Long-Term Patterns in Forest Soil CO2 Flux in a Pacific Northwest Temperate Rainforest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Weather Data
2.3. Net Soil CO2 Efflux Measurements
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirschbaum, M.U.F. The Temperature Dependence of Soil Organic Matter Decomposition, and the Effect of Global Warming on Soil Organic C Storage. Soil Biol. Biochem. 1995, 27, 753–760. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.-D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-Growth Forests as Global Carbon Sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloway, J.; Heimann, M.; et al. The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC Climate: Geneva, Switzerland, 2013. [Google Scholar]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse Gas Emissions from Soils—A Review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexier, M.C.; Wisniewski, J. Carbon Pools and Flux of Global Forest Ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Litton, C.M.; Raich, J.W.; Ryan, M.G. Carbon Allocation in Forest Ecosystems. Glob. Chang. Biol. 2007, 13, 2089–2109. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The Global Carbon Dioxide Flux in Soil Respiration and Its Relationship to Vegetation and Climate. Tellus B 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Raich, J.W.; Potter, C.S. Global Patterns of Carbon Dioxide Emissions from Soils. Glob. Biogeochem. Cycles 1995, 9, 23–36. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The Vertical Distribution of Soil Organic Carbon and Its Relation to Climate and Vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Raich, J.W. Temporal Variability of Soil Respiration in Experimental Tree Plantations in Lowland Costa Rica. Forests 2017, 8, 40. [Google Scholar] [CrossRef]
- Phillips, C.L.; Bond-Lamberty, B.; Desai, A.R.; Lavoie, M.; Risk, D.; Tang, J.; Todd-Brown, K.; Vargas, R. The Value of Soil Respiration Measurements for Interpreting and Modeling Terrestrial Carbon Cycling. Plant Soil 2017, 413, 1–25. [Google Scholar] [CrossRef]
