Multiple Dimensions of Functional Traits in Subtropical Montane Mosses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site, Focal Species, Sampling, and Pretreatment
2.2. Photosynthetic Traits
2.3. Water Retention Capacity
2.4. Architectural Traits
2.5. Chemical Analysis
2.6. Data Analysis
3. Results
4. Discussion
4.1. Morphological Adaptations to the Microhabitat Gradient
4.2. Photosynthetic Adaptations to the Microhabitat Gradient
4.3. Multiple Dimensions of Moss Functional Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomarasca, U.; Migliavacca, M.; Kattge, J.; Nelson, J.A.; Niinemets, Ü.; Wirth, C.; Cescatti, A.; Bahn, M.; Nair, R.; Acosta, A.T.R.; et al. Leaf-Level Coordination Principles Propagate to the Ecosystem Scale. Nat. Commun. 2023, 14, 3948. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Osnas, J.L.D.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global Leaf Trait Relationships: Mass, Area, and the Leaf Economics Spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef]
- Fan, X.-Y.; Liu, W.-Y.; Song, L.; Liu, S.; Shi, X.-M.; Yuan, G.-D. A Combination of Morphological and Photosynthetic Functional Traits Maintains the Vertical Distribution of Bryophytes in a Subtropical Cloud Forest. Am. J. Bot. 2020, 107, 761–772. [Google Scholar] [CrossRef]
- Wang, Z.; Bader, M.Y.; Liu, X.; Zhu, Z.; Bao, W. Comparisons of Photosynthesis-Related Traits of 27 Abundant or Subordinate Bryophyte Species in a Subalpine Old-Growth Fir Forest. Ecol. Evol. 2017, 7, 7454–7461. [Google Scholar] [CrossRef]
- Löbel, S.; Mair, L.; Lönnell, N.; Schröder, B.; Snäll, T. Biological Traits Explain Bryophyte Species Distributions and Responses to Forest Fragmentation and Climatic Variation. J. Ecol. 2018, 106, 1700–1713. [Google Scholar] [CrossRef]
- Coe, K.K.; Howard, N.B.; Slate, M.L.; Bowker, M.A.; Mishler, B.D.; Butler, R.; Greenwood, J.; Stark, L.R. Morphological and Physiological Traits in Relation to Carbon Balance in a Diverse Clade of Dryland Mosses. Plant Cell Environ. 2019, 42, 3140–3151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pi, C.; Bao, W.; Bader, M.Y. Elevational Trends in Photosynthetic Capacity and Trait Relationships of Subtropical Montane Understorey Bryophytes. Ecol. Indic. 2022, 142, 109251. [Google Scholar] [CrossRef]
- Waite, M.; Sack, L. How Does Moss Photosynthesis Relate to Leaf and Canopy Structure? Trait Relationships for 10 Hawaiian Species of Contrasting Light Habitats. New Phytol. 2010, 185, 156–172. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Bader, M.Y.; Feng, D.; Bao, W. The ‘Plant Economic Spectrum’ in Bryophytes, a Comparative Study in Subalpine Forest. Am. J. Bot. 2017, 104, 261–270. [Google Scholar] [CrossRef]
