Effects of Forest Fires on Boreal Permafrost and Soil Microorganisms: A Review
Abstract
:1. Introduction
2. Variations in Soil Microbial Community Structure, Carbon, and Nitrogen in Boreal Permafrost Regions
2.1. Changes in the Soil Microbial Community Structure
2.2. Changes in Soil Microbial Carbon and Nitrogen Characteristics
3. Impact of Forest Fires on Permafrost
3.1. Altered Characteristics Due to Forest Fire-Induced Permafrost Degradation
3.2. Impact of Forest Fires on Permafrost Landscapes
3.3. Effect of Fire Intensity on the Soil Microbial Community Structure
3.4. Effect of Years Post-Fire on the Dynamics of the Soil Microbial Community Structure
3.5. Effects of Forest Fires on the Soil Microbial Community Structure in the Boreal Permafrost Regions
4. Effects of Forest Fire on MBC and MBN in the Boreal Permafrost Regions
4.1. Effect of Fire Severity on Soil MBC and MBN
4.2. Effect of Years Post-Fire on MBC and MBN
5. Discussion
6. Prospects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, H.; Luo, S.; Luo, B. Effects of forest fire disturbance on soil organic carbon in forest ecosystems: A review. Acta Ecol. Sin. 2020, 40, 1839–1850. (In Chinese) [Google Scholar]
- Knorre, A.A.; Siegwolf, R.T.; Kirdyanov, A.V.; Saurer, M.; Churakova, O.V.; Prokushkin, A.S. Fire as a major factor in dynamics of tree-growth and stable δ13C and δ18O variations in larch in the permafrost zone. Forests 2022, 13, 725. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Wang, B.; Tian, Y.; Quan, Y.; Liu, J. Analysis of factors related to forest fires in different forest ecosystems in China. Forests 2022, 13, 1021. [Google Scholar] [CrossRef]
- Liang, S.; Hurteau, M.D. Novel climate–fire–vegetation interactions and their influence on forest ecosystems in the western USA. Funct. Ecol. 2023, 37, 2126–2142. [Google Scholar] [CrossRef]
- Zylstra, P.; Wardell-Johnson, G.; Falster, D.; Howe, M.; McQuoid, N.; Neville, S. Mechanisms by which growth and succession limit the impact of fire in a south-western Australian forested ecosystem. Funct. Ecol. 2023, 37, 1350–1365. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, L. Prediction of forest fire danger rating based on meteorological and space-time factors. J. For. Res. 2018, 3, 102–110. [Google Scholar]
- Hu, Y.; Zhao, F.; Chen, F.; Chen, F.; Shu, L. Impacts of global warming and large-scale climate fluctuation on forest fires and forest carbon emissions. Terr. Ecosyst. Conserv. 2021, 1, 75–81. (In Chinese) [Google Scholar]
- Subcommittee, P. Glossary of permafrost and related ground-ice terms. Assoc. Comm. Geotech. Res. NRC 1988, 156, 63–64. [Google Scholar]
- Obu, J. How much of the earth’s surface is underlain by permafrost? J. Geophys. Res. 2021, 126, e2021JF006123. [Google Scholar] [CrossRef]
- Zhang, T.; Heginbottom, J.A.; Barry, R.G.; Brown, J. Further statistics on the distribution of permafrost and ground ice in the Northern Hemisphere. Polar. Geogr. 2000, 24, 126–131. [Google Scholar] [CrossRef]
- Ran, Y.; Li, X.; Cheng, G.; Che, J.; Aalto, J.; Karjalainen, O.; Hjort, J.; Luoto, M.; Jin, H.; Obu, J.; et al. New high-resolution estimates of the permafrost thermal state and hydrothermal conditions over the Northern Hemisphere. Earth Syst. Sci. Data Discuss. 2021, 14, 865–884. [Google Scholar] [CrossRef]
- Li, G.; Zhang, M.; Pei, W.; Melnikov, A.; Khristoforov, I.; Li, R.; Yu, F. Changes in permafrost extent and active layer thickness in the Northern Hemisphere from 1969 to 2018. Sci. Total Environ. 2021, 804, 150182. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Liu, M.; Chen, H.; Jiang, X.; Zang, S. Effects of snow cover change on soil microbial community structure in permafrost region of Great Hing’an Mountains. Acta Ecol. Sin. 2020, 40, 789–799. (In Chinese) [Google Scholar]
- Taş, N.; Prestat, E.; McFarland, J.W.; Wickland, K.P.; Knight, R.; Berhe, A.A.; Jorgenson, T.; Waldrop, M.P.; Jansson, J.K. Impact of fire on active layer and permafrost microbial communities and metagenomes in an upland Alaskan boreal forest. ISME J. 2014, 8, 1904–1919. [Google Scholar] [CrossRef] [PubMed]
- McGuire, A.D.; Koven, C.; Lawrence, D.M.; Clein, J.S.; Xia, J.; Beer, C.; Burke, E.; Chen, G.; Chen, X.; Delire, C.; et al. Variability in the sensitivity among model simulations of permafrost and carbon dynamics in the permafrost region between 1960 and 2009. Glob. Biogeochem. Cycles 2016, 30, 1015–1037. [Google Scholar] [CrossRef]