- Kern, J.S. Spatial Patterns of Soil Organic Carbon in the Contiguous United States. Soil Sci. Soc. Am. J. 1994, 58, 439–455. [Google Scholar] [CrossRef]
- Turner, D.P.; Koerper, G.J.; Harmon, M.E.; Lee, J.J. A Carbon Budget for Forests of the Conterminous United States. Ecol. Appl. 1995, 5, 421–436. [Google Scholar] [CrossRef]
- Singh, J.S.; Gupta, S.R. Plant Decomposition and Soil Respiration in Terrestrial Ecosystems. Bot. Rev. 1977, 43, 449–528. [Google Scholar] [CrossRef]
- Schlentner, R.E.; Cleve, K.V. Relationships between CO2 Evolution from Soil, Substrate Temperature, and Substrate Moisture in Four Mature Forest Types in Interior Alaska. Can. J. For. Res. 1985, 15, 97–106. [Google Scholar] [CrossRef]
- Carlyle, J.C.; Than, U.B. Abiotic Controls of Soil Respiration Beneath an Eighteen-Year-Old Pinus Radiata Stand in South-Eastern Australia. J. Ecol. 1988, 76, 654–662. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-Associated Increases in the Global Soil Respiration Record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef]
- Raich, J.W.; Lambers, H.; Oliver, D.J. 10.16—Respiration in Terrestrial Ecosystems. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 613–649. ISBN 978-0-08-098300-4. [Google Scholar]
- Cisneros-Dozal, L.M.; Trumbore, S.; Hanson, P.J. Partitioning Sources of Soil-Respired CO2 and Their Seasonal Variation Using a Unique Radiocarbon Tracer. Glob. Chang. Biol. 2005, 12, 194–204. [Google Scholar] [CrossRef]
- Campbell, J.L.; Sun, O.J.; Law, B.E. Supply-Side Controls on Soil Respiration among Oregon Forests. Glob. Chang. Biol. 2004, 10, 1857–1869. [Google Scholar] [CrossRef]
- Campbell, J.L.; Law, B.E. Forest Soil Respiration across Three Climatically Distinct Chronosequences in Oregon. Biogeochemistry 2005, 73, 109–125. [Google Scholar] [CrossRef]
- Taylor, A.J.; Lai, C.-T.; Hopkins, F.M.; Wharton, S.; Bible, K.; Xu, X.; Phillips, C.; Bush, S.; Ehleringer, J.R. Radiocarbon-Based Partitioning of Soil Respiration in an Old-Growth Coniferous Forest. Ecosystems 2015, 18, 459–470. [Google Scholar] [CrossRef]
