Comparative Chloroplast Genomics Reveals a Unique Gene Inversion in Two Cordia Trees (Cordiaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Specimens, DNA Extraction, and Sequencing
2.2. Assembly, Annotation, Codon Usage, and RNA Editing Sites
2.3. Repeat Analysis and Characterization of Substitution Rate
2.4. Divergence Sequences and IR Junctions Analyses
2.5. Phylogenetic Analysis
3. Results
3.1. Characteristics of C. monoica and C. sinensis
3.2. Codon Usage
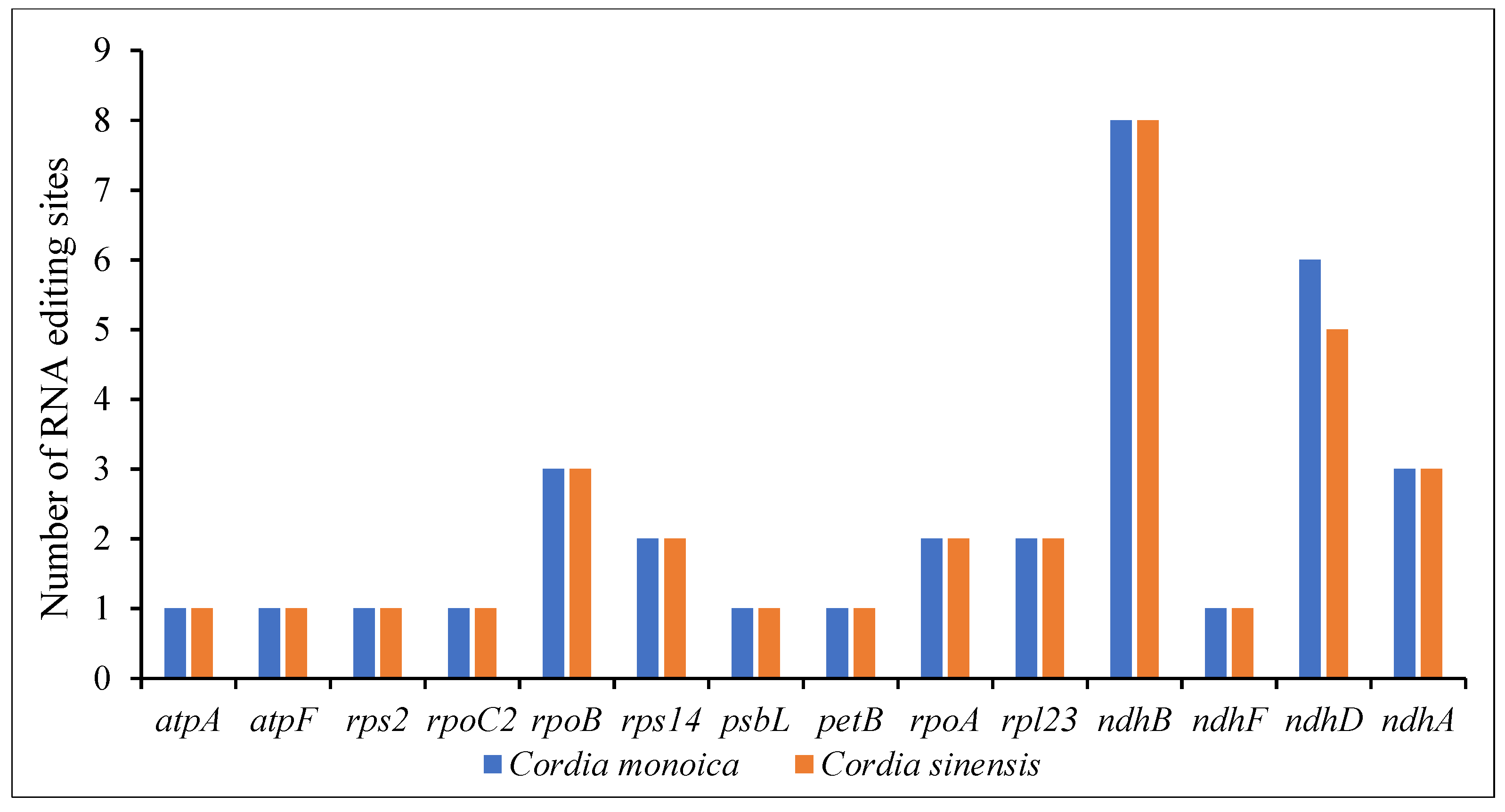
3.3. RNA Editing Sites
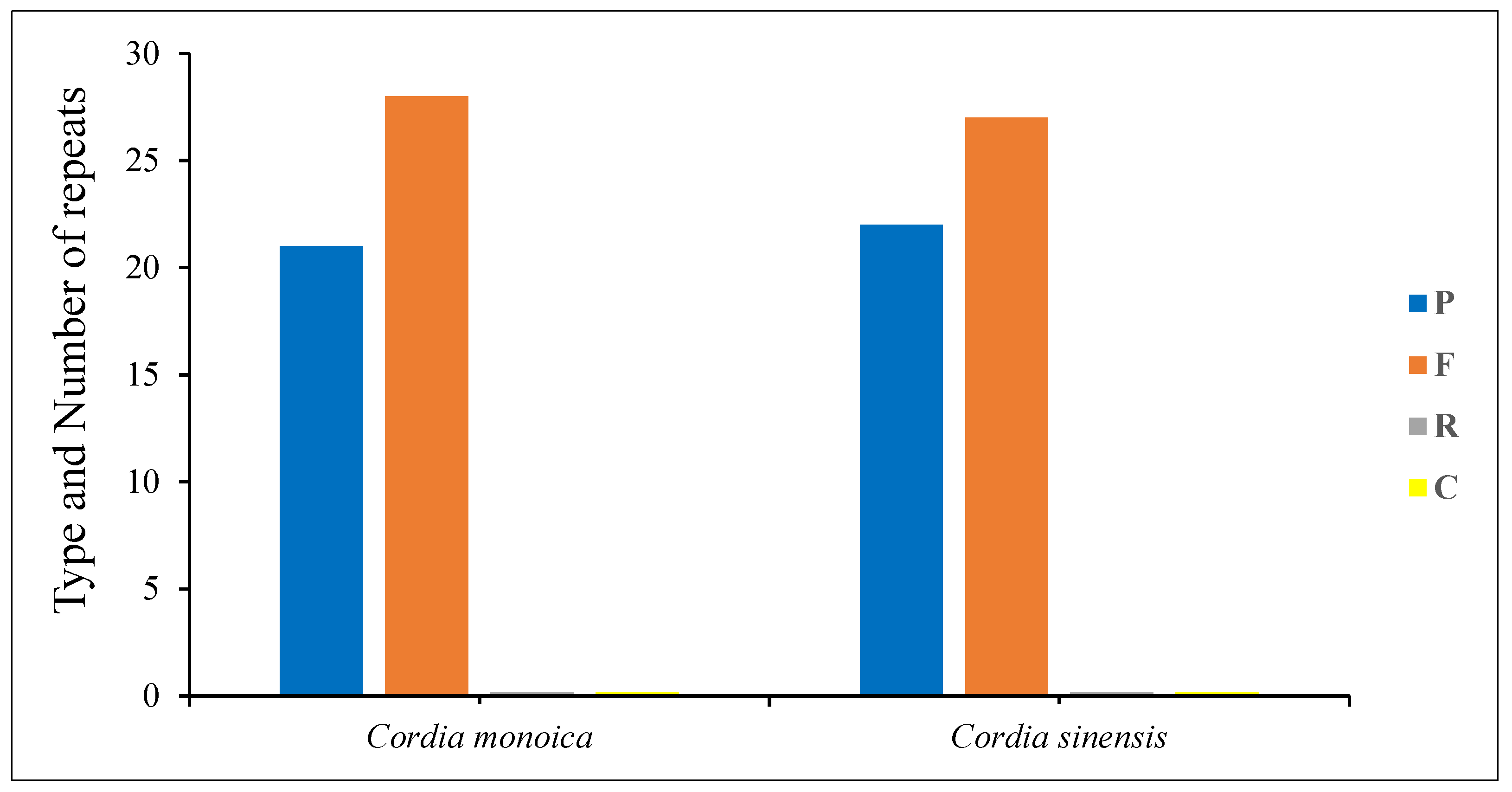
3.4. Long Repeats
3.5. Simple Sequence Repeats (SSRs)
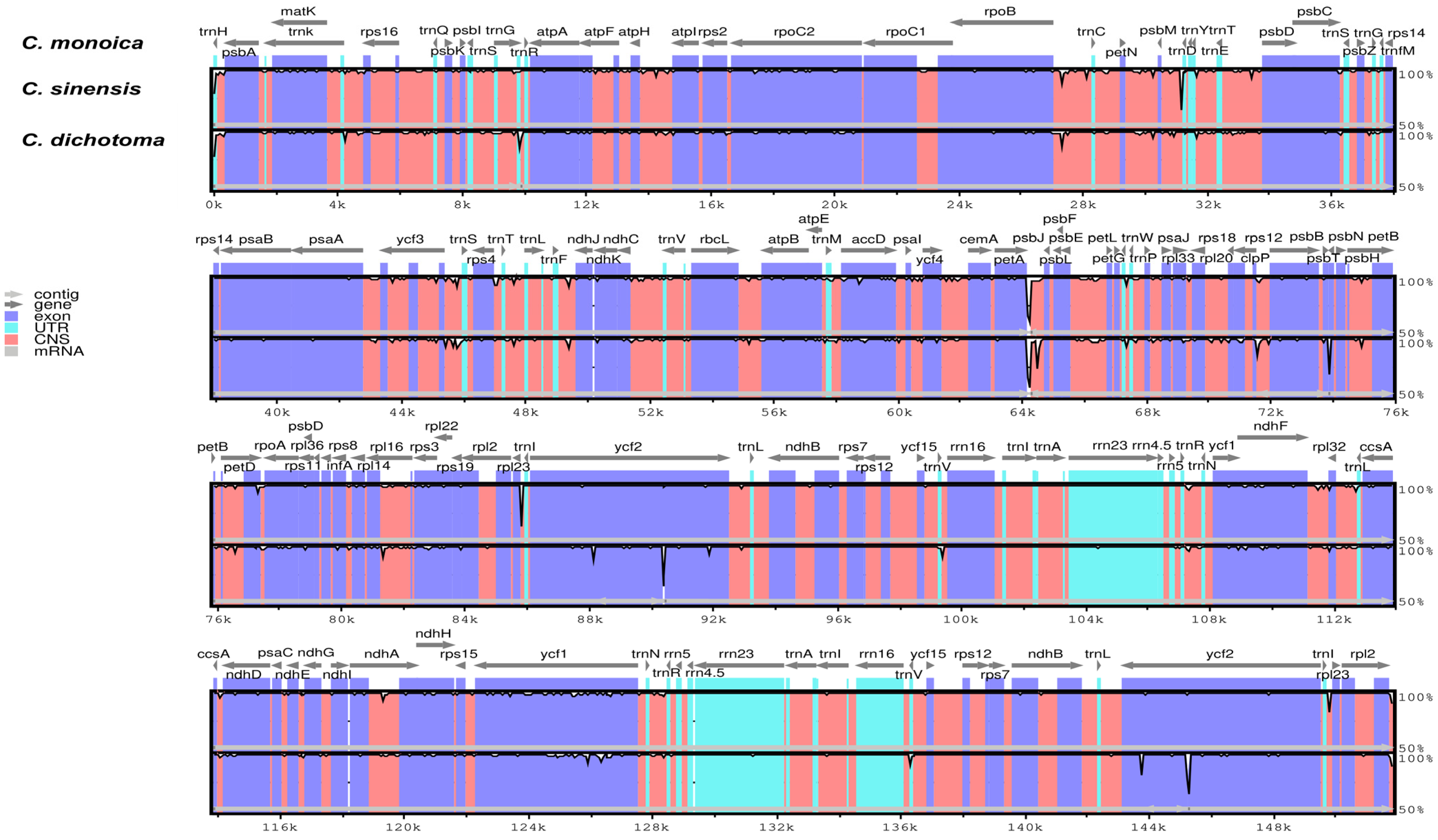
3.6. Comparative Analysis
3.7. Divergence of Protein-Coding Gene Sequence
3.8. Characterization of Substitution Rate
3.9. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luebert, F.; Cecchi, L.; Frohlich, M.W.; Gottschling, M.; Guilliams, C.M.; Hasenstab-Lehman, K.E.; Hilger, H.H.; Miller, J.S.; Mittelbach, M.; Nazaire, M.; et al. Familial Classification of the Boraginales. Taxon 2016, 65, 502–522. [Google Scholar] [CrossRef]
- Simpson, M.G. Diversity and Classification of Flowering Plants: Eudicots. In Plant Systematics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 285–466. [Google Scholar]
- John, H. The Families of Flowering Plants. I. Dicotyledons. Arranged According to a New System Based on Their Probable Phylogeny. J. Hutchinson. Bot. Gaz. 1926, 82, 111–112. [Google Scholar] [CrossRef]
- Dahlgren, R.M.T. A Revised System of Classification of the Angiosperms. Bot. J. Linn. Soc. 1980, 80, 91–124. [Google Scholar] [CrossRef]
- Thorne, R. An Updated Phylogenetic Classification of the Flowering Plants. Aliso 1992, 13, 265–389. [Google Scholar] [CrossRef]
- Takhtajan, A. Diversity and Classification of Flowering Plants; Columbia University Press: New York, NY, USA, 1997; ISBN 9780231100984. [Google Scholar]
- Moore, M.J.; Jansen, R.K. Molecular Evidence for the Age, Origin, and Evolutionary History of the American Desert Plant Genus Tiquilia (Boraginaceae). Mol. Phylogenetics Evol. 2006, 39, 668–687. [Google Scholar] [CrossRef]
- Nazaire, M.; Hufford, L. A Broad Phylogenetic Analysis of Boraginaceae: Implications for the Relationships of Mertensia. Syst. Bot. 2012, 37, 758–783. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Gottschling, M.; Hilger, H.H.; Wolf, M.; Diane, N. Secondary Structure of the ITS1 Transcript and Its Application in a Reconstruction of the Phylogeny of Boraginales. Plant Biol. 2001, 3, 629–636. [Google Scholar] [CrossRef]
