Evaluation of the Deterioration State of Historical Palm Leaf Manuscripts from Burma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Optical Microscopy
2.3. Physical Characterization
2.4. SEM–EDX
2.5. AFM
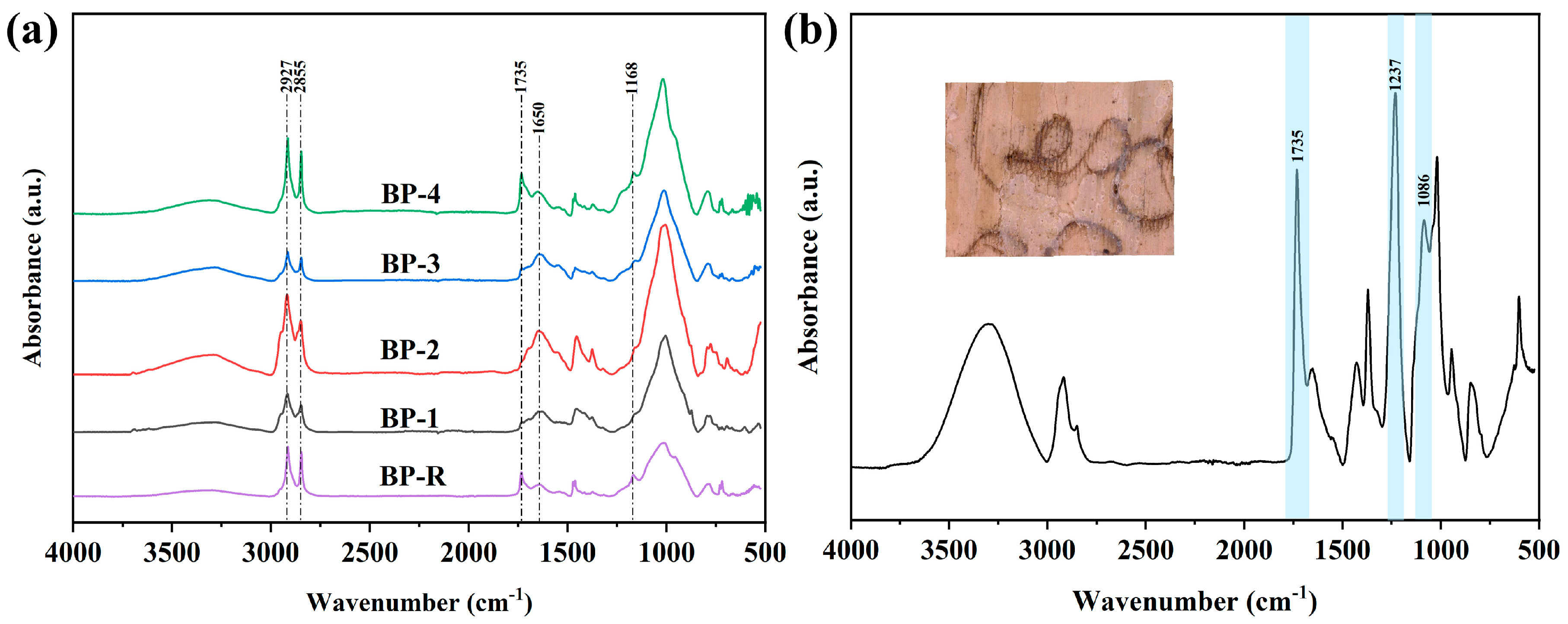
2.6. ATR–FTIR
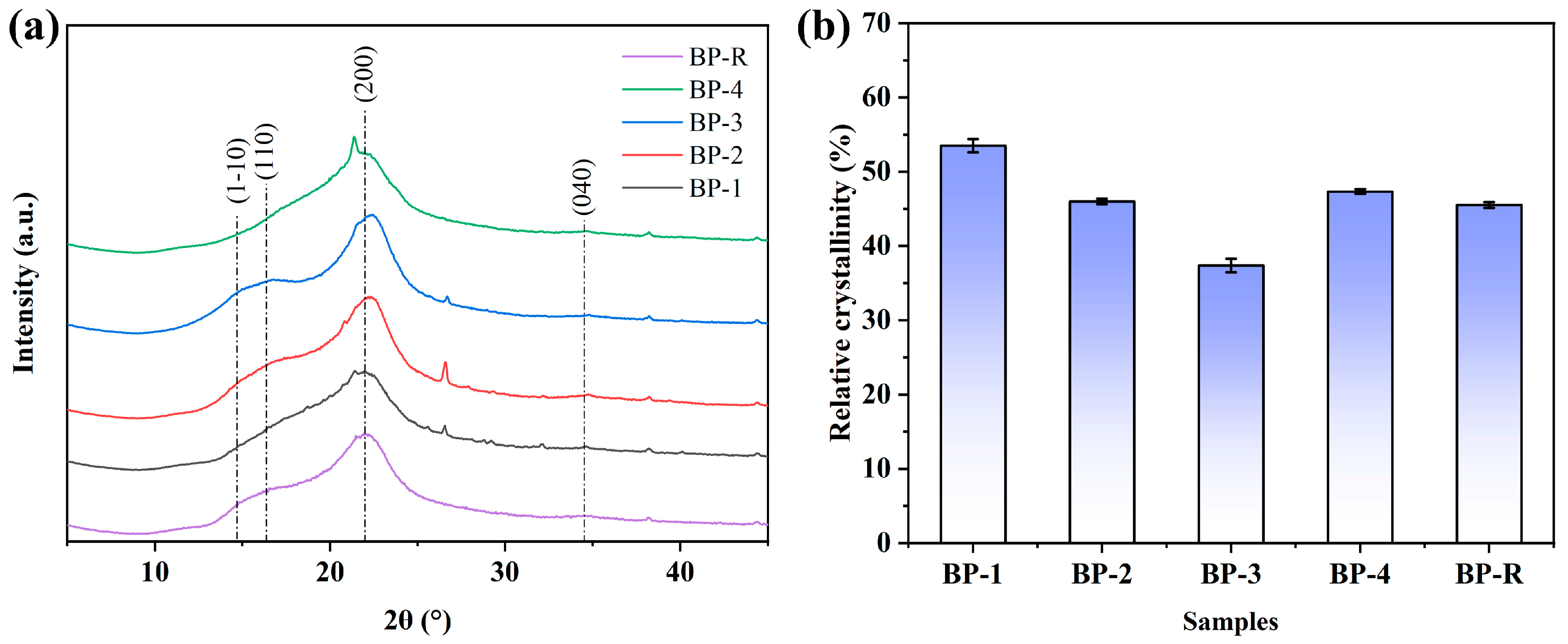
2.7. XRD
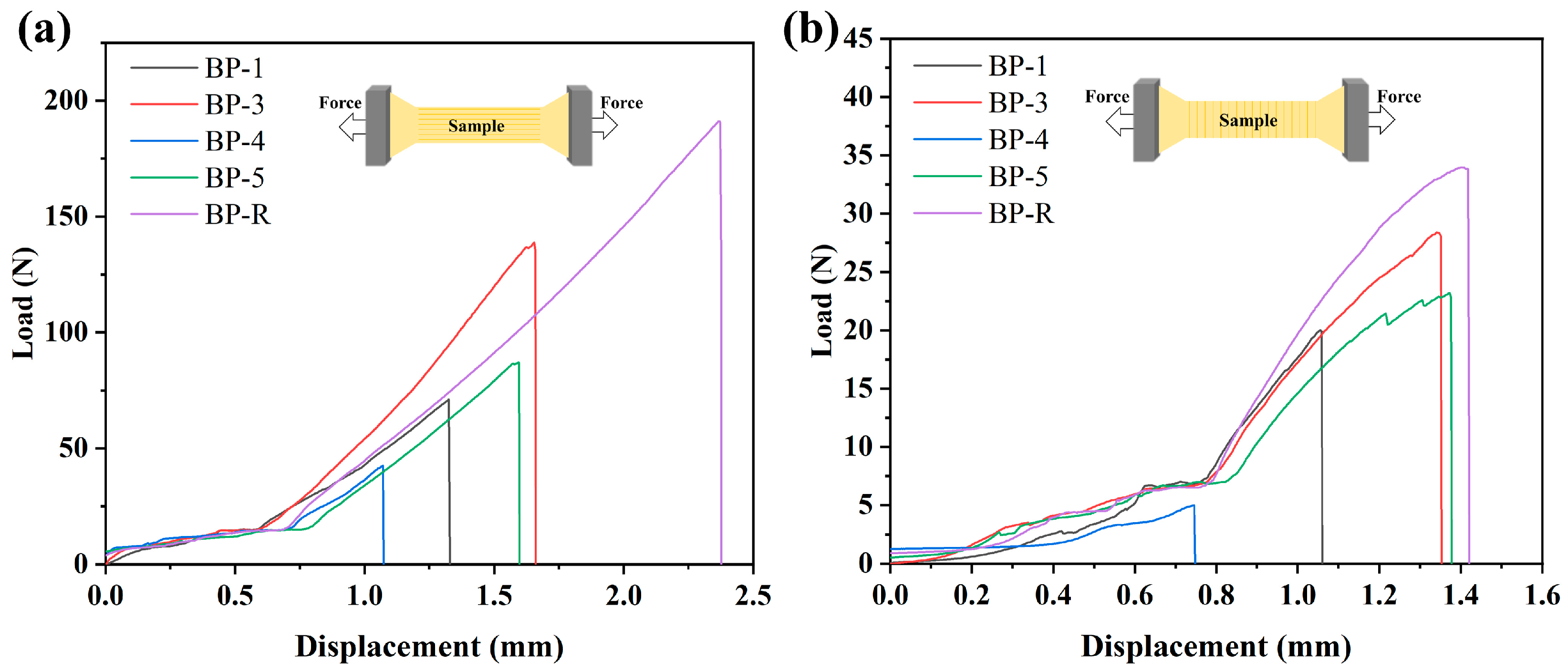
2.8. Tensile Strength
3. Results and Discussion
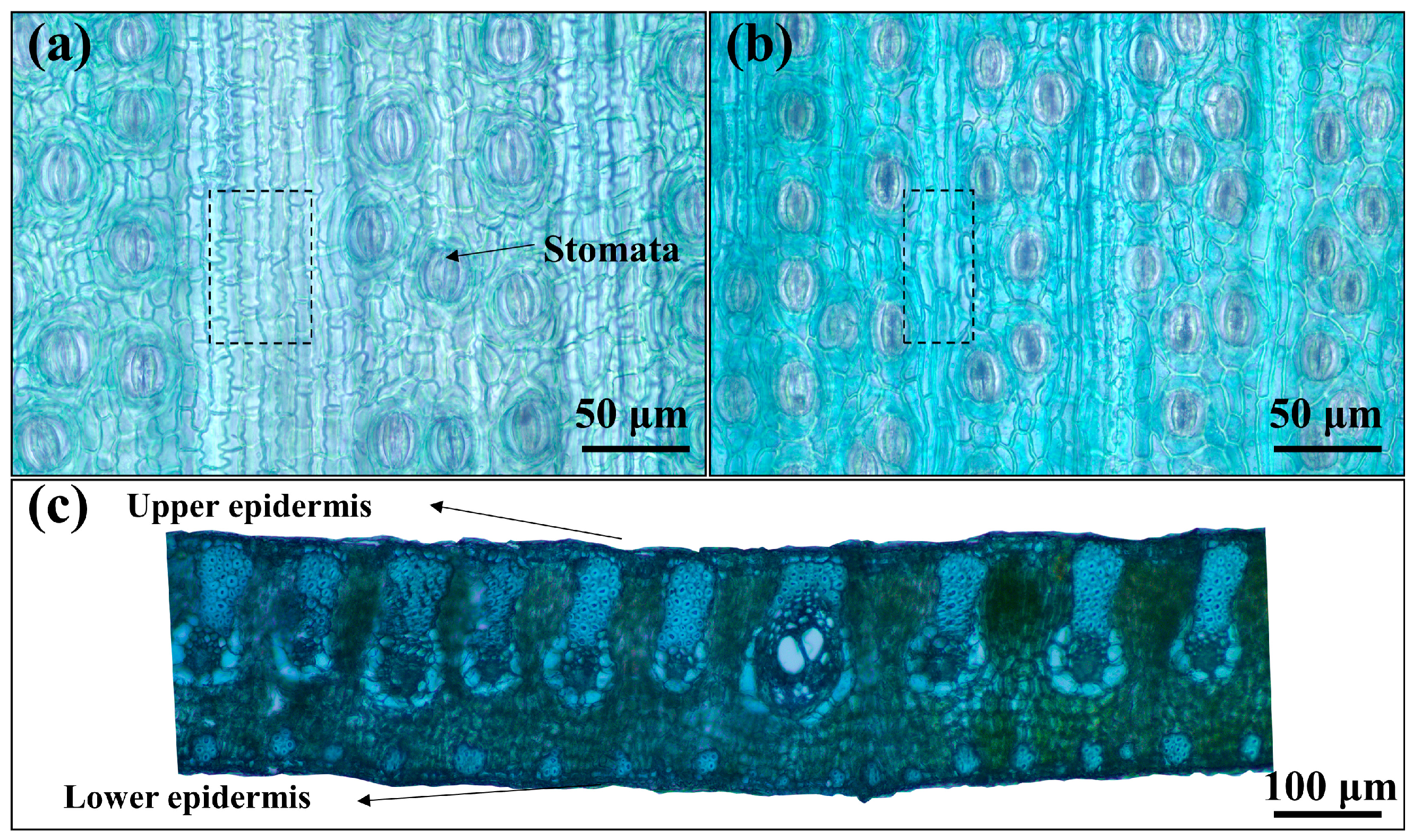
3.1. Anatomy of Palm Leaf
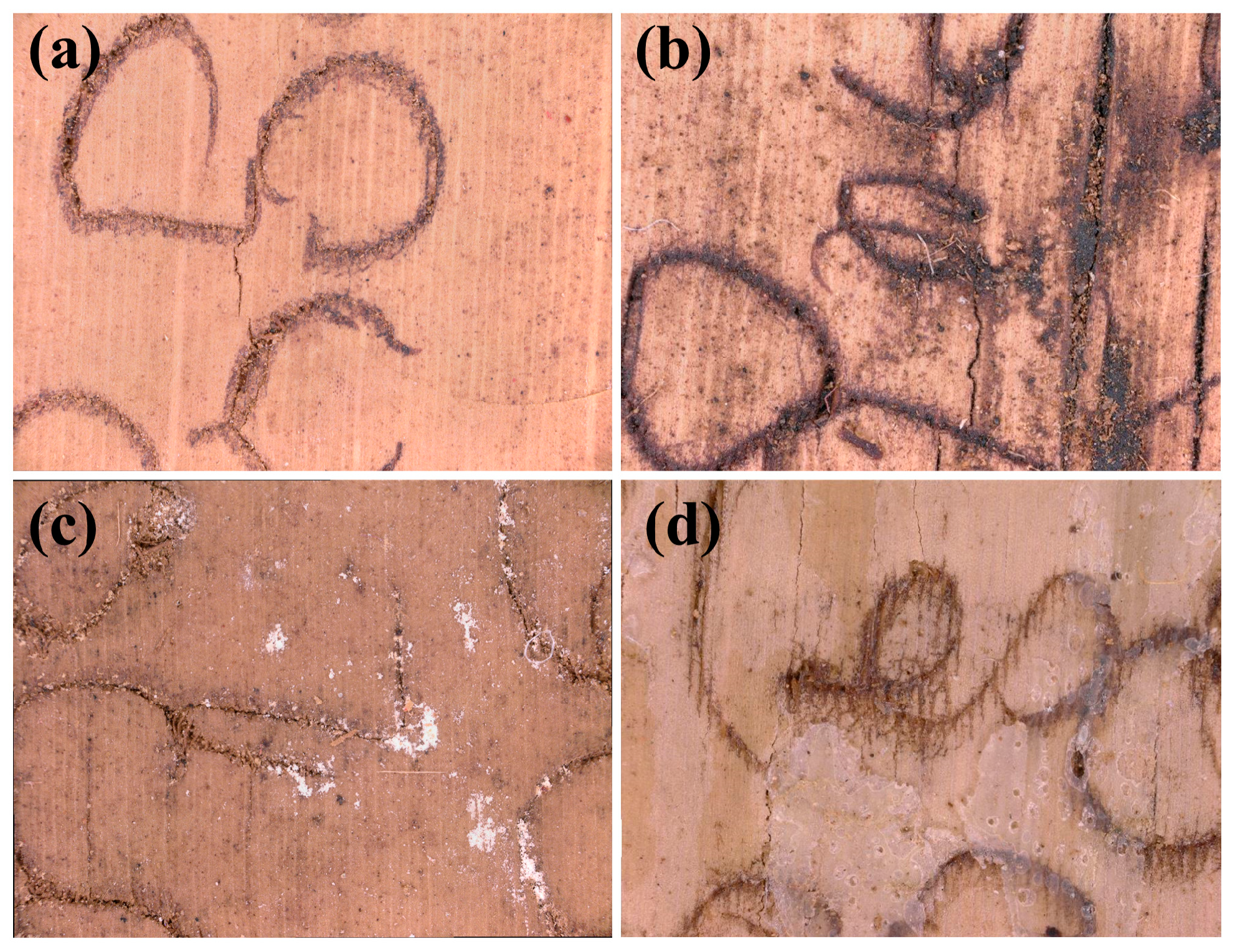
3.2. Basic Physical Parameters
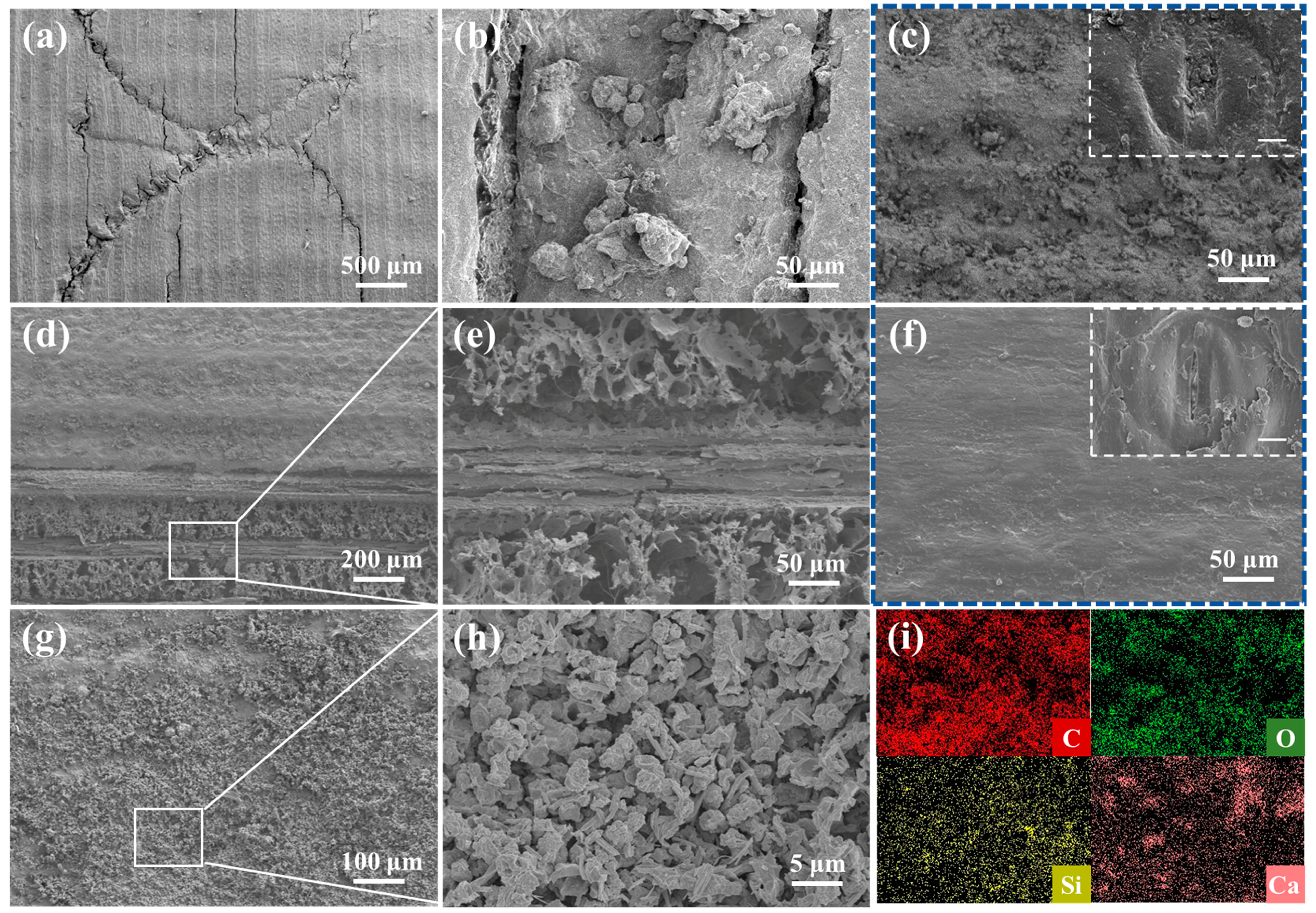
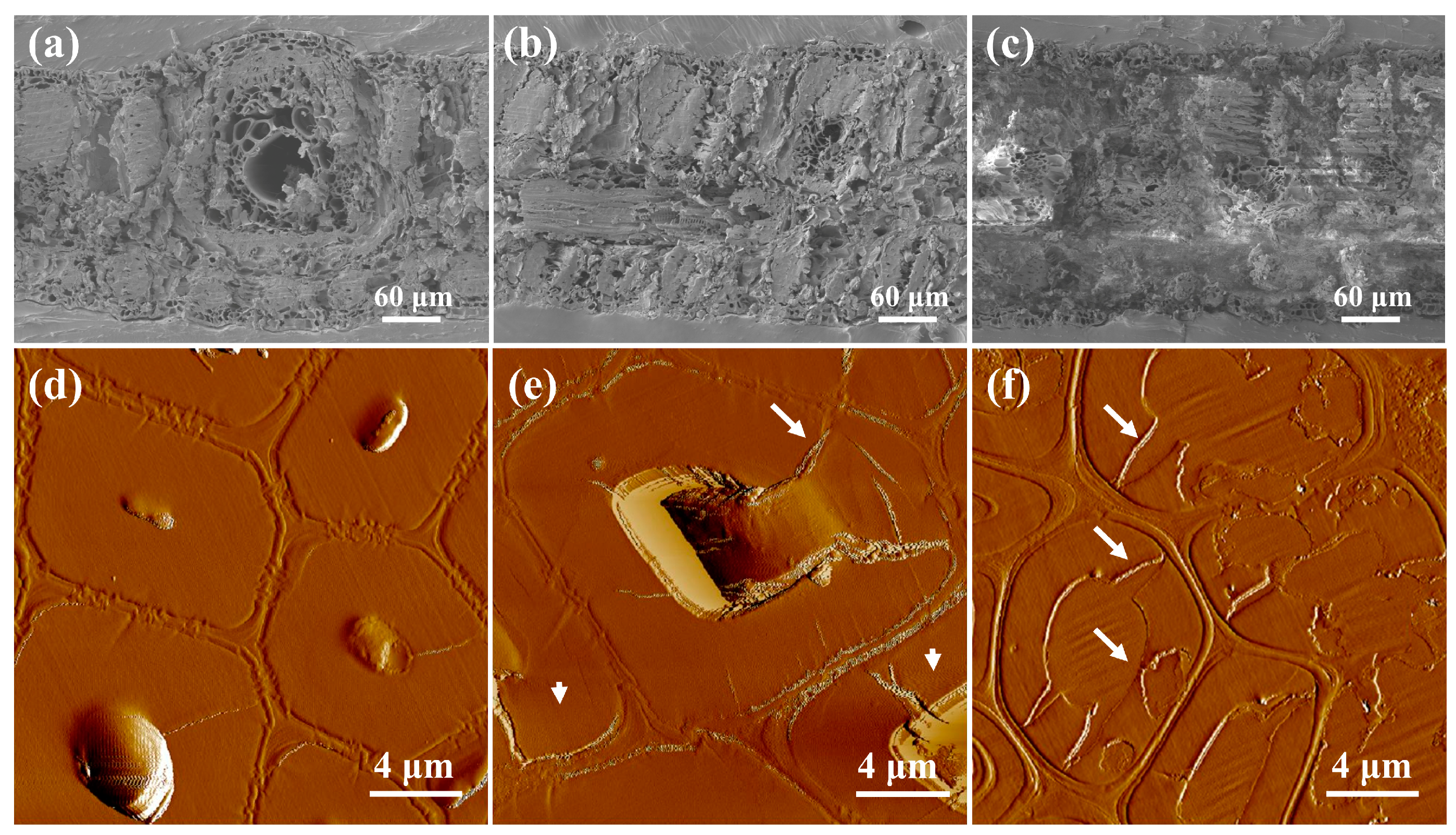
3.3. Morphology Analysis
3.4. Chemical Component Analysis
3.5. Mechanical Properties Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vijaya Lakshmi, T.R.; Sastry, P.N.; Rajinikanth, T.V. Feature selection to recognize text from palm leaf manuscripts. Signal Image Video Process. 2018, 12, 223–229. [Google Scholar] [CrossRef]
- Wang, K.; Xia, W.; Ren, J.; Yu, W.; Feng, H.; Hu, S. Wind energy harvesting inspired by Palm leaf flutter: Observation, mechanism and experiment. Energy Convers. Manag. 2023, 284, 116971. [Google Scholar] [CrossRef]
