Maximum Entropy Model Prediction of the Distributions of Two Sympatric Bean Weevil Species, Megabruchidius dorsalis (Fahraeus, 1839) and Bruchidius coreanus (Chûjô, 1937), under Various Climate Scenarios in Guizhou Province, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Occurrence Data Collection and Processing
2.2. Environmental Variable Selection and Processing
2.3. MaxEnt Model Construction
2.4. Classification of Suitable Areas
3. Results
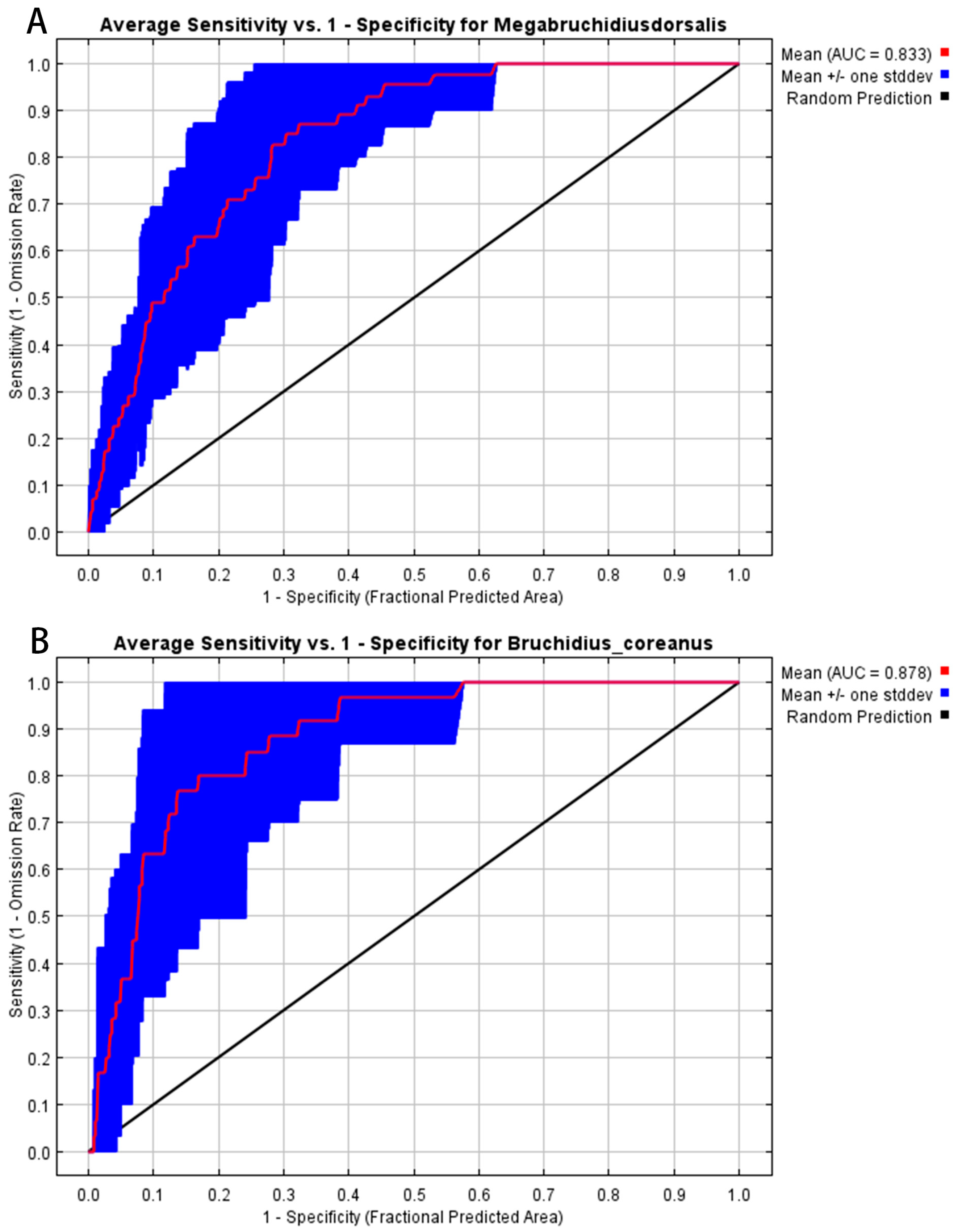
3.1. Model Accuracy Evaluation
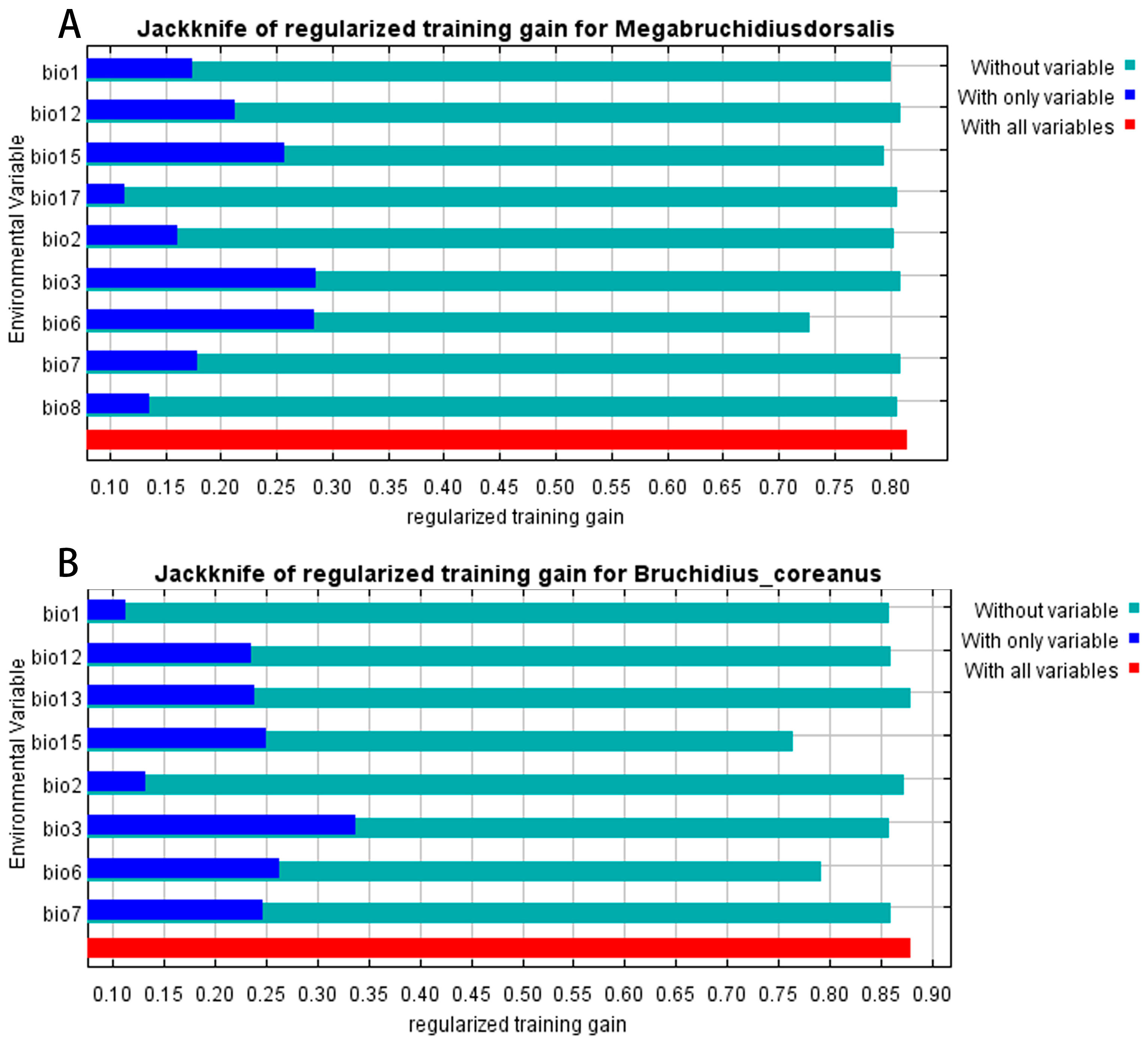
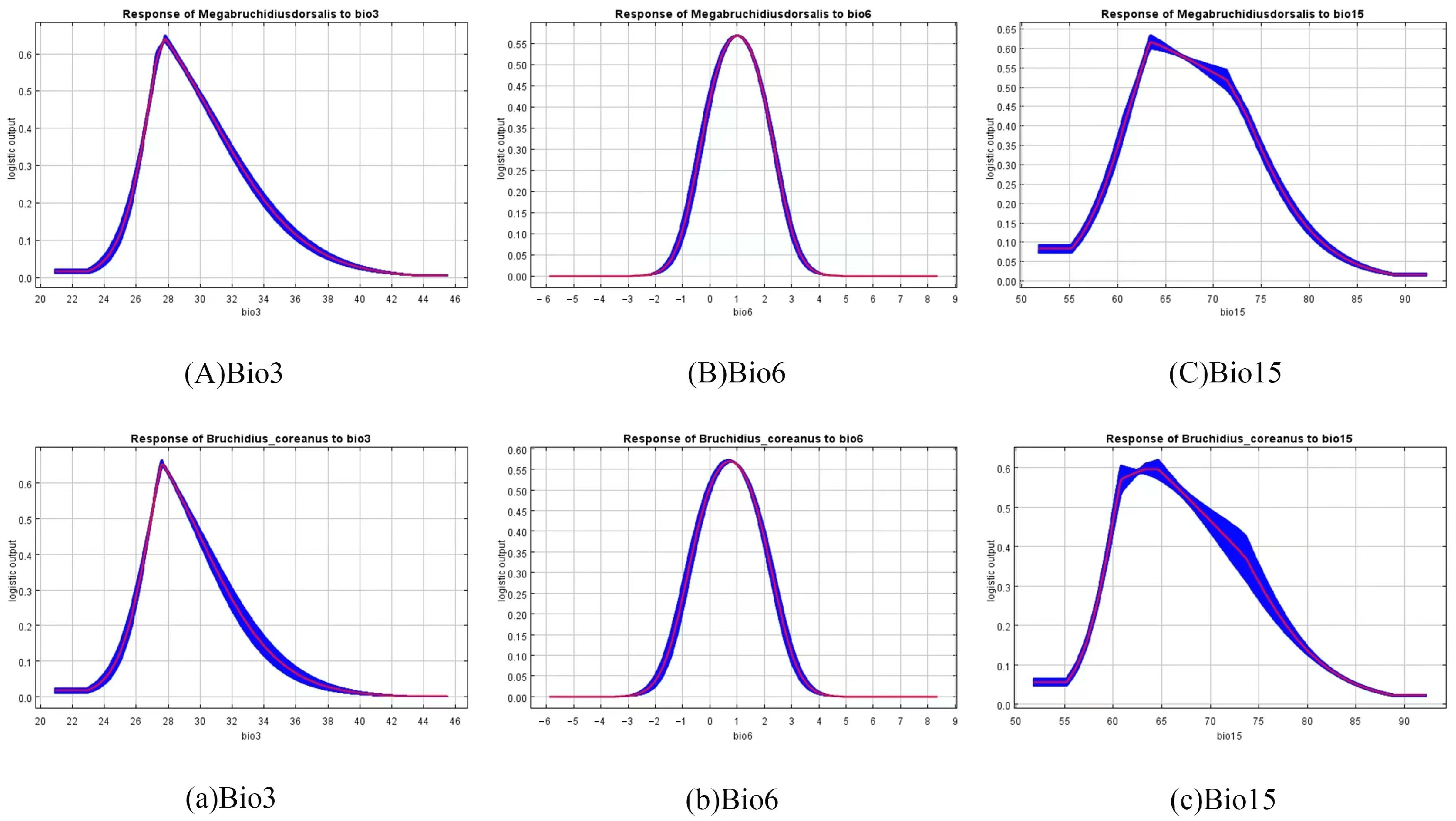
3.2. Analysis of the Contributions of Environmental Variables
3.3. Current and Potential Future Distributions of M. dorsalis and B. coreanus in Guizhou
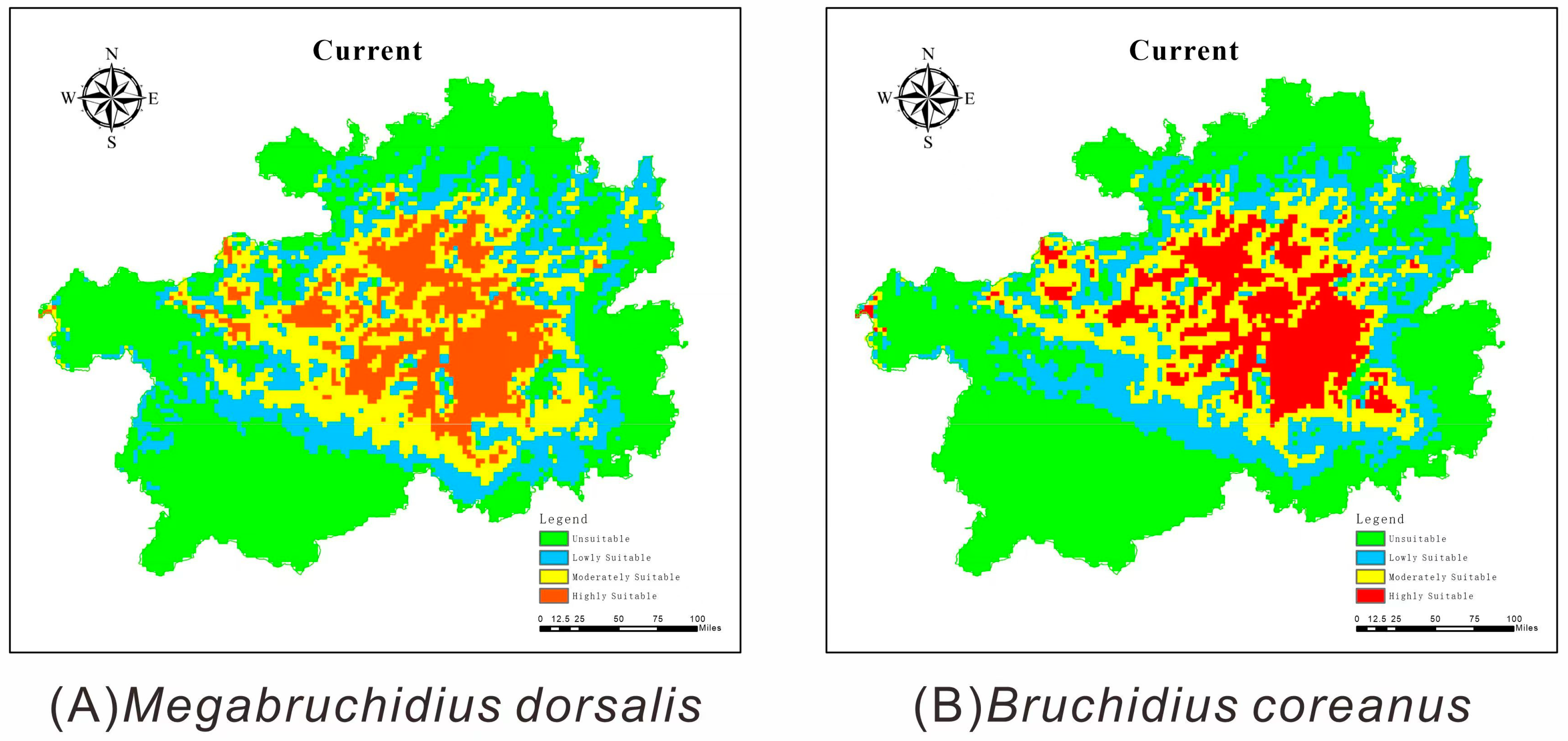
3.3.1. Predicted Current Distributions
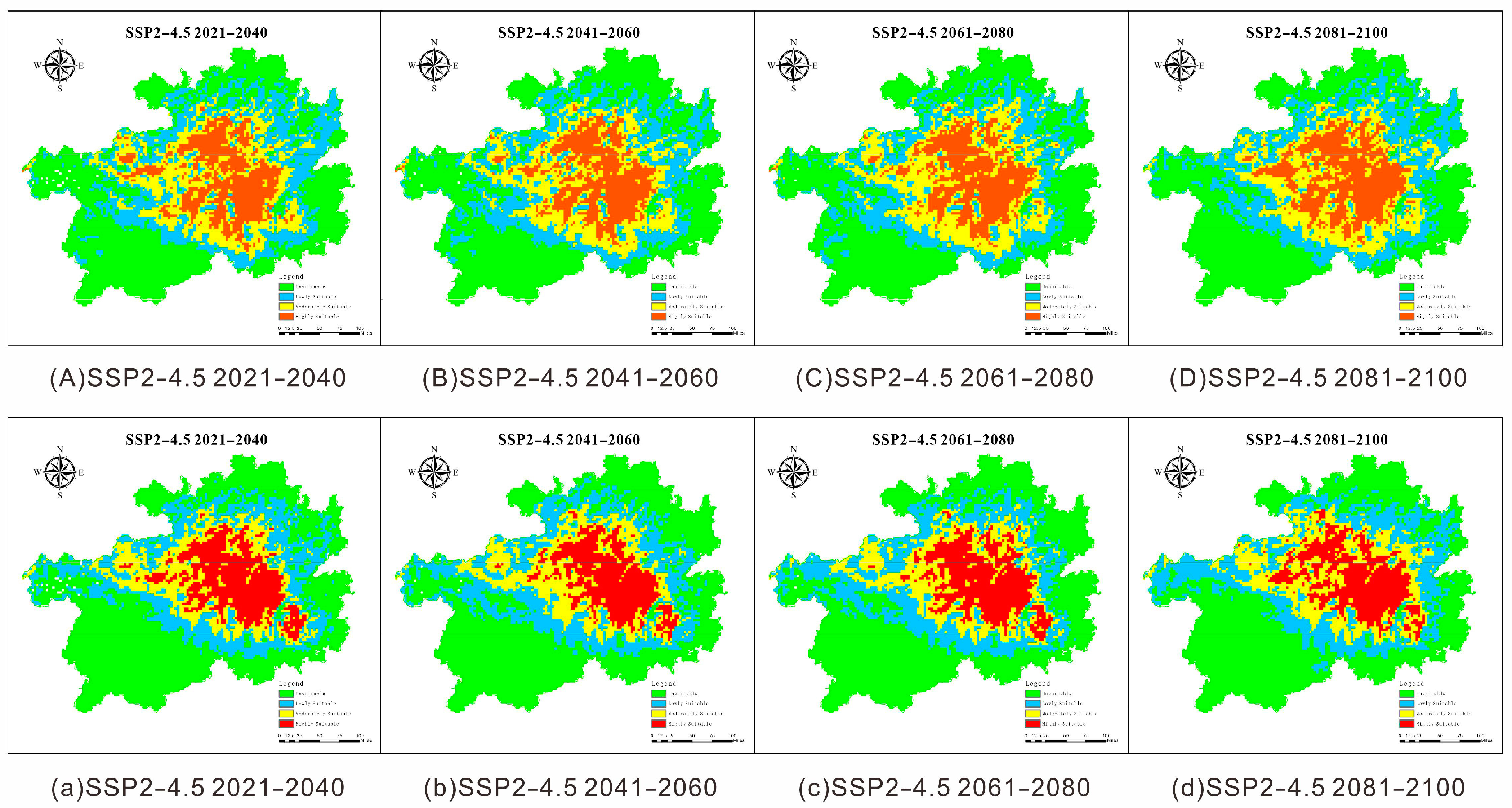
3.3.2. Potential Future Distribution of M. dorsalis and B. coreanus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Species | Location | Reference | Location (China) | Reference |
|---|---|---|---|---|
| Megabruchidius dorsalis | Japan | Li et al. (2014) [24] | Taiwan, China | György and Tuda (2020) [30] |
| Megabruchidius dorsalis | Europe | György and Tuda (2020) [30] | Fujian, China | ŘÍha and Bezděk (2015) [31] |
| Megabruchidius dorsalis | China | Li et al. (2014) [24] | Hongkong, China | ŘÍha and Bezděk (2015) [31] |
| Megabruchidius dorsalis | Mongolia | ŘÍha and Bezděk (2015) [31] | Xinjiang, China | Li et al. (2014) [24] |
| Megabruchidius dorsalis | Turkmenistan | ŘÍha and Bezděk (2015) [31] | Dongling, Shenyang, Liaoning provinces, China | Wang (1984) [32] |
| Megabruchidius dorsalis | Hungary | Ramos (2009) [23]; György and Tuda (2020) [30] | Beiling, Shenyang, China | Wang (1984) [32] |
| Megabruchidius dorsalis | Switzerland | Ramos (2009) [23] | Yiwulushan Nature Reserve, Beizhen County, Liaoning Province, China | Wang (1984) [32] |
| Megabruchidius dorsalis | Papua New Guinea | Li et al. (2014) [24] | Xiong Yue Botanical Garden, Gai County, Liaoning Province, China | Wang (1984) [32] |
| Megabruchidius dorsalis | Italy | Li et al. (2014) [24] | Zhengzhou, Henan Province, China | Yang and Zhou (1974) [33] |
| Megabruchidius dorsalis | Greece | Yus Ramos et al. (2014) [34] | Luoyang, Henan Province, China | Yang and Zhou (1974) [33] |