- Cohen, J.I. A Phylogenetic Analysis of Morphological and Molecular Characters of Boraginaceae: Evolutionary Relationships, Taxonomy, and Patterns of Character Evolution. Cladistics 2013, 30, 139–169. [Google Scholar] [CrossRef]
- Refulio-Rodriguez, N.F.; Olmstead, R.G. Phylogeny of Lamiidae. Am. J. Bot. 2014, 101, 287–299. [Google Scholar] [CrossRef]
- Hasenstab-Lehman, K. Phylogenetics of the Borage Family: Delimiting Boraginales and Assessing Closest Relatives. Aliso 2017, 35, 41–49. [Google Scholar] [CrossRef]
- Gottschling, M.; Weigend, M.; Hilger, H. Congruence of a Phylogeny of Cordiaceae (Boraginales) Inferred from ITS1 Sequence Data with Morphology, Ecology, and Biogeography. Ann. Mo. Bot. Gard. 2005, 92, 425–437. [Google Scholar]
- Grevich, J.J.; Daniell, H. Chloroplast Genetic Engineering: Recent Advances and Future Perspectives. CRC Crit. Rev. Plant Sci. 2005, 24, 83–107. [Google Scholar] [CrossRef]
- Roston, R.L.; Jouhet, J.; Yu, F.; Gao, H. Editorial: Structure and Function of Chloroplasts. Front. Plant Sci. 2018, 9, 1656. [Google Scholar] [CrossRef]
- Shaw, J.; Lickey, E.B.; Schilling, E.E.; Small, R.L. Comparison of Whole Chloroplast Genome Sequences to Choose Noncoding Regions for Phylogenetic Studies in Angiosperms: The Tortoise and the Hare III. Am. J. Bot. 2007, 94, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Mower, J.P.; Vickrey, T.L. Chapter Nine—Structural Diversity Among Plastid Genomes of Land Plants. In Plastid Genome Evolution; Chaw, S.-M., Jansen, R.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 85, pp. 263–292. ISBN 0065-2296. [Google Scholar]
- Bendich, A.J. Circular Chloroplast Chromosomes: The Grand Illusion. Plant Cell 2004, 16, 1661–1666. [Google Scholar] [CrossRef]
- Liu, S.; Ni, Y.; Li, J.; Zhang, X.; Yang, H.; Chen, H.; Liu, C. CPGView: A Package for Visualizing Detailed Chloroplast Genome Structures. Mol. Ecol. Resour. 2023, 23, 694–704. [Google Scholar] [CrossRef]
- Yao, J.; Zhao, F.; Xu, Y.; Zhao, K.; Quan, H.; Su, Y.; Hao, P.; Liu, J.; Yu, B.; Yao, M.; et al. Complete Chloroplast Genome Sequencing and Phylogenetic Analysis of Two Dracocephalum Plants. Biomed. Res. Int. 2020, 2020, 4374801. [Google Scholar] [CrossRef]
- Wolfe, K.H.; Li, W.H.; Sharp, P.M. Rates of Nucleotide Substitution Vary Greatly among Plant Mitochondrial, Chloroplast, and Nuclear DNAs. Proc. Natl. Acad. Sci. USA 1987, 84, 9054–9058. [Google Scholar] [CrossRef]