- Laing, C.G.; Granath, G.; Belyea, L.R.; Allton, K.E.; Rydin, H. Tradeoffs and scaling of functional traits in Sphagnum as drivers of carbon cycling in peatlands. Oikos 2014, 123, 817–828. [Google Scholar] [CrossRef]
- Bengtsson, F.; Granath, G.; Rydin, H. Photosynthesis, growth, and decay traits in Sphagnum—A multispecies comparison. Ecol. Evol. 2016, 6, 3325–3341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bader, M.Y. Associations between Shoot-Level Water Relations and Photosynthetic Responses to Water and Light in 12 Moss Species. AoB Plants 2018, 10, ply034. [Google Scholar] [CrossRef] [PubMed]
- Grau-Andrés, R.; Kardol, P.; Gundale, M.J. Trait Coordination in Boreal Mosses Reveals a Bryophyte Economics Spectrum. J. Ecol. 2022, 110, 2493–2506. [Google Scholar] [CrossRef]
- Mazziotta, A.; Granath, G.; Rydin, H.; Bengtsson, F.; Norberg, J. Scaling functional traits to ecosystem processes: Towards a mechanistic understanding in peat mosses. J. Ecol. 2019, 107, 843–859. [Google Scholar] [CrossRef]
- Carriquí, M.; Roig-Oliver, M.; Brodribb, T.J.; Coopman, R.; Gill, W.; Mark, K.; Niinemets, Ü.; Perera-Castro, A.V.; Ribas-Carbó, M.; Sack, L.; et al. Anatomical Constraints to Nonstomatal Diffusion Conductance and Photosynthesis in Lycophytes and Bryophytes. New Phytol. 2019, 222, 1256–1270. [Google Scholar] [CrossRef]
- Ye, Z.-P.; Suggett, D.J.; Robakowski, P.; Kang, H.-J. A Mechanistic Model for the Photosynthesis–Light Response Based on the Photosynthetic Electron Transport of Photosystem II in C3 and C4 Species. New Phytol. 2013, 199, 110–120. [Google Scholar] [CrossRef]
- Song, L.; Zhang, Y.-J.; Chen, X.; Li, S.; Lu, H.-Z.; Wu, C.-S.; Tan, Z.-H.; Liu, W.-Y.; Shi, X.-M. Water Relations and Gas Exchange of Fan Bryophytes and Their Adaptations to Microhabitats in an Asian Subtropical Montane Cloud Forest. J. Plant Res. 2015, 128, 573–584. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: AnRPackage for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 25 January 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lüdecke, D. Sjstats: Statistical Functions for Regression Models (Version 0.18.2). 2022. Available online: https://CRAN.R-project.org/package=sjstats (accessed on 25 January 2024).
- Asefa, M.; Cao, M.; Zhang, G.; Ci, X.; Li, J.; Yang, J. Environmental Filtering Structures Tree Functional Traits Combination and Lineages across Space in Tropical Tree Assemblages. Sci. Rep. 2017, 7, 132. [Google Scholar] [CrossRef]
- Turberville, C.M.; Fuentes-González, J.A.; Rogers, S.; Pienaar, J. Moss Phyllid Morphology Varies Systematically with Substrate Slope. Plant Ecol. Evol. 2021, 154, 419–431. [Google Scholar] [CrossRef]