- Xu, Q.; Xiao, C.; Feng, Y.; Du, Z.; Wang, L.; Wei, Z. Advances in microbe mediated key processes of the carbon cycle in thermokarst lakes. Adv. Earth Sci. 2023, 38, 470–482. (In Chinese) [Google Scholar]
- Ni, J.; Wu, T.; Zhao, L. Carbon cycle in circum-Arctic permafrost regions: Progress and prospects. J. Glaciol. Geocryol. 2019, 41, 845–857. (In Chinese) [Google Scholar]
- Rantanen, M.; Karpechko, A.Y.; Lipponen, A.; Nordling, K.; Hyvärinen, O.; Ruosteenoja, K.; Vihma, T.; Laaksonen, A. The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 2022, 3, 168–178. [Google Scholar] [CrossRef]
- Li, W.; Yan, D.; Weng, B.; Zhu, L. Research progress on hydrological effects of permafrost degradation in the Northern Hemisphere. Geoderma 2023, 438, 116629. [Google Scholar] [CrossRef]
- Biskaborn, B.K.; Smith, S.L.; Noetzli, J.; Matthes, H.; Vieira, G.; Streletskiy, D.A.; Schoeneich, P.; Romanovsky, V.E.; Lewkowicz, A.G.; Abramov, A.; et al. Permafrost is warming at a global scale. Nat. Commun. 2019, 10, 264–275. [Google Scholar] [CrossRef]
- Wang, G.; Chen, L.; Zhang, D.; Chang, R. Divergent trajectory of soil autotrophic and heterotrophic respiration upon permafrost thaw. Environ. Sci. Technol. 2022, 56, 10483–10493. [Google Scholar] [CrossRef]
- Zhao, M. Analysis situation in the study of elevational patterns of soil microbial diversity. J. Green Sci. Technol 2020, 23–25. (In Chinese) [Google Scholar]
- Qu, Z.; Liu, B.; Ma, Y.; Sun, H. Differences in bacterial community structure and potential functions among eucalyptus plantations with different ages and species of trees. Appl. Soil. Ecol. 2020, 149, 103515. [Google Scholar] [CrossRef]
- Tang, Y.; Wei, Z.; Yan, T.; Yang, L.; Ci, E. Biological indicator of soil quality: A review. Soils 2017, 39, 157–163. (In Chinese) [Google Scholar]
- Yang, Y.-L.; Ma, X.-S.; Xie, H.-T.; Bao, X.-L.; Liang, C.; Zhu, X.-F.; He, H.-B.; Zhang, X.-D. Effects of conservation tillage on soil microbial community and the function of soil carbon cycling. Chin. J. Appl. Ecol. 2021, 32, 2675–2684. (In Chinese) [Google Scholar]
- Fang, Y.; Wang, W.; Yao, X.; Peng, X. Soil microbial community composition and environmental controls in northern temperate steppe of China. Acta Sci. Nat. PKU 2017, 53, 142–150. (In Chinese) [Google Scholar]
- Li, R.-B. Review of Research Advances of soil microbial biomass carbon. Forest Environ Sci. 2008, 24, 65–69. (In Chinese) [Google Scholar]
- Mazzarino, M.J.; Szott, L.; Jimenez, M. Dynamics of soil total C and N, microbial biomass, and water-soluble C in tropical agroecosystems. Soil Biol. Biochem. 1993, 25, 205–214. [Google Scholar] [CrossRef]
- Mabuhay, J.A.; Nakagoshi, N.; Isagi, Y. Influence of erosion on soil microbial biomass, abundance and community diversity. Land Degrad. Dev. 2004, 15, 183–195. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y. Research progress of forest soil microbial metrology. J. Guizhou Norm. Univ. (Nat. Sci.) 2020, 38, 118–122. (In Chinese) [Google Scholar]
- Shi, B.D.; Han, D.D.; Li, Z.G.; Quan, X.K.; Yu, H.Z.; Di, X.Y.; Yang, G. Characteristics of soil microbial biomass carbon and nitrogen in burned area in Larix gmelinii forest. J. Northeast Forest Univ. 2022, 50, 78–82+98. (In Chinese) [Google Scholar]
- Hu, H.Q.; Wei, S.J.; Wei, S.; Sun, L. Effect of fire disturbance on forest ecosystem carbon cycle under the background of climate warming. J. Catastrophol. 2012, 27, 37–41. (In Chinese) [Google Scholar]
- Piao, S.; Nan, H.; Huntingford, C.; Ciais, P.; Friedlingstein, P.; Sitch, S.; Peng, S.; Ahlström, A.; Canadell, J.G.; Cong, N.; et al. Evidence for a weakening relationship between interannual temperature variability and northern vegetation activity. Nat. Commun. 2014, 5, 5018–5025. [Google Scholar] [CrossRef]
- Song, X.; Wang, G.; Ran, F.; Chang, R.; Song, C.; Xiao, Y. Effects of topography and fire on soil CO2 and CH4 flux in boreal forest underlain by permafrost in northeast China. Ecol. Eng. 2017, 106, 35–43. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, J.F.; Liu, J.P.; Du, H. A review of main studies on permafrost degradation in northeast China. Sci. Technol. Eng. 2022, 22, 13151–13161. (In Chinese) [Google Scholar]
- Gibson, C.M.; Chasmer, L.E.; Thompson, D.K.; Quinton, W.L.; Flannigan, M.D.; Olefeldt, D. Wildfire as a major driver of recent permafrost thaw in boreal peatlands. Nat. Commun. 2018, 9, 3041. [Google Scholar] [CrossRef]