| Megabruchidius dorsalis | Argentina | György and Tuda (2020) [30] | Kaifeng, Henan Province, China | Yang and Zhou (1974) [33] |
| Megabruchidius dorsalis | France | György and Tuda (2020) [30] | Anyang, Henan Province, China | Yang and Zhou (1974) [33] |
| Megabruchidius dorsalis | Russia | György and Tuda (2020) [30] | Hebei Province, China | Li et al. (2014) [24] |
| Megabruchidius dorsalis | Ukraine | György and Tuda (2020) [30] | Qinghai Province, China | Li et al. (2014) [24] |
| Megabruchidius dorsalis | Slovakia | ŘÍha and Bezděk (2015) [31] | Gansu Province, China | Li et al. (2014) [24] |
| Megabruchidius dorsalis | Crimea | Korotyaev (2016) [35] | Fencheng town, Xiangfen County, Shanxi Province, China | Xin (2016) [36] |
| Megabruchidius dorsalis | Germany | Korotyaev (2016) [35] | South Campus of Guizhou University, Huaxi District, Guiyang City, Guizhou Province, China | Li et al. (2014) [24] |
| Megabruchidius dorsalis | Croatia | György and Tuda (2020) [30] | Suiyang County, Zunyi, China | This study |
| Megabruchidius dorsalis | Romania | György and Tuda (2020) [30] | Huichuan District, Zunyi, China | This study |
| Megabruchidius dorsalis | Austria | Sajna (2019) [37] | Bozhou District, Zunyi,China | This study |
| Megabruchidius dorsalis | Kazakhstan | Temreshev and Makezhanov (2019) [38] | Honghuagang District, Zunyi, China | This study |
| Megabruchidius dorsalis | South Kazakhstan | Temreshev and Makezhanov (2019) [38] | Sinan County, Tongren, China | This study |
| Megabruchidius dorsalis | Turkey | Temreshev and Makezhanov (2019) [38] | Jiangkou County, Tongren City, Guizhou Province, China | This study |
| Megabruchidius dorsalis | S. Korea | Cho and An (2020) [39] | Weng ‘an County, Qiannan Prefecture, Guizhou Province, China | This study |
| Megabruchidius dorsalis | Republic of Moldova | Pintilioaie et al. (2018) [40] | Fuquan City, Qiannan Prefecture, Guizhou Province, China | This study |
| Megabruchidius dorsalis | S.W. Poland | Ruta et al. (2017) [41] | Dushan County, Qiannan Prefecture, Guizhou Province, China | This study |
| Megabruchidius dorsalis | Slovenia | Sajna (2019) [37] | Majiang County, Qiandongnan Prefecture, Guizhou Province, China | This study |
| Megabruchidius dorsalis | Caucasus | Korotyaev (2016) [35] | Kaili City, Qiandongnan State, Guizhou Province, China | This study |