- Fleischbein, K.; Wilcke, W.; Goller, R.; Boy, J.; Valarezo, C.; Zech, W.; Knoblich, K. Rainfall Interception in a Lower Montane Forest in Ecuador: Effects of Canopy Properties. Hydrol. Process. 2005, 19, 1355–1371. [Google Scholar] [CrossRef]
- Mackelprang, R.; Vaishampayan, P.; Fisher, K. Adaptation to Environmental Extremes Structures Functional Traits in Biological Soil Crust and Hypolithic Microbial Communities. mSystems 2022, 7, e01419-21. [Google Scholar] [CrossRef]
- Zhao, Y.-T.; Ali, A.; Yan, E.-R. The Plant Economics Spectrum Is Structured by Leaf Habits and Growth Forms across Subtropical Species. Tree Physiol. 2017, 37, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; McCormack, M.L.; Ma, C.; Kong, D.; Zhang, Q.; Chen, X.; Zeng, H.; Niinemets, Ü.; Guo, D. Leaf Economics and Hydraulic Traits Are Decoupled in Five Species-rich Tropical-subtropical Forests. Ecol. Lett. 2015, 18, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Hájek, T.; Ballance, S.; Limpens, J.; Zijlstra, M.; Verhoeven, J.T.A. Cell-Wall Polysaccharides Play an Important Role in Decay Resistance of Sphagnum and Actively Depressed Decomposition in Vitro. Biogeochemistry 2011, 103, 45–57. [Google Scholar] [CrossRef]
- Robinson, S.A.; Waterman, M.J. Sunsafe Bryophytes: Photoprotection from Excess and Damaging Solar Radiation. In Photosynthesis in Bryophytes and Early Land Plants; Hanson, D.T., Rice, S.K., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2014; pp. 113–130. ISBN 978-94-007-6988-5. [Google Scholar]
- Wang, Z.; Bao, W.; Feng, D.; Lin, H. Functional trait scaling relationships across 13 temperate mosses growing in wintertime. Ecol. Res. 2014, 29, 629–639. [Google Scholar] [CrossRef]
- Rice, S.K.; Neal, N.; Mango, J.; Black, K. Relationships among Shoot Tissue, Canopy and Photosynthetic Characteristics in the Feathermoss Pleurozium Schreberi. Bryol. 2011, 114, 367–378. [Google Scholar] [CrossRef]
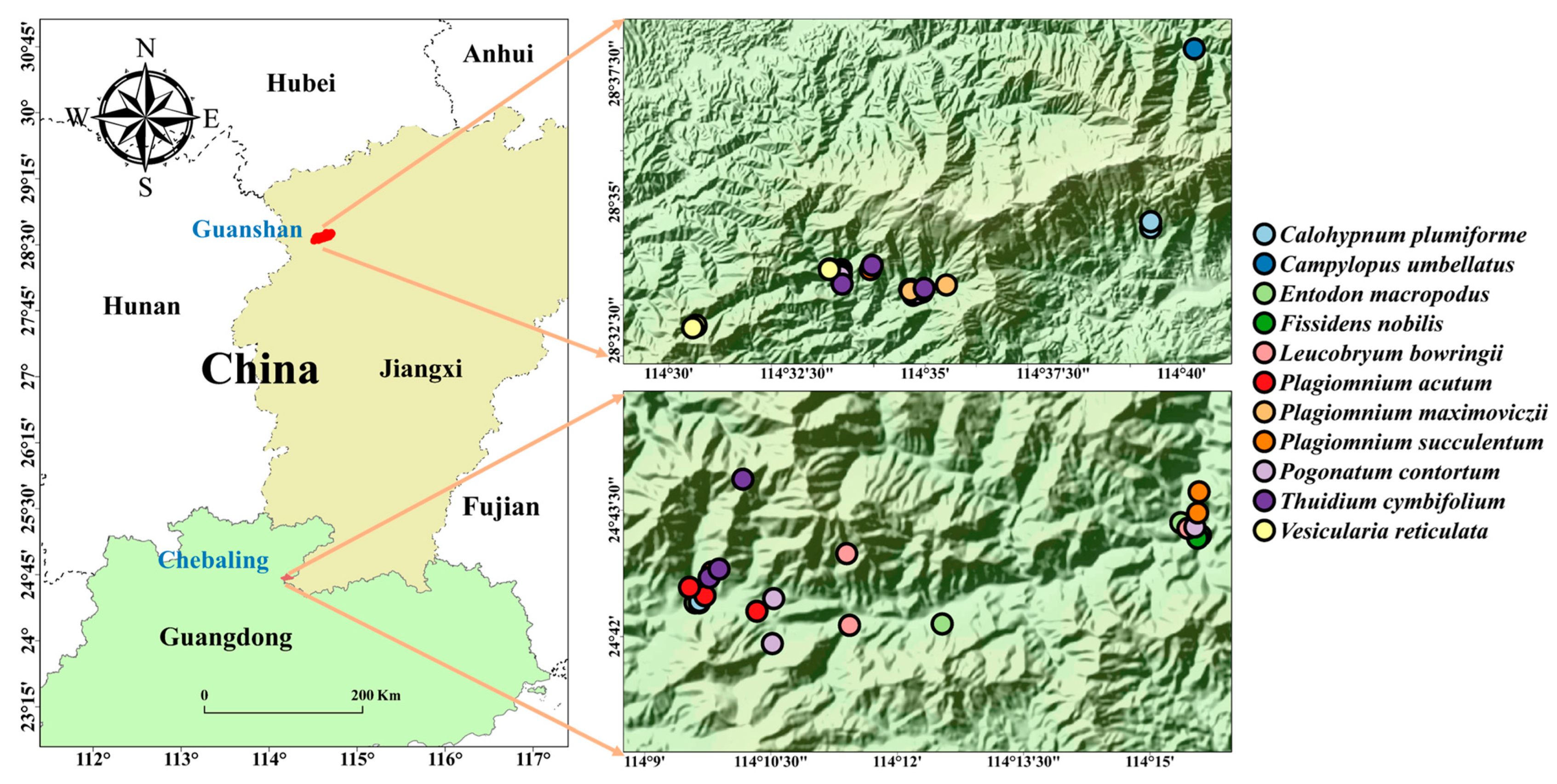


| Family | Species | Life-Form | Substrate | Region |
|---|---|---|---|---|
| Fissidentaceae | Fissidens nobilis | Fan | Soil/Rock | CBL/GS |
| Hypnaceae | Calohypnum plumiforme | Weft | Soil/Rock | CBL/GS |
| Vesicularia reticulata | Weft | Soil/Rock/Rotten wood | GS | |
| Leucobryaceae | Leucobryum bowringii | Cushion | Soil | CBL/GS |
| Campylopus umbellatus | Turf | Soil/Rock | GS | |
| Mniaceae | Plagiomnium acutum | Rough mat | Soil | CBL/GS |
| Plagiomnium succulentum | Rough mat | Rock/Rotten wood | CBL/GS | |
| Plagiomnium maximoviczii | Turf | Soil | GS | |
| Polytrichaceae | Pogonatum contortum | Turf | Soil | CBL/GS |
| Thuidiaceae | Thuidium cymbifolium | Weft | Soil/Rock | CBL/GS |