- Smith, S.L.; Romanovsky, V.E.; Lewkowicz, A.G.; Burn, C.R.; Allard, M.; Clow, G.D.; Yoshikawa, K.; Throop, J. Thermal state of permafrost in North America: A contribution to the international polar year. Permafrost Periglac. 2010, 21, 117–135. [Google Scholar] [CrossRef]
- O’neill, H.B.; Burn, C.R.; Allard, M.; Arenson, L.U.; Bunn, M.I.; Connon, R.F.; Kokelj, S.V.; LeBlanc, A.-M.; Morse, P.D.; Smith, S.L. Permafrost thaw and northern development. Nat. Clim. Chang. 2020, 10, 722–723. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Scott, J.J.; Saltonstall, K.; Broders, K.; Montero-Sanchez, M.; Püspök, J.; Bååth, E.; Meir, P. Microbial diversity declines in warmed tropical soil and respiration rise exceed predictions as communities adapt. Nat. Microbiol. 2022, 7, 1650–1660. [Google Scholar] [CrossRef]
- Zheng, Y.; Shi, C.; Wu, F.; Liu, H.; Zhao, Z.; Hu, C. Research progress on influencing factors of soil microbial activity. Chin. J. Soil Sci. 2009, 40, 1209–1214. (In Chinese) [Google Scholar]
- Wang, D.; Zang, S.; Wang, L.; Ma, D.; Li, M. Effects of permafrost degradation on soil carbon and nitrogen cycling in permafrost wetlands. Front. Earth Sci. 2022, 10, 911314. [Google Scholar] [CrossRef]
- Monteux, S.; Weedon, J.T.; Blume-Werry, G.; Gavazov, K.; Jassey, V.E.; Johansson, M.; Keuper, F.; Olid, C.; Dorrepaal, E. Long-term in situ permafrost thaw effects on bacterial communities and potential aerobic respiration. ISME J. 2018, 12, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Heal, K.; Wang, S.; Cao, S.; Zhou, C. Chemodiversity of soil dissolved organic matter and its association with soil microbial communities along a chronosequence of Chinese fir monoculture plantations. Front. Microbiol. 2021, 12, 729344. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Chen, K.; Li, H.; Wei, J.; Zhao, P.; Zhou, M. Research progress on the effects of forest fire disturbance on soil carbon release in high-latitude permafrost regions. Ecol. Environ. Sci. 2022, 31, 1278–1284. (In Chinese) [Google Scholar]
- Tian, J.; He, N.; Hale, L.; Niu, S.; Yu, G.; Liu, Y.; Blagodatskaya, E.; Kuzyakov, Y.; Gao, Q.; Zhou, J. Soil organic matter availability and climate drive latitudinal patterns in bacterial diversity from tropical to cold temperate forests. Funct. Ecol. 2018, 32, 61–70. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, B.; Chen, C.; Zhang, Z.; Wang, Q.; He, J. Precipitation overrides warming in mediating soil nitrogen pools in an alpine grassland ecosystem on the Tibetan Plateau. Sci. Rep. 2016, 6, 31438. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, S.; Pu, Y.; Xu, X.; Li, Y. Distribution characteristics and impact factors of soil microbial biomass carbon, nitrogen and phosphorus in western Sichuan plain. Chin. J. Eco-Agric. 2019, 27, 1607–1616. (In Chinese) [Google Scholar]
- Euskirchen, E.S.; Edgar, C.W.; Turetsky, M.R.; Waldrop, M.P.; Harden, J.W. Differential response of carbon fluxes to climate in three peatland ecosystems that vary in the presence and stability of permafrost. J. Geophys. Res. Biogeosci. 2014, 119, 1576–1595. [Google Scholar] [CrossRef]
- Schädel, C.; Schuur, E.A.G.; Bracho, R.; Elberling, B.; Knoblauch, C.; Lee, H.; Luo, Y.; Shaver, G.R.; Turetsky, M.R. Circumpolar assessment of permafrost C quality and its vulnerability over time using long-term incubation data. Glob. Chang. Biol. 2014, 20, 641–652. [Google Scholar] [CrossRef]
- Ganie, M.A.; Mukhtar, M.; Dar, M.A.; Ramzan, S. Soil microbiological activity and carbon dynamics in the current climate change scenarios: A Review. Pedosphere 2016, 26, 577–591. [Google Scholar]
- Liang, Y.; Jiang, Y.; Wang, F.; Wen, C.; Deng, Y.; Xue, K.; Qin, Y.; Yang, Y.; Wu, L.; Zhou, J.; et al. Long-term soil transplant simulating climate change with latitude significantly alters microbial temporal turnover. ISME J. 2015, 9, 2561–2572. [Google Scholar] [CrossRef]
- Mitzscherling, J.; Horn, F.; Winterfeld, M.; Mahler, L.; Kallmeyer, J.; Overduin, P.P.; Schirrmeister, L.; Winkel, M.; Grigoriev, M.N.; Wagner, D.; et al. Microbial community composition and abundance after millennia of submarine permafrost warming. Biogeosciences 2019, 16, 3941–3958. [Google Scholar] [CrossRef]
- Liu, F.; Kou, D.; Chen, Y.; Xue, K.; Ernakovich, J.G.; Chen, L.; Yang, G.; Yang, Y. Altered microbial structure and function after thermokarst formation. Global Change Biol. 2021, 27, 823–835. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, T.; Du, Y.; Cong, R.; Yang, L.; He, S.; Wang, X. Research progress on frozen soil and its microorganisms. J. Temp. For. Res. 2021, 4, 13–18. (In Chinese) [Google Scholar]