- Bell, C.D.; Soltis, D.E.; Soltis, P.S. The Age and Diversification of the Angiosperms Re-Revisited. Am. J. Bot. 2010, 97, 1296–1303. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L.; Ballenger, J.; Palmer, J. The Distribution and Phylogenetic Significance of a 50-Kb Chloroplast DNA Inversion in the Flowering Plant Family Leguminosae. Mol. Phylogenet. Evol. 1996, 5, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Tangphatsornruang, S.; Uthaipaisanwong, P.; Sangsrakru, D.; Chanprasert, J.; Yoocha, T.; Jomchai, N.; Tragoonrung, S. Characterization of the Complete Chloroplast Genome of Hevea Brasiliensis Reveals Genome Rearrangement, RNA Editing Sites and Phylogenetic Relationships. Gene 2011, 475, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.F.; Jansen, R.K.; Zanis, M.J.; Emery, N.C. Sources of Inversion Variation in the Small Single Copy (SSC) Region of Chloroplast Genomes. Am. J. Bot. 2015, 102, 1751–1752. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-S.; Lee, B.Y.; Kwak, M. The Complete Chloroplast Genome Sequences of Lychnis Wilfordii and Silene Capitata and Comparative Analyses with Other Caryophyllaceae Genomes. PLoS ONE 2017, 12, e0172924. [Google Scholar] [CrossRef]
- Palmer, J.D.; Nugent, J.M.; Herbon, L.A. Unusual Structure of Geranium Chloroplast DNA: A Triple-Sized Inverted Repeat, Extensive Gene Duplications, Multiple Inversions, and Two Repeat Families. Proc. Natl. Acad. Sci. USA 1987, 84, 769–773. [Google Scholar] [CrossRef]
- Tangphatsornruang, S.; Sangsrakru, D.; Chanprasert, J.; Uthaipaisanwong, P.; Yoocha, T.; Jomchai, N.; Tragoonrung, S. The Chloroplast Genome Sequence of Mungbean (Vigna Radiata) Determined by High-Throughput Pyrosequencing: Structural Organization and Phylogenetic Relationships. DNA Res. 2010, 17, 11–22. [Google Scholar] [CrossRef]
- Ogihara, Y.; Terachi, T.; Sasakuma, T. Intramolecular Recombination of Chloroplast Genome Mediated by Short Direct-Repeat Sequences in Wheat Species. Proc. Natl. Acad. Sci. USA 1988, 85, 8573–8577. [Google Scholar] [CrossRef]
- Hiratsuka, J.; Shimada, H.; Whittier, R.; Ishibashi, T.; Sakamoto, M.; Mori, M.; Kondo, C.; Honji, Y.; Sun, C.-R.; Meng, B.-Y.; et al. The Complete Sequence of the Rice (Oryza Sativa) Chloroplast Genome: Intermolecular Recombination between Distinct TRNA Genes Accounts for a Major Plastid DNA Inversion during the Evolution of the Cereals. Mol. Gen. Genet. 1989, 217, 185–194. [Google Scholar] [CrossRef]