- Waring, R.H.; Franklin, J.F. Evergreen Coniferous Forests of the Pacific Northwest. Science 1979, 204, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Lundegårdh, H. Carbon Dioxide Evolution of Soil and Crop Growth. Soil Sci. 1927, 23, 417. [Google Scholar] [CrossRef]
- Lieth, H.; Ouellette, R. Studies on the Vegetation of the Gaspé Peninsula: Ii. the Soil Respiration of Some Plant Communities. Can. J. Bot. 1962, 40, 127–140. [Google Scholar] [CrossRef]
- Ellis, R.C. The Seasonal Pattern of Nitrogen and Carbon Mineralization in Forest and Pasture Soils in Southern Ontario. Can. J. Soil. Sci. 1974, 54, 15–28. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Carbon Balance in Terrestrial Detritus. Annu. Rev. Ecol. Syst. 1977, 8, 51–81. [Google Scholar] [CrossRef]
- Fischer, D.G.; Hart, S.C.; LeRoy, C.J.; Whitham, T.G. Variation in Below-Ground Carbon Fluxes along a Populus Hybridization Gradient. New Phytol. 2007, 176, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Lojewski, N.R.; Fischer, D.G.; Bailey, J.K.; Schweitzer, J.A.; Whitham, T.G.; Hart, S.C. Genetic Basis of Aboveground Productivity in Two Native Populus Species and Their Hybrids. Tree Physiol. 2009, 29, 1133–1142. [Google Scholar] [CrossRef]
- Kirsch, J.L.; Fischer, D.G.; Kazakova, A.N.; Biswas, A.; Kelm, R.E.; Carlson, D.W.; LeRoy, C.J. Diversity-Carbon Flux Relationships in a Northwest Forest. Diversity 2012, 4, 33–58. [Google Scholar] [CrossRef]
- LeRoy, C.J.; Fischer, D.G. Do Genetically-Specific Tree Canopy Environments Feed Back to Affect Genetically Specific Leaf Decomposition Rates? Plant Soil 2019, 437, 1–10. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Fisher, R.A.; Wardle, D.A. Plant Communities as Drivers of Soil Respiration: Pathways, Mechanisms, and Significance for Global Change. Biogeosciences 2011, 8, 2047–2061. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.H. Plant Diversity Loss Reduces Soil Respiration across Terrestrial Ecosystems. Glob. Chang. Biol. 2019, 25, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Liu, S.; Wang, J.; Chang, S.X.; Liu, X.; Lu, H.; Wang, Y. Tree Species Diversity Promotes Soil Carbon Stability by Depressing the Temperature Sensitivity of Soil Respiration in Temperate Forests. Sci. Total Environ. 