- Dong, X.; Man, H.; Liu, C.; Wu, X.; Zhu, J.; Zheng, Z.; Ma, D.; Li, M.; Zang, S. Changes in soil bacterial community along a gradient of permafrost degradation in Northeast China. Catena 2023, 222, 106870. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, F.; Kang, L.; Zhang, D.; Kou, D.; Mao, C.; Qin, S.; Zhang, Q.; Yang, Y. Large-scale evidence for microbial response and associated carbon release after permafrost thaw. Glob. Chang. Biol. 2021, 24, 3218–3229. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, D.; Jiang, X.; Liu, M.; Zang, S. Effects of seasonal freeze-thaw on soil microbial community structures and extracellular enzyme activities in Zhalong wetland. Acta Sci. Circumst. 2020, 40, 1443–1451. (In Chinese) [Google Scholar]
- Zhang, N.; Chen, K.; Wang, H.; Yang, Y. Changes of microbial community characteristics in freezing-thaw degradation of alpine wetland. Ecol. Sci. 2022, 41, 20–28. (In Chinese) [Google Scholar]
- Wu, M.-H.; Chen, S.-Y.; Chen, J.-W.; Xue, K.; Chen, S.-L.; Wang, X.-M.; Chen, T.; Kang, S.-C.; Rui, J.-P.; Thies, J.E.; et al. Reduced microbial stability in the active layer is associated with carbon loss under alpine permafrost degradation. Proc. Nat. Acad. Sci. USA 2021, 118, e2025321118. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ma, D.; Zang, S.; Wu, X. Characteristics of soil microbial community structure under different forest types of permafrost regions in the Greater Khingan Mountains. J. Glaciol. Geocryol. 2018, 40, 1028–1036. (In Chinese) [Google Scholar]
- Frank-Fahle, B.A.; Yergeau, É.; Greer, C.W.; Lantuit, H.; Wagner, D. Microbial functional potential and community composition in permafrost-affected soils of the NW Canadian Arctic. PLoS ONE 2014, 9, e84761. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Kobabe, S.; Liebner, S. Bacterial community structure and carbon turnover in permafrost-affected soils of the Lena Delta, northeastern Siberia. Can. J. Microbiol. 2009, 55, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Kong, W.; Liang, C.; Zhou, T.; Jia, H.; Dong, X. Permafrost thawing exhibits a greater influence on bacterial richness and community structure than permafrost age in Arctic permafrost soils. Cryosphere 2020, 14, 3907–3916. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Shi, R.; Han, S.; Li, H.; Xu, H. Temporal variations of soil microbial biomass and enzyme activities during the secondary succession of primary broadleaved-pinuskoraiensis forests in Changbai Mountains of Northeast China. Chin. J. Appl. Ecol. 2013, 24, 366–372. (In Chinese) [Google Scholar]
- Chen, S.; Zhao, Q.; Liu, W.; Zhang, Z.; Li, S.; Li, H.; Nie, Z.; Zhou, L.; Kang, S. Effects of freeze-thaw cycles on soil N2O concentration and flux in the permafrost regions of the Qinghai-Tibetan Plateau. Sci. Cold Arid. Reg. 2018, 10, 69–79. [Google Scholar]
- Wang, J.; Han, Y.; Song, C.; Xi, F. Effects of freezing-thawing cycles on soil organic carbon mineralization in the peatland ecosystems from continuous permafrost zone, Great Hinggan Mountains. Clim. Chang. Res. 2018, 14, 59–66. (In Chinese) [Google Scholar]
- Chen, L.; Gao, Y. Global climate change effects on soil microbial biomass stoichiometry in alpine ecosystems. Land 2022, 11, 1661. [Google Scholar] [CrossRef]
- Cheng, G.; Zhao, L.; Li, R.; Wu, X.; Sheng, Y.; Hu, G.; Zou, D.; Jin, H.; Li, X.; Wu, Q. Characteristics, changes and impacts of permafrost on the Tibetan Plateau. Chin. Sci. Bull. 2019, 64, 2783–2795. (In Chinese) [Google Scholar]
- Oliva, M.; Pereira, P.; Antoniades, D. The environmental consequences of permafrost degradation in a changing climate. Sci. Total Environ. 2017, 616, 435–437. [Google Scholar] [CrossRef]
- Wu, M.; Qu, D.; Li, T.; Liu, F.; Gao, Y.; Chen, Y.; Chen, T. Effects of permafrost degradation on soil microbial biomass carbon and nitrogen in the Shule River Headwaters, the Qilian Mountains. Sci. Geogr. Sin. 2021, 41, 177–186. (In Chinese) [Google Scholar]
- Lucash, M.S.; Marshall, A.M.; Weiss, S.A.; McNabb, J.W.; Nicolsky, D.J.; Flerchinger, G.N.; Link, T.E.; Vogel, J.G.; Scheller, R.M.; Abramoff, R.Z.; et al. Burning trees in frozen soil: Simulating fire, vegetation, soil, and hydrology in the boreal forests of Alaska. Ecol. Model. 2023, 481, 110367. [Google Scholar] [CrossRef]