| Megabruchidius dorsalis | Indian | Li et al. (2014) [24] | Liupanshui special administrative region of Guizhou Province, China | This study |
| Megabruchidius dorsalis | Bangladesh | György and Tuda (2020) [30] | Qingzhen County, Guiyang City, Guizhou Province, China | This study |
| Megabruchidius dorsalis | Bulgaria | Li et al. (2014) [24] | Nanming District, Guiyang, China | This study |
| Megabruchidius dorsalis | Turkmenistan | Li et al. (2014) [24] | Dafang County, Bijie City, Guizhou Province, China | This study |
| Megabruchidius dorsalis | Indonesia | Li et al. (2014) [24] | Pingba District, Anshun, Guizhou Province, China | This study |
| Bruchidius coreanus | Kyoto, Japan | Morimoto (1990) [42] | Suiyang County, Zunyi, China | This study |
| Bruchidius coreanus | Kumamoto, Japan | Morimoto (1990) [42] | Huichuan District, Zunyi, China | This study |
| Bruchidius coreanus | Guizhou province, China | Peng et al. (2024) [21] | Bozhou District, Zunyi, China | This study |
| Bruchidius coreanus | Korea | Peng et al. (2024) [21]; Cho and An (2020) [39] | Honghuagang District, Zunyi, China | This study |
| Bruchidius coreanus | Sinan County, Tongren, China | This study | ||
| Bruchidius coreanus | Weng‘an County, Qiannan Prefecture, Guizhou Province, China | This study | ||
| Bruchidius coreanus | Fuquan City, Qiannan Prefecture, Guizhou Province, China | This study | ||
| Bruchidius coreanus | Majiang County, Qiandongnan Prefecture, Guizhou Province, China | This study | ||
| Bruchidius coreanus | Kaili City, Qiandongnan State, Guizhou Province, China | This study | ||
| Bruchidius coreanus | Dafang County, Bijie City, Guizhou Province, China | This study | ||
| Bruchidius coreanus | Pingba District, Anshun, Guizhou Province, China | This study | ||
| Bruchidius coreanus | Huaxi District, Guiyang City, Guizhou Province, China | This study |
References
- IPCC. Climate Change 2021—The Physical Science Basis. Chem. Int. 2021, 43, 22–23. [Google Scholar] [CrossRef]
- Ye, X.J. Spatial and temporal characteristics of climate change in Guizhou in recent 30 years. Anhui Agric. Sci. Bull. 2018, 24, 129–132+138. [Google Scholar]
- Zhang, Y. Projections of 2.0 °C Warming over the globe and China under RCP4.5. Atmos. Ocean. Sci. Lett. 2012, 5, 514–520. [Google Scholar]