- Fullerton, S.M.; Bernardo Carvalho, A.; Clark, A.G. Local Rates of Recombination Are Positively Correlated with GC Content in the Human Genome. Mol. Biol. Evol. 2001, 18, 1139–1142. [Google Scholar] [CrossRef]
- Smith, N.G.C.; Webster, M.T.; Ellegren, H. Deterministic Mutation Rate Variation in the Human Genome. Genome Res. 2002, 12, 1350–1356. [Google Scholar] [CrossRef]
- Walker, J.F.; Zanis, M.J.; Emery, N.C. Comparative Analysis of Complete Chloroplast Genome Sequence and Inversion Variation in Lasthenia Burkei (Madieae, Asteraceae). Am. J. Bot. 2014, 101, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; He, J.; Ding, C.; Lyu, R.; Pei, L.; Cheng, J.; Xie, L. Comparative Analysis of Complete Chloroplast Genomes of Anemoclema, Anemone, Pulsatilla, and Hepatica Revealing Structural Variations Among Genera in Tribe Anemoneae (Ranunculaceae). Front. Plant Sci. 2018, 9, 1097. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chen, Y.Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce Acceleration-Supported Software for Integrated Quality Control and Preprocessing of High-Throughput Sequencing Data. Gigascience 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De Novo Assembly of Organelle Genomes from Whole Genome Data. Nucleic Acids Res. 2017, 45, e18. [Google Scholar] [CrossRef]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq—Versatile and Accurate Annotation of Organelle Genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) Version 1.3.1: Expanded Toolkit for the Graphical Visualization of Organellar Genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Lenz, H.; Knoop, V. PREPACT 2.0: Predicting C-to-U and U-to-C RNA Editing in Organelle Genome Sequences with Multiple References and Curated RNA Editing Annotation. Bioinform. Biol. Insights 2013, 7, 1–19. [Google Scholar] [CrossRef]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The Manifold Applications of Repeat Analysis on a Genomic Scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-Web: A Web Server for Microsatellite Prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A Software for Comprehensive Analysis of DNA Polymorphism Data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Mayor, C.; Brudno, M.; Schwartz, J.R.; Poliakov, A.; Rubin, E.M.; Frazer, K.A.; Pachter, L.S.; Dubchak, I. VISTA: Visualizing Global DNA Sequence Alignments of Arbitrary Length. Bioinformatics 2000, 16, 1046–1047. [Google Scholar] [CrossRef] [PubMed]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: An Online Program to Visualize the Junction Sites of Chloroplast Genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Posada, D. JModelTest: Phylogenetic Model Averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Luo, J.; Hou, B.