- Junio, R.F.P.; de Mendonça Neuba, L.; Souza, A.T.; Pereira, A.C.; Nascimento, L.F.C.; Monteiro, S.N. Thermochemical and structural characterization of promising carnauba novel leaf fiber (Copernicia prunifera). J. Mater. Res. Technol. 2022, 18, 4714–4723. [Google Scholar] [CrossRef]
- Abdel-Azim, M.S. Development of prototype structure for low-cost and energy-efficient house by utilizing palm tree fronds. Build. Environ. 1997, 32, 375–380. [Google Scholar] [CrossRef]
- Wiland, J.; Brown, R.; Fuller, L.; Havelock, L.; Johnson, J.; Kenn, D.; Kralka, P.; Muzart, M.; Pollard, J.; Snowdon, J. A literature review of palm leaf manuscript conservation—Part 1: A historic overview, leaf preparation, materials and media, palm leaf manuscripts at the British Library and the common types of damage. J. Inst. Conserv. 2022, 45, 236–259. [Google Scholar] [CrossRef]
- Zhang, M.; Song, X.; Wang, Y. Two Different storage environments for palm leaf manuscripts: Comparison of deterioration phenomena. Restaurator 2021, 42, 147–168. [Google Scholar] [CrossRef]
- Zhang, M.; Song, X.; Wang, J.; Lyu, X. Preservation characteristics and restoration core technology of palm leaf manuscripts in Potala Palace. Arch. Sci. 2022, 22, 501–519. [Google Scholar] [CrossRef]
- Alexander, T.J.; Kumar, S.S. A novel binarization technique based on Whale Optimization Algorithm for better restoration of palm leaf manuscript. J. Ambient. Intell. Humaniz. Comput. 2020, 1–8. [Google Scholar] [CrossRef]