- Zhao, D.S.; Gao, X.; Wu, S.H.; Zheng, D. Trend of climate variation in China from 1960 to 2018 based on natural regionalization. Adv. Earth Sci. 2020, 35, 750–760. [Google Scholar]
- Guo, Y.L.; Li, X.; Zhao, Z.F.; Nawaz, Z. Predicting the impacts of climate change, soils and vegetation types on the geographic distribution of Polyporus umbellatus in China. Sci. Total Environ. 2019, 648, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Zhang, W.W.; Zhao, X.; Zhu, H.Q.; Ma, L.M.; Qian, Z.Q.; Zhang, Z. Modeling the potential distribution of three taxa of Akebia Decne. under climate change scenarios in China. Forests 2021, 12, 1710. [Google Scholar] [CrossRef]
- Dawson, P.T.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond predictions: Biodiversity conservation in a changing climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef]
- Huang, B.S.; Mao, J.W.; Zhao, Y.J.; Sun, Y.K.; Cao, Y.; Xiong, Z. Similar pattern of potential distribution of Pinus yunnanensis Franch and Tomicus yunnanensis Kirkendall under climate change in China. Forests 2022, 13, 1379. [Google Scholar] [CrossRef]
- Balbontín, J. Identifying suitable habitat for dispersal in Bonelli's eagle: An important issue in halting its decline in Europe. Biol. Conserv. 2005, 126, 74–83. [Google Scholar] [CrossRef]
- Manel, S.; Ormerod, H. Evaluating presence-absence models in ecology: The need to account for prevalence. J. Appl. Ecol. 2010, 38, 921–931. [Google Scholar] [CrossRef]
- Li, Y.; Cao, W.; He, X.Y.; Chen, W.; Xu, S. Prediction of suitable habitat for Lycophytes and Ferns in northeast China: A case study on Athyrium brevifrons. Chin. Geogr. Sci. 2019, 29, 1011–1023. [Google Scholar] [CrossRef]
- Qiao, H.J.; Hu, J.H.; Huang, J.H. Theoretical basis, future directions, and challenges for ecological niche models. Sci. Sin. Vitae 2013, 43, 915–927. [Google Scholar] [CrossRef]
- Dang, A.T.N.; Kumar, L.; Reid, M. Modelling the Potential Impacts of Climate Change on Rice Cultivation in Mekong Delta, Vietnam. Sustainability 2020, 12, 9608. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, X.; Li, K.; Wang, C.; Guna, A.; Zhang, J. Prediction of Potential Geographical Distribution Patterns of Actinidia arguta under Different Climate Scenarios. Sustainability 2021, 13, 3526. [Google Scholar] [CrossRef]
- Saeedi, H.; Costello, M.J.; Warren, D.; Brandt, A. Latitudinal and bathymetrical species richness patterns in the NW Pacific and adjacent Arctic Ocean. Sci. Rep. 2019, 9, 9303. [Google Scholar] [CrossRef]