- Han, A.; Qing, S.; Bao, Y.; Na, L.; Bao, Y.; Liu, X.; Zhang, J.; Wang, C. Short-term effects of fire severity on vegetation based on Sentinel-2 satellite ddata. Sustainability 2021, 13, 432. [Google Scholar] [CrossRef]
- Xiang, X.; Shi, Y.; Yang, J.; Kong, J.; Lin, X.; Zhang, H.; Zeng, J.; Chu, H. Rapid recovery of soil bacterial communities after wildfire in a Chinese boreal forest. Sci. Rep. 2014, 4, 3829. [Google Scholar] [CrossRef]
- Strauss, J.; Schirrmeister, L.; Grosse, G.; Fortier, D.; Hugelius, G.; Knoblauch, C.; Romanovsky, V.; Schädel, C.; von Deimling, T.S.; Schuur, E.A.; et al. Deep Yedoma permafrost: A synthesis of depositional characteristics and carbon vulnerability. Earth-Sci. Rev. 2017, 172, 75–86. [Google Scholar] [CrossRef]
- Antonova, S.; Sudhaus, H.; Strozzi, T.; Zwieback, S.; Kääb, A.; Heim, B.; Langer, M.; Bornemann, N.; Boike, J. Thaw subsidence of a yedoma landscape in northern Siberia, measured in situ and estimated from TerraSAR-X interferometry. Remote Sens. 2018, 10, 494–521. [Google Scholar] [CrossRef]
- Jin, H.; Wang, S.; Lu, L.; Yu, S. Features of permafrost degradation in Hinggan Mountains, Northeastern China. Sci. Geogr. Sin. 2009, 29, 223–228. (In Chinese) [Google Scholar]
- Li, Y.; Qing, Y.; Lyu, M.; Chen, S.; Yang, Z.; Lin, C.; Yang, Y. Effects of artificial warming on different soil organic carbon and nitrogen pools in a subtropical plantation. Soil Biol. Biochem. 2018, 124, 161–167. [Google Scholar] [CrossRef]
- Guo, L.; Chang, J.; Xu, H.; Sun, W. Modelling plant canopy effects on water-heat exchange in the freezing-thawing processes of active layer on the Qinghai-Tibet Plateau. J. Mt. Sci. 2021, 18, 1564–1579. [Google Scholar] [CrossRef]
- Yang, H.; Hong, X.; Yuan, Z.; He, X. Modelling the influence of vegetation on the hydrothermal processes of frozen soil in the Qinghai–Tibet Plateau. Water 2023, 15, 1692–1710. [Google Scholar] [CrossRef]
- Kelley, A.M.; Epstein, H.E.; Walker, D.A. Role of vegetation and climate in permafrost active layer depth in arctic tundra of northern Alaska and Canada. J. Glaciol. Geocryol. 2004, 26, 269–274. [Google Scholar]
- Chang, X.L.; Jin, H.J.; Wang, Y.P.; Zhang, Y.L.; Zhou, G.Y.; Che, F.Q.; Zhao, Y.M. Influences of vegetation on permafrost: A review. Acta Ecol. Sin. 2012, 32, 7981–7990. (In Chinese) [Google Scholar] [CrossRef]
- Schaphoff, S.; Reyer, C.P.O.; Schepaschenko, D.; Gerten, D.; Shvidenko, A. Tamm Review: Observed and projected climate change impacts on Russia’s forests and its carbon balance. For. Ecol. Manag. 2016, 361, 432–444. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Kane, E.S.; Harden, J.W.; Ottmar, R.D.; Manies, K.L.; Hoy, E.; Kasischke, E.S. Recent acceleration of biomass burning and carbon losses in Alaskan forests and peatlands. Nat. Geosci. 2011, 4, 27–31. [Google Scholar] [CrossRef]
- Kong, J.; Yang, J. Effects of fire on soil properties and nutrient availability in a Dahurian larch forest in Great Xing’an Mountains of Northeast China. Chin. J. Ecol. 2013, 32, 2837–2843. [Google Scholar]
- Jiang, Y.; Rocha, A.V.; O’Donnell, J.A.; Drysdale, J.A.; Rastetter, E.B.; Shaver, G.R.; Zhuang, Q. Contrasting soil thermal responses to fire in Alaskan tundra and boreal forest. J. Geophys. Res. Earth Surf. 2015, 120, 363–378. [Google Scholar] [CrossRef]
- Smith, S.L.; Riseborough, D.W.; Bonnaventure, P.P. Eighteen year record of forest fire effects on ground thermal regimes and permafrost in the Central Mackenzie Valley, NWT, Canada. Permafr. Periglac. Process 2015, 26, 289–303. [Google Scholar] [CrossRef]
- Shenoy, A.; Johnstone, J.F.; Kasischke, S.; Kielland, K. Persistent effects of fire severity on early successional forests in interior Alaska. For. Ecol. Manag. 2011, 261, 381–390. [Google Scholar] [CrossRef]
- Alexander, H.D.; Natali, S.M.; Loranty, M.M.; Ludwig, S.M.; Spektor, V.V.; Davydov, S.; Zimov, N.; Trujillo, I.; Mack, M.C. Impacts of increased soil burn severity on larch forest regeneration on permafrost soils of far northeastern Siberia. For. Ecol. Manag. 2018, 417, 144–153. [Google Scholar] [CrossRef]
- Li, X.; Jin, H.; He, R.; Huang, Y.; Luo, D.; Jin, X.; Lü, L. Impacts of hazardous fires on permafrost environment: A review. J. Glaciol. Geocryol. 2017, 39, 317–327. (In Chinese) [Google Scholar]
- Holloway, J.E.; Lewkowicz, A.G.; Douglas, T.A.; Li, X.; Turetsky, M.R.; Baltzer, J.L.; Jin, H. Impact of wildfire on permafrost landscapes: A review of recent advances and future prospects. Permafr. Periglac. Process. 2020, 31, 371–382. [Google Scholar] [CrossRef]