2018, 645, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Rex, I.; Fischer, D.G.; Bartlett, R. A Decade of Understory Community Dynamics and Stability in a Mature Second-Growth Forest in Western Washington. Northwest Sci. 2023, 96, 164–183. [Google Scholar] [CrossRef]
- Cueva, A.; Bullock, S.H.; López-Reyes, E.; Vargas, R. Potential Bias of Daily Soil CO2 Efflux Estimates Due to Sampling Time. Sci. Rep. 2017, 7, 11925. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Van’t Hoff, J.H. Lectures on Theoretical and Physical Chemistry. In Part I. Chemical Dynamics; Lehfeld, R.A., Translator; Edward Arnold: London, UK, 1898. [Google Scholar]
- Orchard, V.A.; Cook, F.J. Relationship between Soil Respiration and Soil Moisture. Soil Biol. Biochem. 1983, 15, 447–453. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The Temperature Dependence of Organic-Matter Decomposition—Still a Topic of Debate. Soil Biol. Biochem. 2006, 38, 2510–2518. [Google Scholar] [CrossRef]
- Giardina, C.P.; Ryan, M.G. Evidence That Decomposition Rates of Organic Carbon in Mineral Soil Do Not Vary with Temperature. Nature 2000, 404, 858–861. [Google Scholar] [CrossRef]
- Drewitt, G.B.; Black, T.A.; Nesic, Z.; Humphreys, E.R.; Jork, E.M.; Swanson, R.; Ethier, G.J.; Griffis, T.; Morgenstern, K. Measuring Forest Floor CO2 Fluxes in a Douglas-Fir Forest. Agric. For. Meteorol. 2002, 110, 299–317. [Google Scholar] [CrossRef]
- Vogt, K.A.; Edmonds, R.L.; Antos, G.C.; Vogt, D.J. Relationships between CO2 Evolution, ATP Concentrations and Decomposition in Four Forest Ecosystems in Western Washington. Oikos 1980, 35, 72–79. [Google Scholar] [CrossRef]
- Kane, E.S.; Pregitzer, K.S.; Burton, A.J. Soil Respiration along Environmental Gradients in Olympic National Park. Ecosystems 2003, 6, 326–335. [Google Scholar] [CrossRef]