| Entodonaceae | Entodon macropodus | Weft | Rock/Rotten Wood | CBL |
| Traits | Code | Unit | Mean ± SD | CV |
|---|---|---|---|---|
| Photosynthesis progress | ||||
| Saturation irradiance | Is | µmol photons m−2 s−1 | 748.71 ± 247.57 | 0.33 |
| Compensation irradiance | Ic | µmol photons m−2 s−1 | 21.72 ± 13.45 | 0.62 |
| Light-saturated assimilation rate per mass | Amass | nmol CO2 g−1 s−1 | 21.01 ± 11.79 | 0.56 |
| Light-saturated assimilation rate per area | Aarea | μmol CO2 m−2 s−1 | 75.24 ± 44.34 | 0.69 |
| Light-saturated assimilation rate per chlorophyll | AChl | nmol CO2 g−1 s−1 | 2.86 ± 2.15 | 0.75 |
| Photosynthetic N use efficiency | AN | nmol CO2 g−1 s−1 | 1.14 ± 0.62 | 0.54 |
| Photosynthetic P use efficiency | AP | nmol CO2 g−1 s−1 | 16.9 ± 11.52 | 0.68 |
| Dark respiration per mass | Rdmass | nmol CO2 g−1 s−1 | 20.51 ± 9.60 | 0.47 |
| Dark respiration per area | Rdarea | μmol CO2 m−2 s−1 | 0.79 ± 0.47 | 0.60 |
| Dark respiration rate/maximum photosynthetic rate | Rd/A | Dimensionless | 1.55 ± 2.06 | 1.32 |
| Optimal photochemical efficiency of PSII | Fv/Fm | Dimensionless | 0.66 ± 0.03 | 0.05 |
| Actual photochemical efficiency of PSII | φPSII | Dimensionless | 0.39 ± 0.14 | 0.35 |
| Photochemical quenching | qP | Dimensionless | 0.44 ± 0.12 | 0.28 |
| Nonphotochemical quenching | NPQ | Dimensionless | 3.36 ± 1.56 | 0.46 |
| Chlorophyll per mass | Chlmass | mg g−1 | 8.36 ± 2.91 | 0.35 |
| Chlorophyll per area | Chlarea | g m−2 | 3.58 ± 1.63 | 0.47 |
| Water retention capacity | ||||
| Maximum water content | WCmax | % of d.w. | 432.16 ± 191.13 | 0.44 |
| Water loss decaying constant | DC | ×10−3 | 16.01 ± 7.82 | 0.48 |
| Nutrient content | ||||
| Organic carbon per mass | Cmass | mg g−1 | 377.57 ± 31.26 | 0.08 |
| Organic carbon per area | Carea | g m−2 | 14.87 ± 7.94 | 0.47 |
| Total nitrogen per mass | Nmass | mg g−1 | 18.82 ± 4.60 | 0.24 |
| Total nitrogen per area | Narea | g m−2 | 0.69 ± 0.28 | 0.40 |
| Total phosphorus per mass | Pmass | mg g−1 | 1.44 ± 0.60 | 0.41 |
| Total nitrogen per area | Parea | g m−2 | 0.05 ± 0.02 | 0.45 |
| C:N ratio | C/N | Dimensionless | 21.22 ± 5.23 | 0.25 |
| C:P ratio | C/P | Dimensionless | 313.67 ± 141.89 | 0.45 |
| N:P ratio | N/P | Dimensionless | 14.45 ± 4.13 | 0.28 |
| Architectural traits | ||||
| Costa length | CL | mm | 3.75 ± 2.70 | 0.72 |
| Costa width | CW | mm | 24.52 ± 21.51 | 0.88 |
| Leaf length | LL | mm | 4.12 ± 2.32 | 0.56 |
| Maximum leaf width | LW | mm | 1.29 ± 0.84 | 0.65 |
| Leaf area | LA | mm | 4.09 ± 4.47 | 1.09 |
| Shoot mass per area | SMA | g m−2 | 39.01 ± 17.88 | 0.46 |
| Cross-axis area of central strand | CAA | mm2 | 0.003 ± 0.002 | 0.79 |