- Subramani, K.; Subramaniam, M. Creation of original Tamil character dataset through segregation of ancient palm leaf manuscripts in medicine. Expert Syst. 2021, 38, e12538. [Google Scholar] [CrossRef]
- Wiland, J.; Brown, R.; Fuller, L.; Havelock, L.; Johnson, J.; Kenn, D.; Kralka, P.; Muzart, M.; Pollard, J.; Snowdon, J. A literature review of palm leaf manuscript conservation—Part 2: Historic and current conservation treatments, boxing and storage, religious and ethical issues, recommendations for best practice. J. Inst. Conserv. 2023, 46, 64–91. [Google Scholar] [CrossRef]
- Singh, M.R.; Sharma, D. Investigation of pigments on an Indian palm leaf manuscript (18th–19th century) by SEM-EDX and other techniques. Restaurator 2020, 41, 49–65. [Google Scholar] [CrossRef]
- Agrawal, O.P. Conservation of Manuscripts and Paintings of South-East Asia; Butterworths: Boston, MA, USA, 1984. [Google Scholar]
- Sah, A. Palm leaf manuscripts of the world: Material, technology and conservation. Stud. Conserv. 2002, 47, 15–24. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-Ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Giraud-Guille, M.M. Plywood structures in nature. Curr. Opin. Solid State Mater. Sci. 1998, 3, 221–227. [Google Scholar] [CrossRef]