- Vogt, K.A.; Grier, C.C.; Meier, C.E.; Edmonds, R.L. Mycorrhizal Role in Net Primary Priduction and Nutrient Cytcling in Abies Amabilis Ecosystems in Western Washington. Ecology 1982, 63, 370–380. [Google Scholar] [CrossRef]
- Acosta, M.; Darenova, E.; Krupková, L.; Pavelka, M. Seasonal and Inter-Annual Variability of Soil CO2 Efflux in a Norway Spruce Forest over an Eight-Year Study. Agric. For. Meteorol. 2018, 256–257, 93–103. [Google Scholar] [CrossRef]
- Ryan, M.G.; Law, B.E. Interpreting, Measuring, and Modeling Soil Respiration. Biogeochemistry 2005, 73, 3–27. [Google Scholar] [CrossRef]
- Huang, N.; Gu, L.; Black, T.A.; Wang, L.; Niu, Z. Remote Sensing-Based Estimation of Annual Soil Respiration at Two Contrasting Forest Sites. J. Geophys. Res. Biogeosci. 2015, 120, 2306–2325. [Google Scholar] [CrossRef]
- Crabbe, R.A.; Janouš, D.; Dařenová, E.; Pavelka, M. Exploring the Potential of LANDSAT-8 for Estimation of Forest Soil CO2 Efflux. Int. J. Appl. Earth Obs. Geoinf. 2019, 77, 42–52. [Google Scholar] [CrossRef]
- Johnston, A.S.A.; Sibly, R.M. The Influence of Soil Communities on the Temperature Sensitivity of Soil Respiration. Nat. Ecol. Evol. 2018, 2, 1597–1602. [Google Scholar] [CrossRef]
- Marlier, M.E.; Xiao, M.; Engel, R.; Livneh, B.; Abatzoglou, J.T.; Lettenmaier, D.P. The 2015 Drought in Washington State: A Harbinger of Things to Come? Environ. Res. Lett. 2017, 12, 114008. [Google Scholar] [CrossRef]



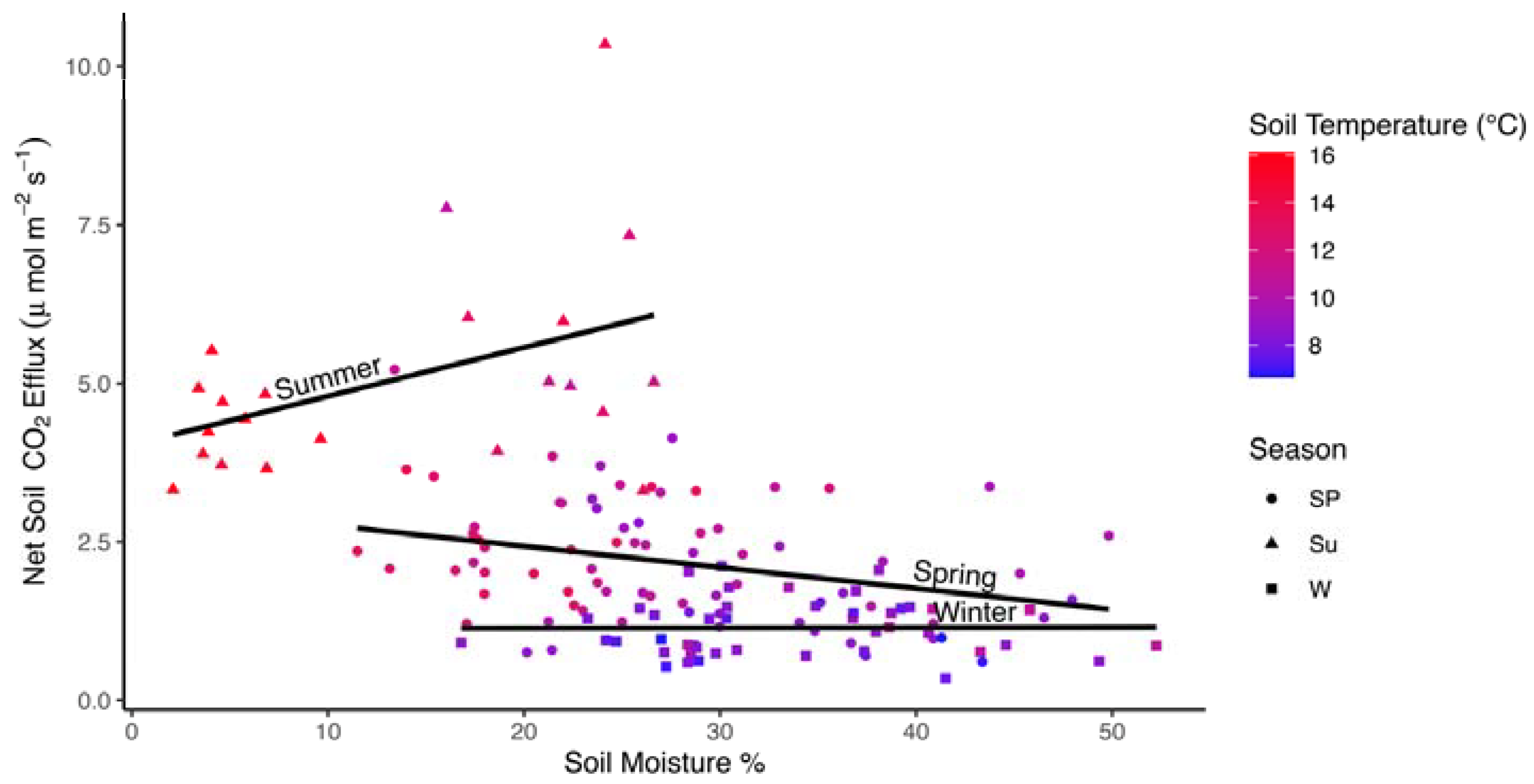
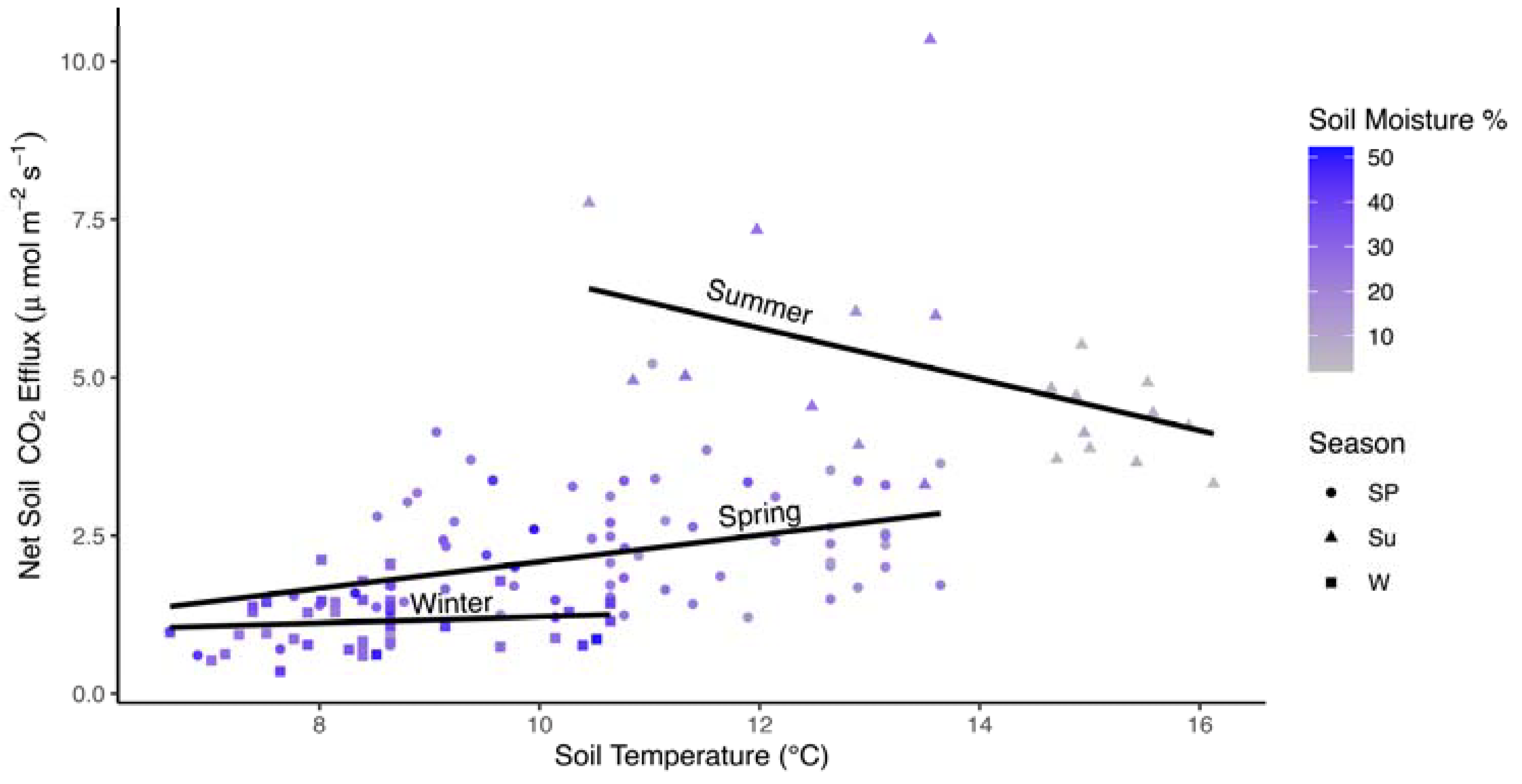
| Factor | SS | MS | dfnum/dfden | F | p |
|---|---|---|---|---|---|
| Season | 9.40 | 3.14 | 3/36.08 | 4.47 | 0.009 |
| Stand Type | 1.75 | 0.87 | 2/22.88 | 1.25 | 0.306 |
| Precipitation (10-day mm) | 0.62 | 0.620 | 1/63.28 | 0.88 | 0.351 |
| Temperature (°C) | 5.92 | 5.92 | 1/62.19 | 8.44 | 0.005 |
| Season: Stand Type | 8.89 | 1.48 | 6/355.86 | 2.11 | 0.051 |
| Precipitation: Temperature | 0.48 | 0.48 | 1/69.12 | 0.68 | 0.412 |
| Season: Precipitation | 17.00 | 5.67 | 3/102.31 | 8.08 | <0.001 |
| Season: Temperature | 10.17 | 3.39 | 3/71.65 | 4.83 | 0.004 |
| Season: Precipitation: Temperature | 19.11 | 6.37 | 3/105.84 | 9.08 | <0.001 |