| Cross-sectional area of shoot | CSA | mm2 | 0.08 ± 0.03 | 0.40 |
| Perimeter2⁄area | P2/A | Dimensionless | 52.29 ± 30.75 | 0.58 |
| Leaf length-to-width ratio | L/W | Dimensionless | 3.75 ± 2.38 | 0.63 |
| Cells per area | CPA | cells mm−2 | 666.70 ± 536.87 | 0.81 |
| Cell lumen length | CLL | µm | 41.98 ± 30/32 | 0.72 |
| Cell lumen width | CLW | µm | 12.83 ± 12.4 | 0.97 |
| Cell lumen area | CLA | µm2 | 582.44 ± 981.12 | 1.68 |
| Cell wall thickness | CWT | µm | 2.23 ± 0.80 | 0.36 |
| Models | Statistics | PC1 | PC2 | PC3 | PC4 | |
| Fixed | Estimate | Slope | −0.01 | −0.04 ** | −0.03 * | −0.02 |
| Canopy density | −1.62 * | 0.63 | 0.41 | −1.01 * | ||
| Water availability | −2.16 ** | 0.40 | −0.71 | −1.52 ** | ||
| R2 | Slope | 0.01 | 0.12 ** | 0.12 * | 0.05 | |
| Canopy density | 0.10 * | 0.02 | 0.01 | 0.09 | ||
| Water availability | 0.16 ** | 0.01 | 0.03 | 0.18 ** | ||
| Mixed (Species) | Estimate | Slope | 0.00 | −0.03 * | 0.00 | 0.00 |
| Canopy density | −0.10 | 0.44 | −0.13 | −0.00 | ||
| Water availability | −0.14 | −0.26 | 0.11 | −1.23 * | ||
| Marginal R2 | Slope | 0.00 | 0.09 * | 0.00 | 0.00 | |
| Canopy density | 0.00 | 0.01 | 0.00 | 0.00 | ||
| Water availability | 0.00 | 0.00 | 0.00 | 0.12 * | ||
| Conditional R2 | Slope | 0.938 *** | 0.30 * | 0.81 *** | 0.71 *** | |
| Canopy density | 0.935 *** | 0.33 ** | 0.81 *** | 0.72 *** | ||
| Water availability | 0.935 *** | 0.36 ** | 0.81 *** | 0.72 *** | ||
| Mixed (Species nested within region) | Estimate | Slope | −0.00 | −0.03 * | 0.01 | 0.00 |
| Canopy density | −0.14 | 0.79 | −0.14 | 0.04 | ||
| Water availability | −0.19 | −0.54 | 0.02 | −1.29 * | ||
| Marginal R2 | Slope | 0.00 | 0.10 * | 0.00 | 0.00 | |
| Canopy density | 0.00 | 0.04 | 0.00 | 0.00 | ||
| Water availability | 0.00 | 0.02 | 0.00 | 0.13 * | ||
| Conditional R2 | Slope | 0.95 *** | 0.56 *** | 0.85 *** | 0.73 *** | |
| Canopy density | 0.95 *** | 0.59 *** | 0.83 *** | 0.74 *** | ||
| Water availability | 0.95 *** | 0.60 *** | 0.83 *** | 0.83 *** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yi, L.; Zhou, X.; Xiong, Y.; Liu, J.; Qiu, H.; Liu, W. Multiple Dimensions of Functional Traits in Subtropical Montane Mosses. Forests 2024, 15, 587. https://doi.org/10.3390/f15040587
Liu Z, Yi L, Zhou X, Xiong Y, Liu J, Qiu H, Liu W. Multiple Dimensions of Functional Traits in Subtropical Montane Mosses. Forests. 2024; 15(4):587. https://doi.org/10.3390/f15040587
Chicago/Turabian StyleLiu, Zhiwei, Lingli Yi, Xiaohang Zhou, Yong Xiong, Jinhui Liu, Haiyan Qiu, and Weiqiu Liu. 2024. "Multiple Dimensions of Functional Traits in Subtropical Montane Mosses" Forests 15, no. 4: 587. https://doi.org/10.3390/f15040587