- Horn, J.W.; Fisher, J.B.; Tomlinson, P.B.; Lewis, C.E.; Laubengayer, K. Evolution of lamina anatomy in the palm family (Arecaceae). Am. J. Bot. 2009, 96, 1462–1486. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, T.; Zimmer, B.; Petutschnigg, A.J. On the modelling of colour changes of wood surfaces. Eur. J. Wood Wood Prod. 2009, 67, 141–149. [Google Scholar] [CrossRef]
- Shahani, C.J.; Harrison, G. Spontaneous formation of acids in the natural aging of paper. Stud. Conserv. 2002, 47, 189–192. [Google Scholar] [CrossRef]
- Sugano, J.; Linnakoski, R.; Huhtinen, S.; Pappinen, A.; Niemelä, P.; Asiegbu, F.O. Cellulolytic activity of brown-rot Antrodia sinuosa at the initial stage of cellulose degradation. Holzforschung 2019, 73, 673–680. [Google Scholar] [CrossRef]
- Tanabe, J.; Ishiguri, F.; Ohshima, J.; Iizuka, K.; Yokota, S. Changes in lignocellulolytic enzyme activity during the degradation of Picea jezoensis wood by the white-rot fungus Porodaedalea pini. Int. Biodeterior. Biodegrad. 2016, 110, 108–112. [Google Scholar] [CrossRef]
- Mo, W.; Li, B.; Chen, K. Low-temperature thermal drying-induced pore expansion effects of cellulosic fibers. Cellulose 2023, 30, 3441–3453. [Google Scholar] [CrossRef]