- Li, T.; Jeřábek, J.; Winkler, J.; Vaverková, M.D.; Zumr, D. Effects of prescribed fire on topsoil properties: A small-scale straw burning experiment. J. Hydrol. Hydromech. 2022, 70, 450–461. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, B.; Sun, M.; Zhou, Z. Impact of forest fire on understory vegetation and soil in typical forest types of Daxing’an Mountains, northeastern China. J. Beijing For. Univ. 2012, 34, 7–13. (In Chinese) [Google Scholar]
- Bai, Q.; Zhou, M.; Qi, Q.; Meng, R.; Cui, X.; Wen, D. The changes of the frozen soil temperature and humidity of the forest fire forest in the Great Higgnan mountains. J. Arid. Land Res. Environ. 2013, 27, 69–75. (In Chinese) [Google Scholar]
- Nossov, D.R.; Jorgenson, M.T.; Kielland, K.; Kanevskiy, M.Z. Edaphic and microclimatic controls over permafrost response to fire in interior Alaska. Environ. Res. Lett. 2013, 8, 035013. [Google Scholar] [CrossRef]
- Li, X.Y.; Jin, H.J.; Wang, H.W.; Marchenko, S.S.; Shan, W.; Luo, D.L.; He, R.X.; Spektor, V.; Huang, Y.D.; Li, X.Y.; et al. Influences of forest fires on the permafrost environment: A review. Adv. Clim. Chang. Res. 2021, 12, 48–65. [Google Scholar] [CrossRef]
- Li, X.; Jin, H.; He, R.; Huang, Y.; Wang, H.; Luo, D.; Jin, X.; Lü, L.; Wang, L.; Li, W.; et al. Effects of forest fires on the permafrost environment in the northern Da Xing’anling (Hinggan) mountains, Northeast China. Permafr. Periglac. Process. 2019, 30, 163–177. [Google Scholar] [CrossRef]
- Jiang, X.; Cai, H.; Yang, X. Response of active layer thickening to wildfire in the pan-Arctic region: Permafrost type and vegetation type influences. Sci. Total Environ. 2023, 902, 166132. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Jorgenson, M.T.; Douglas, T.A.; Romanovsky, V.E.; Kielland, K.; Hiemstra, C.; Euskirchen, E.S.; Ruess, R.W. Interactive effects of wildfire and climate on permafrost degradation in Alaskan lowland forests. J. Geophys. Res. Biogeosci. 2015, 120, 1619–1637. [Google Scholar] [CrossRef]
- Hou, C.; Xie, F.; Li, X.; Xiao, D. Changes of permafrost active layer in greater Khingan mountains under fire disturbance. J. Univ. Jinan Sci. Technol. 2010, 24, 277–281. (In Chinese) [Google Scholar]
- Zhang, Z.; Wu, Q.; Zhou, Z. Risk assessment of freeze thawing disaster in permafrost zone. J. Nat. Disasters 2012, 21, 142–149. [Google Scholar]
- Anders, K.; Marx, S.; Boike, J.; Herfort, B.; Wilcox, E.J.; Langer, M.; Marsh, P.; Höfle, B. Multitemporal terrestrial laser scanning point clouds for thaw subsidence observation at Arctic permafrost monitoring sites. Earth Surf. Process. Landf. 2020, 45, 1589–1600. [Google Scholar] [CrossRef]
- Bouchard, F.; MacDonald, L.A.; Turner, K.W.; Thienpont, J.R.; Medeiros, A.S.; Biskaborn, B.K.; Korosi, J.; Hall, R.I.; Pienitz, R.; Wolfe, B.B. Paleolimnology of thermokarst lakes: A window into permafrost landscape evolution. Arct. Sci. 2016, 3, 91–117. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Abbott, B.W.; Jones, M.C.; Anthony, K.W.; Olefeldt, D.; Schuur, E.A.G.; Grosse, G.; Kuhry, P.; Hugelius, G.; Koven, C.; et al. Carbon release through abrupt permafrost thaw. Nat. Geosci. 2020, 13, 138–143. [Google Scholar] [CrossRef]
- Mu, C. Thermokarst terrains change landscape and earth surface processes. Chin. J. Nat. 2020, 42, 386–392. (In Chinese) [Google Scholar]
- Luo, J.; Niu, F.; Lin, Z.; Liu, M.; Yin, G. Thermokarst lake changes between 1969 and 2010 in the Beilu River Basin, Qinghai–Tibet Plateau, China. Sci. Bull. 2015, 60, 556–564. [Google Scholar] [CrossRef]
- Smith, N.R.; Kishchuk, B.E.; Mohn, W.W. Effects of wildfire and harvest disturbances on forest soil Bacterial communities. Appl. Environ. Microbiol. 2008, 74, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.M.; Grosse, G.; Arp, C.D.; Miller, E.; Liu, L.; Hayes, D.J.; Larsen, C.F. Recent Arctic tundra fire initiates widespread thermokarst development. Sci. Rep. 2015, 5, 15865. [Google Scholar] [CrossRef] [PubMed]
- Ferrenberg, S.; O’neill, S.P.; Knelman, J.E.; Todd, B.; Duggan, S.; Bradley, D.; Robinson, T.; Schmidt, S.K.; Townsend, A.R.; Williams, M.W.; et al. Changes in assembly processes in soil bacterial communities following a wildfire disturbance. ISME J. 2013, 7, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Santalahti, M.E.; Pumpanen, J.; Köster, K.; Berninger, F.; Raffaello, T.; Asiegbu, F.O.; Heinonsalo, J. Bacterial community structure and function shift across a northern boreal forest fire chronosequence. Sci. Rep. 2016, 6, 32411. [Google Scholar] [CrossRef] [PubMed]