- Jones, M.C.; Dye, S.R.; Pinnegar, J.K.; Warren, R.; Cheung, W.W.L. Modelling commercial fish distributions: Prediction and assessment using different approaches. Ecol. Model. 2012, 225, 133–145. [Google Scholar] [CrossRef]
- Wang, R.L.; Li, Q.; Feng, C.H.; Shi, Z.P. Predicting potential ecological distribution of Locusta migratoria tibetensis in China using MaxEnt ecological niche modeling. Acta Ecol. Sin. 2017, 37, 8556–8566. [Google Scholar]
- Yang, X.; Xiong, Z.P.; Dong, Y.G.; Zhang, K.C.; Zhang, H.Y.; Yang, W.X.; Shi, Y.P. Prediction of potential geographical distribution of Acanthoscelides macrophthalmus in Yunnan province. Chin. J. Trop. Crops 2014, 35, 1653–1657. [Google Scholar]
- Chen, S.Y.; Li, Y.; Wang, X.R.; Wu, C.X. Research Progress on Sex Pheromone of Seed Beetle, Bruchinae. J. Mt. Agric. Biol. 2021, 40, 44–52. [Google Scholar]
- Tan, J.J.; Yu, P.Y. Economic Insects of China: Coleoptera Chrysomeloidea; Science Press: Beijing, China, 1980; pp. 39–40. [Google Scholar]
- Peng, Q.Y.; Xie, M.H.; Pan, X.K.; Li, Y.; Gao, L.; Xu, F.L.; Wu, C.X.; Yang, M.F. Morphology and distribution of sensilla on antennae and mouthparts of the adult bruchid beetles, Bruchidius coreanus (Coleoptera: Bruchidae). Microsc. Res. Tech. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Silfverberg, H. Review: Bruchids and legumes: Economics, ecology and coevolution. Entomol. Fenn. 1991, 2, 78. [Google Scholar] [CrossRef]
- Ramos, R.Y. Revision of the genus Megabruchidius Borowiec, 1984 (Coleoptera: Bruchidae) with some first records from Europe. Bol. SEA 2009, 45, 371–382. [Google Scholar]
- Li, Y.; Zhang, R.Z.; Guo, J.J.; Qin, M.; Zhao, S.Q.; Chen, X.L. Effectiveness of three pesticides on Megabruchidius dorsalis. J. Appl. Insects 2014, 51, 221–225. [Google Scholar]
- Hiroyuki, K.; Masakazu, S. Geographical variation in the seasonal population dynamics of Bruchidius dorsalis (Coleoptera: Bruchidae): Constraints of temperature and host plant phenology. Environ. Entomol. 2002, 31, 469–475. [Google Scholar]
- Ohbayashi, K.; Ishikawa, N.; Hodoki, Y.; Okada, Y.; Nakano, S.I.; Ito, M.; Shimada, M. Rapid development and characterization of EST-SSR markers for the honey locust seed beetle, Megabruchidius dorsalis (Coleoptera: Bruchidae), using de novo transcriptome analysis based on next-generation sequencing. Appl. Entomol. Zool. 2019, 54, 141–145. [Google Scholar] [CrossRef]