| Season | Canopy Type | n | Average | SE | CV | ±95% CI |
|---|---|---|---|---|---|---|
| Spring | Conifer | 74 | 2.16 | 0.12 | 49.61 | 0.2 |
| Mixed Conifer and Hardwood | 37 | 2.28 | 0.21 | 56.15 | 0.4 | |
| Hardwood | 49 | 2.17 | 0.13 | 41.45 | 0.3 | |
| Summer | Conifer | 49 | 3.57 | 0.21 | 40.77 | 0.4 |
| Mixed Conifer and Hardwood | 18 | 4.76 | 0.49 | 43.41 | 1.0 | |
| Hardwood | 29 | 3.99 | 0.30 | 39.94 | 0.6 | |
| Autumn | Conifer | 35 | 2.49 | 0.16 | 38.83 | 0.3 |
| Mixed Conifer and Hardwood | 20 | 3.07 | 0.21 | 30.85 | 0.4 | |
| Hardwood | 27 | 2.77 | 0.15 | 28.29 | 0.3 | |
| Winter | Conifer | 29 | 1.13 | 0.13 | 61.52 | 0.3 |
| Mixed Conifer and Hardwood | 17 | 1.23 | 0.15 | 51.69 | 0.3 | |
| Hardwood | 24 | 1.40 | 0.12 | 40.50 | 0.2 |
| Factor | SS | MS | dfnum/dfden | F | p |
|---|---|---|---|---|---|
| Soil Moisture (10 cm depth) | 2.20 | 2.20 | 1/113.95 | 4.13 | 0.044 |
| Soil Temperature (°C) | 0.70 | 0.70 | 1/113.89 | 1.32 | 0.254 |
| Season | 5.33 | 2.67 | 2/115.9 | 5.00 | 0.008 |
| Soil Moisture: Soil Temperature | 2.04 | 2.04 | 1/114.06 | 3.82 | 0.053 |
| Season: Soil Moisture | 2.02 | 1.01 | 2/117.02 | 1.89 | 0.155 |
| Season: Temperature | 4.34 | 2.17 | 2/116.47 | 4.07 | 0.020 |
| Season: Precipitation: Temperature | 2.08 | 1.04 | 2/117.61 | 1.95 | 0.147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, D.G.; Chamberlain, Z.R.; Cook, C.E.; Martin, R.A.; Mueller, L.O. Long-Term Patterns in Forest Soil CO2 Flux in a Pacific Northwest Temperate Rainforest. Forests 2024, 15, 161. https://doi.org/10.3390/f15010161
Fischer DG, Chamberlain ZR, Cook CE, Martin RA, Mueller LO. Long-Term Patterns in Forest Soil CO2 Flux in a Pacific Northwest Temperate Rainforest. Forests. 2024; 15(1):161. https://doi.org/10.3390/f15010161
Chicago/Turabian StyleFischer, Dylan G., Zoe R. Chamberlain, Claire E. Cook, Randall Adam Martin, and Liam O. Mueller. 2024. "Long-Term Patterns in Forest Soil CO2 Flux in a Pacific Northwest Temperate Rainforest" Forests 15, no. 1: 161. https://doi.org/10.3390/f15010161