-W.; Niu, Z.-T.; Liu, W.; Xue, Q.-Y.; Ding, X.-Y. Comparative Chloroplast Genomes of Photosynthetic Orchids: Insights into Evolution of the Orchidaceae and Development of Molecular Markers for Phylogenetic Applications. PLoS ONE 2014, 9, e99016. [Google Scholar] [CrossRef]
- Carvalho Leonardo, I.; Barreto Crespo, M.T.; Capelo, J.; Bustos Gaspar, F. The Complete Plastome of Echium Plantagineum L. (Boraginaceae), the First Chloroplast Genome Belonging to the Echium Genus. Mitochondrial DNA B Resour. 2022, 7, 1154–1156. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-H.; Li, H.-M.; Lei, J.-M.; Liang, Z.-R. The Complete Chloroplast Genome Sequence of Trigonotis Peduncularis (Boraginaceae). Mitochondrial DNA B Resour. 2022, 7, 456–457. [Google Scholar] [CrossRef]
- Li, Q.; Wei, R. Comparison of Boraginales Plastomes: Insights into Codon Usage Bias, Adaptive Evolution, and Phylogenetic Relationships. Diversity 2022, 14, 1104. [Google Scholar] [CrossRef]
- Liu, K.; Wang, R.; Guo, X.-X.; Zhang, X.-J.; Qu, X.-J.; Fan, S.-J. Comparative and Phylogenetic Analysis of Complete Chloroplast Genomes in Eragrostideae (Chloridoideae, Poaceae). Plants 2021, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Li, Y.; Wang, S.; Liu, Z.; Wang, J.; Yang, M. Complete Chloroplast Genomes and Comparative Analysis of Ligustrum Species. Sci. Rep. 2023, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Shaul, O. How Introns Enhance Gene Expression. Int. J. Biochem. Cell Biol. 2017, 91, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Giner-Delgado, C.; Villatoro, S.; Lerga-Jaso, J.; Gayà-Vidal, M.; Oliva, M.; Castellano, D.; Pantano, L.; Bitarello, B.D.; Izquierdo, D.; Noguera, I.; et al. Evolutionary and Functional Impact of Common Polymorphic Inversions in the Human Genome. Nat. Commun. 2019, 10, 4222. [Google Scholar] [CrossRef]
- Alshegaihi, R.M.; Mansour, H.; Alrobaish, S.A.; Al Shaye, N.A.; Abd El-Moneim, D. The First Complete Chloroplast Genome of Cordia Monoica: Structure and Comparative Analysis. Genes 2023, 14, 976. [Google Scholar] [CrossRef]
- Kim, Y.; Cullis, C. A Novel Inversion in the Chloroplast Genome of Marama (Tylosema Esculentum). J. Exp. Bot. 2017, 68, 2065–2072. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Liu, B.; Chen, J.; Zhao, Y.; Huang, Y. Comparative Analysis of 17 Complete Chloroplast Genomes Reveals Intraspecific Variation and Relationships among Pseudostellaria Heterophylla (Miq.) Pax Populations. Front. Plant Sci. 2023, 14, 1163325. [Google Scholar] [CrossRef]