- Mvondo, R.R.N.; Meukam, P.; Jeong, J.; Meneses, D.D.S.; Nkeng, E.G. Influence of water content on the mechanical and chemical properties of tropical wood species. Results Phys. 2017, 7, 2096–2103. [Google Scholar] [CrossRef]
- Björdal, C.G. Microbial degradation of waterlogged archaeological wood. J. Cult. Herit. 2012, 13, S118–S122. [Google Scholar] [CrossRef]
- Björdal, C.; Nilsson, T.; Bardage, S. Three-dimensional visualisation of bacterial decay in individual tracheids of Pinus sylvestris. Holzforschung 2005, 59, 178–182. [Google Scholar] [CrossRef]
- Guo, J.; Chen, J.; Meng, Q.; Ploszczanski, L.; Liu, J.A.; Luo, R.; Jin, T.; Siedlaczek, P.; Lichtenegger, H.C.; Yin, Y.; et al. Molecular and crystal structures of cellulose in severely deteriorated archaeological wood. Cellulose 2022, 29, 9549–9568. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, M.; Qiang, M.; Li, X.; Morrell, J.J.; Yao, Y. Preparation of cellulose nanoparticles from foliage by bio-enzyme methods. Materials 2021, 14, 4557. [Google Scholar] [CrossRef]
- Fang, S.; Lyu, X.; Tong, T.; Lim, A.I.; Li, T.; Bao, J.; Hu, Y.H. Turning dead leaves into an active multifunctional material as evaporator, photocatalyst, and bioplastic. Nat. Commun. 2023, 14, 1203. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, A.S.; Abd, A.M.; Awad, K.M. The role of nano-selenium in alleviating the effects of salt stress in date palm trees (Phoenix dactylifera L.): A fourier transform infrared (FTIR) spectroscopy study. BioNanoScience 2023, 13, 74–80. [Google Scholar] [CrossRef]
- Kayabas, A.; Yildirim, E. Biochemical fingerprints of some endemic plants growing in gypsum soils: Attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectroscopic study. Eur. J. Biol. 2021, 80, 97–106. [Google Scholar] [CrossRef]
- Gorgulu, S.T.; Dogan, M.; Severcan, F. The characterization and differentiation of higher plants by Fourier transform infrared spectroscopy. Appl. Spectrosc. 2007, 61, 300–308. [Google Scholar] [CrossRef]
- Ivanova, D.G.; Singh, B.R. Nondestructive FTIR monitoring of leaf senescence and elicitin-induced changes in plant leaves. Biopolym. Orig. Res. Biomol. 2003, 72, 79–85. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, E.; Fu, F.; Lin, L.; Yi, S. Characterization of wood cell walls treated by high-intensity microwaves: Effects on physicochemical structures and micromechanical properties. Ind. Crops Prod. 2022, 187, 115341. [Google Scholar] [CrossRef]
- Fernandes, A.N.; Thomas, L.H.; Altaner, C.M.; Callow, P.; Forsyth, V.T.; Apperley, D.C.; Kennedy, C.J.; Jarvis, M.C. Nanostructure of cellulose microfibrils in spruce wood. Proc. Natl. Acad. Sci. USA 2011, 108, E1195–E1203. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, W.; Meng, D.; Li, X.; Goodell, B. Non-enzymatic modification of the crystalline structure and chemistry of Masson pine in brown-rot decay. Carbohydr. Polym. 2022, 286, 119242. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Huang, Y.; Yu, W. An in-depth study of molecular and supramolecular structures of bamboo cellulose upon heat treatment. Carbohydr. Polym. 2020, 241, 116412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Ke, D.; Wang, C.; Pan, H.; Chen, K.; Zhang, H. Modified lignin nanoparticles as potential conservation materials for waterlogged archaeological wood. ACS Appl. Nano Mater. 2023, 6, 12351–12363. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, L.; Han, L.; Wu, H.; Yang, T.; Wu, S.; Yin, Y. Deterioration of the cell wall in waterlogged wooden archeological artifacts, 2400 years old. IAWA J. 2019, 40, 820–844. [Google Scholar] [CrossRef]
- Volkmer, T.; Arietano, L.; Plummer, C.; Strautmann, J.; Noël, M. Loss of tensile strength in cellulose tissue on the surface of spruce (Picea abies) caused by natural photodegradation and delignification. Polym. Degrad. Stab. 2013, 98, 1118–1125. [Google Scholar] [CrossRef]
