 | 6.21 | 72.56 | −0.11 | Cell wall | NO |
| LgDGAT3.2 | Lg03G198 | 259 | 27,851.62 | 8.58 | 73.13 | −0.557 | Nucleus | NO |
| LgDGAT3.3 | Lg06G3301 | 360 | 38,915.39 | 5.68 | 66.89 | −0.399 | Nucleus | NO |
| LgWSD1 | Lg10G479 | 168 | 18,727.96 | 8.72 | 96.37 | 0.083 | Nucleus | NO |
| LgWSD2 | Lg10G489 | 399 | 44,459.4 | 5.86 | 100.13 | −0.026 | Chloroplast | NO |
| LgWSD3 | Lg11G2666 | 385 | 43,035.94 | 8.47 | 94.21 | −0.023 | Chloroplast | NO |
| Duplicated Gene 1 | Duplicated Gene 2 | Ka | Ks | Ka/Ks | Duplicated Type | Selective Type | Divergence Time (Mya) |
|---|---|---|---|---|---|---|---|
| LgDGAT2.2 | LgDGAT2.1 | 0.223508 | 0.663243 | 0.336993 | WGD or Segmental | Purifying | 109.808444 |
| LgDGAT2.2 | LgDGAT2.3 | 0.001261 | 0.00406 | 0.310504 | WGD or Segmental | Purifying | 0.672185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Yang, Y.; Xie, L.; Li, Q.; Xiong, B. Genome-Wide Identification and Analysis of the DGAT Gene Family in Lindera glauca and Expression Analysis during Fruit Development Stages. Forests 2023, 14, 1633. https://doi.org/10.3390/f14081633
Bai X, Yang Y, Xie L, Li Q, Xiong B. Genome-Wide Identification and Analysis of the DGAT Gene Family in Lindera glauca and Expression Analysis during Fruit Development Stages. Forests. 2023; 14(8):1633. https://doi.org/10.3390/f14081633
Chicago/Turabian StyleBai, Xue, Yongyi Yang, Lun Xie, Qingqing Li, and Biao Xiong. 2023. "Genome-Wide Identification and Analysis of the DGAT Gene Family in Lindera glauca and Expression Analysis during Fruit Development Stages" Forests 14, no. 8: 1633. https://doi.org/10.3390/f14081633