- Smithwick, E.A.H.; Naithani, K.J.; Balser, T.C.; Romme, W.H.; Turner, M.G. Post-fire spatial patterns of soil nitrogen mineralization and microbial abundance. PLoS ONE 2012, 7, e50597. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, H.; Sietiö, O.M.; Pumpanen, J.; Heinonsalo, J.; Köster, K.; Berninger, F. Wildfire effects on soil bacterial community and its potential functions in a permafrost region of Canada. Appl. Soil Ecol. 2020, 156, 103713. [Google Scholar] [CrossRef]
- Pressler, Y.; Moore, J.C.; Cotrufo, M.F. Belowground community responses to fire: Meta-analysis reveals contrasting responses of soil microorganisms and mesofauna. Oikos 2019, 128, 309–327. [Google Scholar] [CrossRef]
- Jian, M.; Berli, M.; Ghezzehei, T.A. Soil structural degradation during low-severity burns. Geophys. Res. Lett. 2018, 45, 5553–5561. [Google Scholar] [CrossRef]
- She, R.; Yang, X.; Xiao, W. Research status on forest soil microbes under fire disturbance. J. Sichuan For. Sci. Technol. 2021, 42, 94–101. (In Chinese) [Google Scholar]
- Chen, F.; Zheng, H.; Ou-Yang, Z.; Zhang, K.; Tu, N. Responses of microbial community structure to the leaf litter composition. Acta Pedol. Sin. 2011, 48, 603–611. (In Chinese) [Google Scholar]
- Tao, Y.; Di, X. Fire interference on forest soil microbial communities and the mechanism: A review. Sci. Silvae. Sin. 2013, 49, 146–157. (In Chinese) [Google Scholar]
- Köster, K.; Aaltonen, H.; Berninger, F.; Heinonsalo, J.; Köster, E.; Ribeiro-Kumara, C.; Sun, H.; Tedersoo, L.; Zhou, X.; Pumpanen, J. Impacts of wildfire on soil microbiome in Boreal environments. Curr. Opin. Environ. Sci. Health 2021, 22, 100258. [Google Scholar] [CrossRef]
- Wang, D. Analysis of the influence of forest fire interference on the structure of soil microbial community in Pinus tabulaeformis forests. J. Temp. For. Res. 2023, 6, 84–86. (In Chinese) [Google Scholar]
- Whitman, T.; Whitman, E.; Woolet, J.; Flannigan, M.D.; Thompson, D.K.; Parisien, M.-A. Soil bacterial and fungal response to wildfires in the Canadian boreal forest across a burn severity gradient. Soil Biol. Biochem. 2019, 138, 107571. [Google Scholar] [CrossRef]
- Peng, Y.; Cao, Y.; Qu, L. Effects of fire intensity on the soil microbial community of larch gmelinii forests. Bull. Bot. Res. 2017, 37, 549–555. (In Chinese) [Google Scholar]
- Gao, H.; Wang, Q. Research progress on the effects of logging and burning on forest soil microorganisms. Intern. J. Ecol. 2021, 10, 472–480. (In Chinese) [Google Scholar] [CrossRef]
- Granged, A.J.; Zavala, L.M.; Jordán, A.; Bárcenas-Moreno, G. Post-fire evolution of soil properties and vegetation cover in a Mediterranean heathland after experimental burning: A 3-year study. Geoderma 2011, 164, 85–94. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Farguell, J.; Úbeda, X. Long-term dynamics of soil chemical properties after a prescribed fire in a Mediterranean forest (Montgrí Massif, Catalonia, Spain). Sci. Total Environ. 2016, 572, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Santalahti, M.; Pumpanen, J.; Köster, K.; Berninger, F.; Raffaello, T.; Jumpponen, A.; Asiegbu, F.O.; Heinonsalo, J. Fungal community shifts in structure and function across a boreal forest fire chronosequence. Appl. Environ. Microbiol. 2015, 81, 7869–7880. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.I.; Thomson, B.C.; James, P.; Bell, T.; Bailey, M.; Whiteley, A.S. The bacterial biogeography of British soils. Environ. Microbiol. 2011, 13, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Wieting, C.; Ebel, B.A.; Singha, K. Quantifying the effects of wildfire on changes in soil properties by surface burning of soils from the Boulder Creek Critical Zone Observatory. J. Hydrol. Reg. Stud. 2017, 13, 43–57. [Google Scholar] [CrossRef]
- Aaltonen, H.; Köster, K.; Köster, E.; Berninger, F.; Zhou, X.; Karhu, K.; Biasi, C.; Bruckman, V.; Palviainen, M.; Pumpanen, J. Forest fires in Canadian permafrost region: The combined effects of fire and permafrost dynamics on soil organic matter quality. Biogeochemistry 2019, 143, 257–274. [Google Scholar] [CrossRef]
- Hu, M.; Wang, J.; Lu, L.; Gui, H.; Wan, S. Global recovery patterns of soil microbes after fire. Soil Biol. Biochem. 2023, 183, 109057. [Google Scholar] [CrossRef]