- Lian, C.; Yang, H.; Lan, J.; Zhang, X.; Zhang, F.; Yang, J.; Chen, S. Comparative Analysis of Chloroplast Genomes Reveals Phylogenetic Relationships and Intraspecific Variation in the Medicinal Plant Isodon Rubescens. PLoS ONE 2022, 17, e0266546. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, Y.; Suyama, Y.; Yoshimura, K. Chloroplast DNA Inversion Polymorphism in Populations of Abies and Tsuga. Mol. Biol. Evol. 2000, 17, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Q.; Li, Y.; Qian, J.; Han, J. Chloroplast Genome of Aconitum Barbatum Var. Puberulum (Ranunculaceae) Derived from CCS Reads Using the PacBio RS Platform. Front. Plant Sci. 2015, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Emery, L.R.; Zeng, K. Forces That Influence the Evolution of Codon Bias. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Luo, C. Molecular and Functional Diversity of RNA Editing in Plant Mitochondria. Mol. Biotechnol. 2018, 60, 935–945. [Google Scholar] [CrossRef]
- Shikanai, T. RNA Editing in Plant Organelles: Machinery, Physiological Function and Evolution. Cell Mol. Life Sci. 2006, 63, 698–708. [Google Scholar] [CrossRef]
- Konhar, R.; Debnath, M.; Vishwakarma, S.; Bhattacharjee, A.; Sundar, D.; Tandon, P.; Dash, D.; Biswal, D. The Complete Chloroplast Genome of Dendrobium Nobile, an Endangered Medicinal Orchid from North-East India and Its Comparison with Related Dendrobium Species. PeerJ 2019, 7, e7756. [Google Scholar] [CrossRef]
- Guisinger, M.M.; Kuehl, J.V.; Boore, J.L.; Jansen, R.K. Extreme Reconfiguration of Plastid Genomes in the Angiosperm Family Geraniaceae: Rearrangements, Repeats, and Codon Usage. Mol. Biol. Evol. 2010, 28, 583–600. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Li, Y.; Jiang, M.; Liu, C.; He, M.; Wu, B. Chloroplast Genomes of Two Pueraria DC. Species: Sequencing, Comparative Analysis and Molecular Marker Development. FEBS Open Bio. 2022, 12, 349–361. [Google Scholar] [CrossRef]
- Tian, C.; Li, X.; Wu, Z.; Li, Z.; Hou, X.; Li, F.Y. Characterization and Comparative Analysis of Complete Chloroplast Genomes of Three Species From the Genus Astragalus (Leguminosae). Front. Genet. 2021, 12, 1163325. [Google Scholar] [CrossRef]
- Gan, J.; Li, Y.; Tang, D.; Guo, B.; Li, D.; Cao, F.; Sun, C.; Yu, L.; Yan, Z. The Complete Chloroplast Genomes of Gynostemma Reveal the Phylogenetic Relationships of Species within the Genus. Genes 2023, 14, 929. [Google Scholar] [CrossRef] [PubMed]