- Yang, L.J.; Li, H.W.; Teng, K.; Shen, A.D.; Li, X.Y.; Yu, Y.X. Potential geographical distributions of three species of locusts in China. Plant Quar. 2022, 36, 60–66. [Google Scholar]
- Alcala-Canto, Y.; Alberti-Navarro, A.; Figueroa-Castillo, J.A.; Ibarra-Velarde, F.; Vera-Montenegro, Y.; Cervantes-Valencia, M.E. Maximum entropy ecological niche prediction of the current potential geographical distribution of eimeria species of cattle, sheep and goats in Mexico. Open J. Anim. Sci. 2019, 9, 15. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Zhao, M.Y.; Zhang, L.; Wang, C.Y.; Xu, Y.L. Predicting possible distribution of tea (Camellia sinensis L.) under climate change scenarios using maxent model in China. Agriculture 2021, 11, 1122. [Google Scholar] [CrossRef]
- György, Z.; Tuda, M. Host-plant range expansion to Gymnocladus dioica by an introduced seed predatory beetle Megabruchidius dorsalis. Entomol. Sci. 2020, 23, 28–32. [Google Scholar] [CrossRef]
- Říha, M.; Bezděk, J. Checklist of Slovak seed-beetles (Coleoptera: Chrysomelidae: Bruchinae), with the first record of invasive Megabruchidius dorsalis (Fåhraeus, 1839). Stud. Rep. Taxonomical Ser. 2015, 11, 167–173. [Google Scholar]
- Wang, H.K. Preliminary investigation on damage of Megabruchidius dorsalis in northeast of our country. For. Pest Dis. 1984, 2, 36–37. [Google Scholar]
- Yang, Y.; Zhou, Y. There are two kinds of insect pests that harm Gleditsia sinensis, namely a heart eaters of Gleditsia sinensis and Megabruchidius dorsalis. For. Sci. Technol. 1974, 11, 11–13. [Google Scholar]
- Yus Ramos, R.; Ventura, D.; Bensusan, K.; Coello-García, P.; György, Z.; Stojanova, A. Alien seed beetles (Coleoptera: Chrysomelidae: Bruchinae) in Europe. Zootaxa 2014, 3826, 401–448. [Google Scholar] [CrossRef]
- Korotyaev, B.A. First records of an East Asian seed beetle Megabruchidius dorsalis Fåhraeus (Coleoptera, Bruchidae) from Germany and the Black Sea coast of Crimea and Caucasus. Entomol. Rev. 2016, 96, 460–461. [Google Scholar] [CrossRef]
- Xin, S.L. The control effects of three insecticides on Megabruchidius dorsalis. Inn. Mong. For. 2016, 8, 14–15. [Google Scholar]