- Das, S.; Deb, S.; Sahoo, S.S.; Sahoo, U.K. Soil microbial biomass carbon stock and its relation with climatic and other environmental factors in forest ecosystems: A review. Acta Ecol. Sin. 2023, 43, 933–945. [Google Scholar] [CrossRef]
- Holden, S.R.; Rogers, B.M.; Treseder, K.K.; Randerson, J.T. Fire severity influences the response of soil microbes to a boreal forest fire. Environ. Res. Lett. 2016, 11, 035004. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, Y.; Zhang, Z. Influence of environmental factors on distribution and development of Xing’an-Baikal permafrost. J. Jilin Univ. Earth Sci. Ed. 2021, 51, 1427–1440. (In Chinese) [Google Scholar]
- Wang, F.; Tian, L.; Song, A.; Sang, Y.; Zhang, J.; Gao, J. Seasonal dynamics of microbial biomass carbon and nitrogen in soil of Robinia pseudoacacia forests and near-naturally restored vegetation in Northern China. Sci. Silvae Sin. 2015, 51, 16–24. (In Chinese) [Google Scholar]
- Jia, W.; Zhou, H.; Xie, W.; Guan, C.; Gao, C.; Shi, Y. Effects of long-term inorganic fertilizer combined with organic manure on microbial biomass C, N and enzyme activity in cinnamon soil. Plant Nutr. Fert. Sci. 2008, 14, 700–705. (In Chinese) [Google Scholar]
- Zhang, Y.; Pang, X.; Bao, W.; You, C.; Tang, H.; Hu, T. A review of soil organic matter and its research methods. World Sci. Tech. RD 2005, 27, 72–78. (In Chinese) [Google Scholar]
- Li, W.; Liu, X.; Niu Li, B.; Liu, G.; Chu, Y. Impact of fire on soil microbial biomass of Pinus tabuliformis forest in Pingquan County, Hebei of northern China. J. Beijing For. Univ. 2017, 39, 70–77. (In Chinese) [Google Scholar]
- Dove, N.C.; Taş, N.; Hart, S.C. Ecological and genomic responses of soil microbiomes to high-severity wildfire: Linking com-munity assembly to functional potential. ISME J. 2022, 16, 1853–1863. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, D.; Fu, H. Engineering geological characterstics and evaluations of permafrost in Daxing′ an Mountains. J. Eng. Geol. 2008, 16, 657–662. (In Chinese) [Google Scholar]
- Min, K.; Slessarev, E.; Kan, M.; McFarlane, K.; Oerter, E.; Pett-Ridge, J.; Nuccio, E.; Berhe, A.A. Active microbial biomass decreases, but microbial growth potential remains similar across soil depth profiles under deeply-vs. shallow-rooted plants. Soil Biol. Biochem. 2021, 162, 108401. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, L.; Hu, H.; Sun, Z. Post-fire soil microbial biomass and nutrient content of Larix gmelinii forest in autumn. J. Nat. Res. 2011, 26, 450–459. (In Chinese) [Google Scholar]
- Allison, G. The influence of species diversity and stress intensity on community resistance and resilience. Ecol. Monogr. 2004, 74, 117–134. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottovā, D.; Krištůfek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2012, 109, 1159–1164. [Google Scholar] [CrossRef]
- Shen, J.-P.; Chen, C.R.; Lewis, T. Long term repeated fire disturbance alters soil bacterial diversity but not the abundance in an Australian wet sclerophyll forest. Sci. Rep. 2016, 6, 19639. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Ahlström, A.; Hobbie, S.E.; Reich, P.B.; Nieradzik, L.P.; Staver, A.C.; Scharenbroch, B.C.; Jumpponen, A.; Anderegg, W.R.L.; Randerson, J.T.; et al. Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nature 2018, 553, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.H. Diversity in tropical rain forests and coral reefs: High diversity of trees and corals is maintained only in a nonequilibrium state. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Hoy, E.E.; Turetsky, M.R.; Kasischke, E.S. More frequent burning increases vulnerability of Alaskan boreal black spruce forests. Environ. Res. Lett. 2016, 11, 095001. [Google Scholar] [CrossRef]

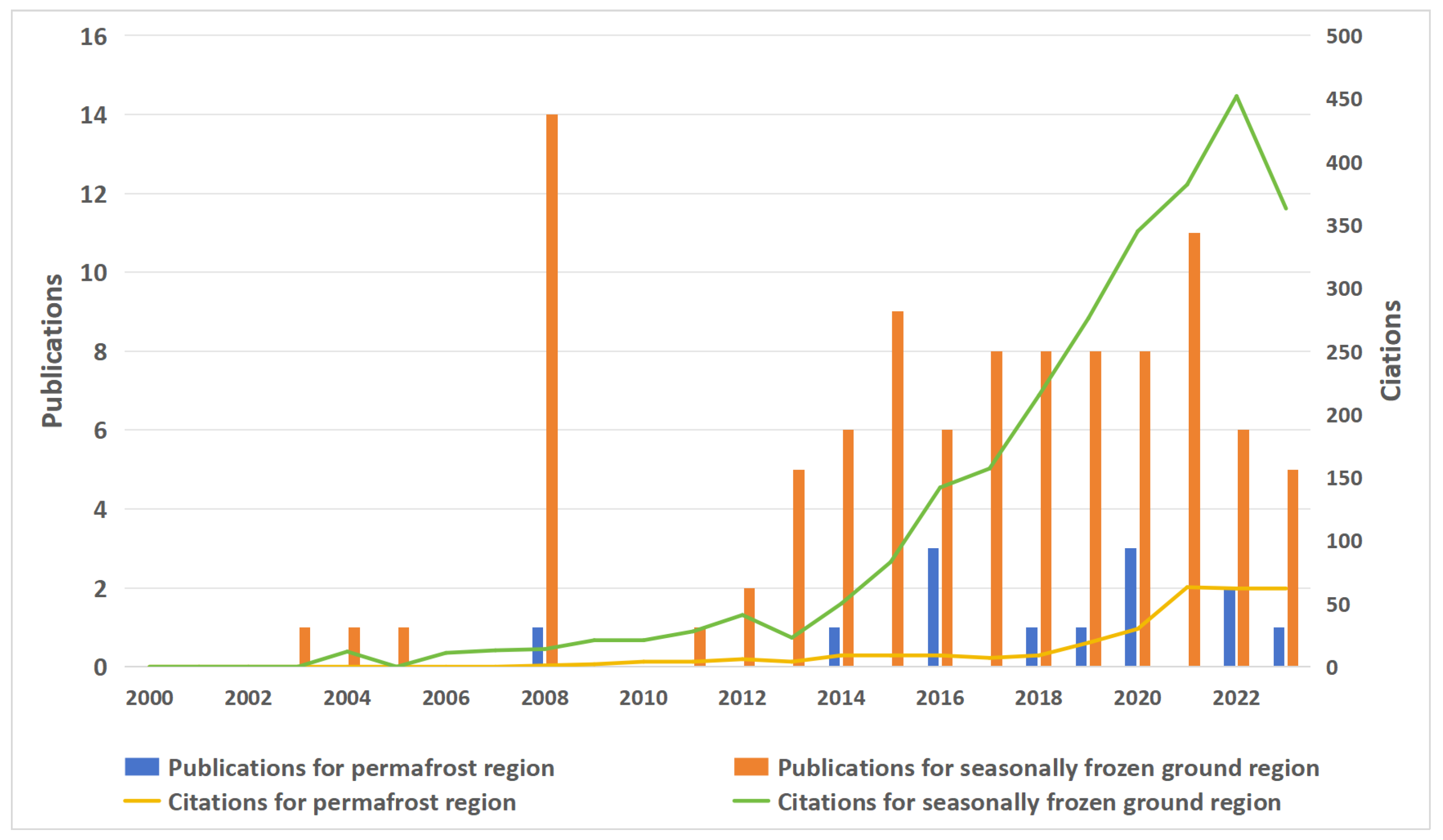
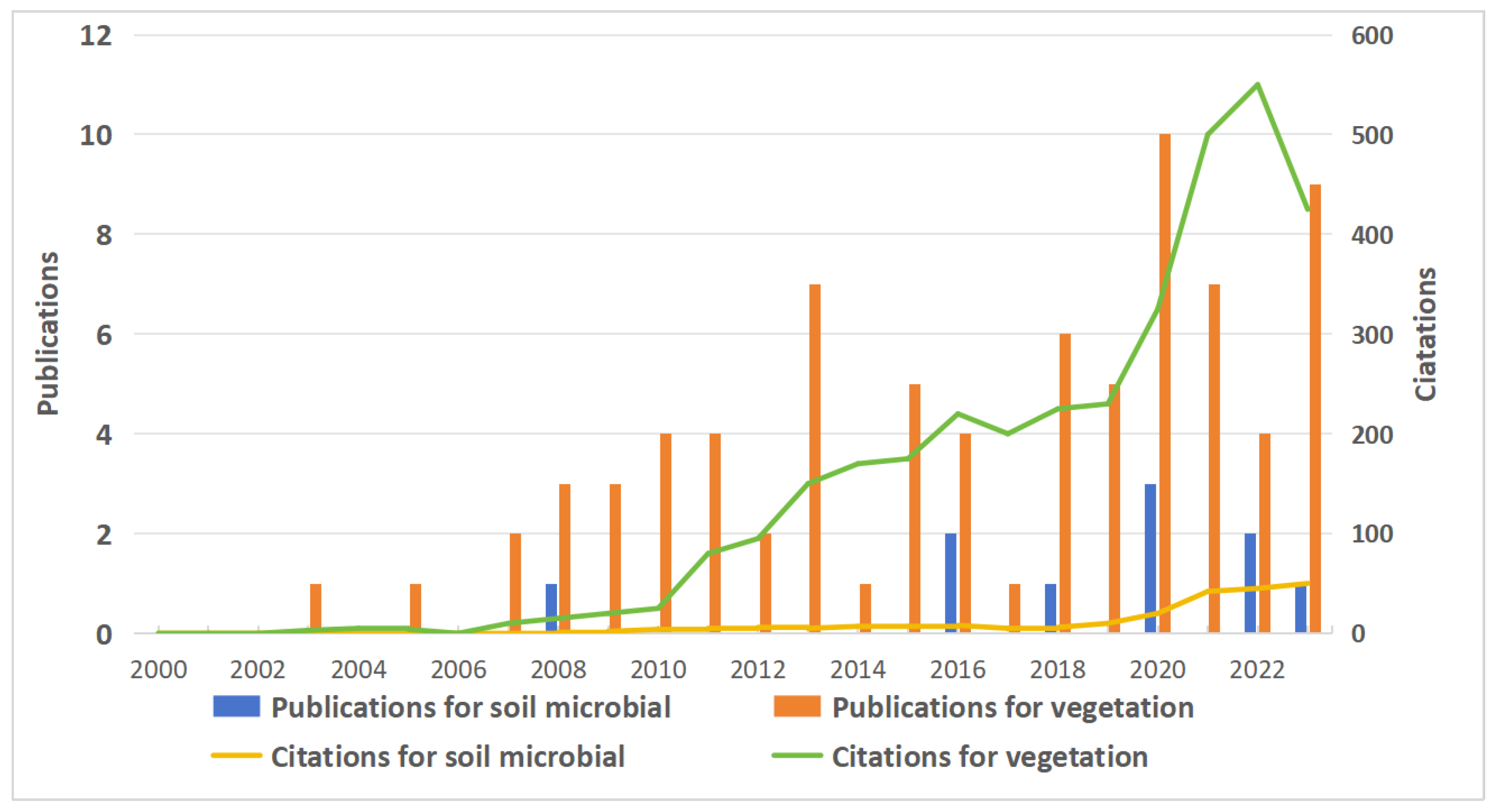
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, X.; Xu, T.; Han, Y.; Li, J.; Shen, Y.; Chen, K. Effects of Forest Fires on Boreal Permafrost and Soil Microorganisms: A Review. Forests 2024, 15, 501. https://doi.org/10.3390/f15030501
Liu J, Li X, Xu T, Han Y, Li J, Shen Y, Chen K. Effects of Forest Fires on Boreal Permafrost and Soil Microorganisms: A Review. Forests. 2024; 15(3):501. https://doi.org/10.3390/f15030501
Chicago/Turabian StyleLiu, Jing, Xiaoying Li, Tao Xu, Yilun Han, Jingtao Li, Yang Shen, and Kui Chen. 2024. "Effects of Forest Fires on Boreal Permafrost and Soil Microorganisms: A Review" Forests 15, no. 3: 501. https://doi.org/10.3390/f15030501