- Provan, J.; Powell, W.; Hollingsworth, P.M. Chloroplast Microsatellites: New Tools for Studies in Plant Ecology and Evolution. Trends Ecol. Evol. 2001, 16, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Addisalem, A.B.; Esselink, G.D.; Bongers, F.; Smulders, M.J.M. Genomic Sequencing and Microsatellite Marker Development for Boswellia Papyrifera, an Economically Important but Threatened Tree Native to Dry Tropical Forests. AoB Plants 2015, 7, plu086. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Peakall, R. Chloroplast Simple Sequence Repeats (CpSSRs): Technical Resources and Recommendations for Expanding CpSSR Discovery and Applications to a Wide Array of Plant Species. Mol. Ecol. Resour. 2009, 9, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, M.N.; Ehirim, B.O.; Nwanyanwu, G.C.; Abubaka, R.I. DNA Fingerprinting Simple Sequence Repeat (SSR) Marker-Basedof Some Varieties of Rice (Oryza Sativa L.) Released in Nigeria. Afr. J. Biotechnol. 2019, 18, 242–248. [Google Scholar] [CrossRef]
- Kuang, D.-Y.; Wu, H.; Wang, Y.-L.; Gao, L.-M.; Zhang, S.-Z.; Lu, L. Complete Chloroplast Genome Sequence of Magnolia Kwangsiensis (Magnoliaceae): Implication for DNA Barcoding and Population Genetics. Genome 2011, 54, 663–673. [Google Scholar] [CrossRef]
- Raubeson, L.A.; Peery, R.; Chumley, T.W.; Dziubek, C.; Fourcade, H.M.; Boore, J.L.; Jansen, R.K. Comparative Chloroplast Genomics: Analyses Including New Sequences from the Angiosperms Nuphar Advena and Ranunculus Macranthus. BMC Genom. 2007, 8, 174. [Google Scholar] [CrossRef]
- Wang, W.; Messing, J. High-Throughput Sequencing of Three Lemnoideae (Duckweeds) Chloroplast Genomes from Total DNA. PLoS ONE 2011, 6, e24670. [Google Scholar] [CrossRef]
- Huo, Y.; Gao, L.; Liu, B.; Yang, Y.; Kong, S.; Sun, Y.; Yang, Y.; Wu, X. Complete Chloroplast Genome Sequences of Four Allium Species: Comparative and Phylogenetic Analyses. Sci. Rep. 2019, 9, 12250. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Y.; Xu, J.; Li, W.; Li, M. Characterization of the Complete Chloroplast Genome Sequence of Dalbergia Species and Its Phylogenetic Implications. Sci. Rep. 2019, 9, 20401. [Google Scholar] [CrossRef]
- Huang, J.L.; Sun, G.L.; Zhang, D.M. Molecular Evolution and Phylogeny of the Angiosperm Ycf2 Gene. J. Syst. Evol. 2010, 48, 240–248. [Google Scholar] [CrossRef]