- Sajna, N. First record of non-native Asian seed beetle, Megabruchidius dorsalis (Fåhraeus, 1839) and its parasitoid. Slov. BioInvasions Rec. 2019, 8, 515–520. [Google Scholar] [CrossRef]
- Temreshev, I.I.; Makezhanov, A.M. Expansion of invasive seed beetle Megabruchidius dorsalis Fahreus, 1839 (Coleoptera, Chrysomelidae, Bruchinae) in the Turkestan Region (South Kazakhstan). Acta Biol. Sibirica 2019, 5, 1–4. [Google Scholar] [CrossRef]
- Cho, H.W.; An, S.L. An annotated checklist of leaf beetles (Coleoptera: Chrysomelidae) of Korea, with comments and new records. Far East. Entomol. 2020, 404, 1–36. [Google Scholar] [CrossRef]
- Pintilioaie, A.M.; Manci, C.O.; Fusu, L.; Mitroiu, M.D.; Rădac, A.I. New invasive bruchine species (Chrysomelidae: Bruchinae) in the fauna of Romania, with a review on their distribution and biology. Ann. Soc. Entomol Fr. 2018, 54, 401–409. [Google Scholar] [CrossRef]
- Ruta, R.; Jałoszyński, P.; Wanat, M. Megabruchidius dorsalis (Fåhraeus, 1839)–inwazyjny strąkowiec nowy dla Polski (Coleoptera: Chrysomelidae: Bruchinae). Wiadomości Entomol. 2017, 36, 162–166. [Google Scholar]
- Morimoto, K. A Synopsis of the Bruchid Fauna of Japan; Springer: Dordrecht, The Netherlands, 1990; pp. 131–140. [Google Scholar]

| Type | Variable Abbreviation | Description | Contribution Rate (%) | |
|---|---|---|---|---|
| M. dorsalis | B. coreanus | |||
| Temperature | Bio1 | Annual mean temperature | 14.5 | 12 |
| Bio2 | Mean diurnal range | 4.6 | 0.7 | |
| Bio3 | Isothermality | 5.2 | 9.1 | |
| Bio4 | Temperature seasonality | |||
| Bio5 | Max temperature of warmest month | |||
| Bio6 | Min temperature of coldest month | 35.6 | 20.1 | |
| Bio7 | Temperature annual range | 9.6 | 25.4 | |
| Bio8 | Mean temperature of wettest quarter | 10.4 | ||
| Bio9 | Mean temperature of driest quarter | |||
| Bio10 | Mean temperature of warmest quarter | |||
| Bio11 | Mean temperature of coldest quarter | |||
| Precipitation | Bio12 | Annual precipitation | 4.2 | 10.6 |
| Bio13 | Precipitation of wettest month | 0.1 | ||
| Bio14 | Precipitation of driest month | |||
| Bio15 | Precipitation seasonality | 8.5 | 22.1 | |
| Bio16 | Precipitation of wettest quarter | |||
| Bio17 | Precipitation of driest quarter | 7.4 | ||
| Bio18 | Precipitation of warmest quarter | |||
| Bio19 | Precipitation of coldest quarter | |||
| Terrain | ELEV | Elevation variable | ||