- Zhai, S.; Horikawa, Y.; Imai, T.; Sugiyama, J. Anatomical and mechanical characteristics of leaf-sheath fibrovascular bundles in palms. IAWA J. 2013, 34, 285–300. [Google Scholar] [CrossRef]

| No. | Color Parameters | pH | Moisture Content (%) | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| BP-1 | 44.46 (±1.30) | 10.10 (±0.64) | 24.12 (±0.87) | 4.95 (±0.21) | 8.13 (±0.30) |
| BP-2 | 38.59 (±2.67) | 8.23 (±0.84) | 18.36 (±2.33) | 5.96 (±0.28) | 10.20 (±0.43) |
| BP-3 | 43.91 (±0.86) | 9.63 (±0.17) | 24.03 (±0.75) | 4.85 (±0.25) | 7.13 (±0.25) |
| BP-4 | 56.86 (±2.25) | 5.58 (±0.74) | 24.39 (±1.25) | 5.81 (±0.33) | 9.00 (±0.16) |
| BP-R | 70.52 (±0.55) | 0.91 (±0.31) | 17.96 (±0.50) | 6.36 (±0.04) | 8.87 (±0.21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, S.; Lin, L.; Tian, X. Evaluation of the Deterioration State of Historical Palm Leaf Manuscripts from Burma. Forests 2023, 14, 1775. https://doi.org/10.3390/f14091775
Chu S, Lin L, Tian X. Evaluation of the Deterioration State of Historical Palm Leaf Manuscripts from Burma. Forests. 2023; 14(9):1775. https://doi.org/10.3390/f14091775
Chicago/Turabian StyleChu, Shimin, Lanying Lin, and Xingling Tian. 2023. "Evaluation of the Deterioration State of Historical Palm Leaf Manuscripts from Burma" Forests 14, no. 9: 1775. https://doi.org/10.3390/f14091775