- Dong, W.; Xu, C.; Li, C.; Sun, J.; Zuo, Y.; Shi, S.; Cheng, T.; Guo, J.; Zhou, S. Ycf1, the Most Promising Plastid DNA Barcode of Land Plants. Sci. Rep. 2015, 5, 8348. [Google Scholar] [CrossRef] [PubMed]
- Chacón, J.; Luebert, F.; Hilger, H.H.; Ovchinnikova, S.; Selvi, F.; Cecchi, L.; Guilliams, C.M.; Hasenstab-Lehman, K.; Sutorý, K.; Simpson, M.G.; et al. The Borage Family (Boraginaceae s.Str.): A Revised Infrafamilial Classification Based on New Phylogenetic Evidence, with Emphasis on the Placement of Some Enigmatic Genera. Taxon 2016, 65, 523–546. [Google Scholar] [CrossRef]
- Gottschling, M.; Luebert, F.; Hilger, H.H.; Miller, J.S. Molecular Delimitations in the Ehretiaceae (Boraginales). Mol. Phylogenetics Evol. 2014, 72, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Li, S.; Wang, Y.; Wang, X. Comparative Genome/Transcriptome Analysis Probes Boraginales’ Phylogenetic Position, WGDs in Boraginales, and Key Enzyme Genes in the Alkannin/Shikonin Core Pathway. Mol. Ecol. Resour. 2019, 20, 228–241. [Google Scholar] [CrossRef]





| Species | C. monoica | C. sinensis |
|---|---|---|
| Cp genome size (bp) | 151,813 | 152,050 |
| IR (bp) | 25,077 | 25,043 |
| LSC (bp) | 83,812 | 84,124 |
| SSC (bp) | 17,847 | 17,840 |
| Total number of genes | 134 | 134 |
| rRNA | 4 | 4 |
| tRNA | 30 | 30 |
| Protein-coding genes | 80 | 80 |
| T (U) % | 31.17 | 31.15 |
| C % | 19.42 | 19.42 |
| A % | 30.65 | 30.66 |
| G % | 18.74 | 18.75 |
| Overall GC content % | 38,16 | 38,17 |
| GC in LSC % | 36.23 | 36.23 |
| GC in SSC % | 32.49 | 32.49 |
| GC in IR % | 43.41 | 43.48 |
| SSR Type | Repeat Unit | Species | |
|---|---|---|---|
| C. monoica | C. sinensis | ||
| Mono | A/T | 131 | 134 |
| C/G | 10 | 8 | |
| Di | AT/AT | 2 | 2 |
| Tetra | AAAC/GTTT | 2 | 2 |
| AAAG/CTTT | 2 | 2 | |
| AAAT/ATTT | 5 | 5 | |
| AATC/ATTG | 1 | 0 | |
| AATT/AATT | 1 | 1 | |
| AGGC/CCTG | 0 | 1 | |
| Penta | AAAAT/ATTTT | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alawfi, M.S.; Albokhari, E.J. Comparative Chloroplast Genomics Reveals a Unique Gene Inversion in Two Cordia Trees (Cordiaceae). Forests 2023, 14, 1778. https://doi.org/10.3390/f14091778
Alawfi MS, Albokhari EJ. Comparative Chloroplast Genomics Reveals a Unique Gene Inversion in Two Cordia Trees (Cordiaceae). Forests. 2023; 14(9):1778. https://doi.org/10.3390/f14091778
Chicago/Turabian StyleAlawfi, Mohammad S., and Enas J. Albokhari. 2023. "Comparative Chloroplast Genomics Reveals a Unique Gene Inversion in Two Cordia Trees (Cordiaceae)" Forests 14, no. 9: 1778. https://doi.org/10.3390/f14091778