| Species | Climate Scenario | Unsuitable Area | Poorly Suitable Area | Moderately Suitable Area | Highly Suitable Area | ||||
|---|---|---|---|---|---|---|---|---|---|
| Area (×104 km2) | Trend (%) | Area (×104 km2) | Trend (%) | Area (×104 km2) | Trend (%) | Area (×104 km2) | Trend (%) | ||
| Megabruchidius dorsalis | Current | 8.0606 | 45.7552 | 3.5738 | 20.2862 | 3.4897 | 19.8092 | 2.4927 | 14.1494 |
| 2021–2040 | 7.5041 | 42.5964 | 4.3884 | 24.9104 | 3.2918 | 18.6855 | 2.4325 | 13.8077 | |
| 2041–2060 | 7.5555 | 42.8881 | 4.3253 | 24.5525 | 3.2798 | 18.6178 | 2.4561 | 13.9416 | |
| 2061–2080 | 7.3906 | 41.9523 | 4.1424 | 23.5141 | 3.4947 | 19.8373 | 2.5890 | 14.6963 | |
| 2081–2100 | 7.2125 | 40.9411 | 4.4581 | 25.3063 | 3.1765 | 18.0310 | 2.7696 | 15.7216 | |
| Bruchidius coreanus | Current | 8.5362 | 48.4549 | 3.6750 | 20.8609 | 3.0848 | 17.5106 | 2.3208 | 13.1736 |
| 2021–2040 | 8.3806 | 47.5720 | 4.1281 | 23.4329 | 2.6851 | 15.2417 | 2.4229 | 13.7534 | |
| 2041–2060 | 8.3162 | 47.2062 | 4.2164 | 23.9340 | 2.8517 | 16.1875 | 2.2324 | 12.6722 | |
| 2061–2080 | 8.2313 | 46.7245 | 4.2093 | 23.8937 | 2.7839 | 15.8026 | 2.3922 | 13.5792 | |
| 2081–2100 | 7.9899 | 45.3540 | 4.2805 | 24.2980 | 3.0007 | 17.0335 | 2.3456 | 13.3145 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; Peng, Q.; Pan, X.; Xie, M.; Liao, J.; Wu, C.; Yang, M. Maximum Entropy Model Prediction of the Distributions of Two Sympatric Bean Weevil Species, Megabruchidius dorsalis (Fahraeus, 1839) and Bruchidius coreanus (Chûjô, 1937), under Various Climate Scenarios in Guizhou Province, China. Forests 2024, 15, 300. https://doi.org/10.3390/f15020300
Ma G, Peng Q, Pan X, Xie M, Liao J, Wu C, Yang M. Maximum Entropy Model Prediction of the Distributions of Two Sympatric Bean Weevil Species, Megabruchidius dorsalis (Fahraeus, 1839) and Bruchidius coreanus (Chûjô, 1937), under Various Climate Scenarios in Guizhou Province, China. Forests. 2024; 15(2):300. https://doi.org/10.3390/f15020300
Chicago/Turabian StyleMa, Guanying, Qiyan Peng, Xiukui Pan, Minghui Xie, Jun Liao, Chengxu Wu, and Maofa Yang. 2024. "Maximum Entropy Model Prediction of the Distributions of Two Sympatric Bean Weevil Species, Megabruchidius dorsalis (Fahraeus, 1839) and Bruchidius coreanus (Chûjô, 1937), under Various Climate Scenarios in Guizhou Province, China" Forests 15, no. 2: 300. https://doi.org/10.3390/f15020300






