The Sign and Strength of Plant-Soil Feedback for the Invasive Shrub, Lonicera maackii, Varies in Different Soils
Abstract
:1. Introduction
2. Methods
2.1. Soil Sources
2.2. Plant Species
2.3. Soil Conditioning
2.4. Feedback Experiment
2.5. Soil Chemical Properties
2.6. Community Level Physiological Profiles (CLPP) Using Biolog® EcoPlateTM
3. Results
3.1. Effects of Soil Type and Conditioning on Soil Properties
| Soil variables | Loamy soil | Sandy soil | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DL | FP | LM | UN | DL | FP | LM | UN | ||||
| pH | 7.9 | 7.8 | 8.0 | 7.4 | 7.0 | 6.9 | 7.1 | 6.1 | |||
| Organic matter (%) | 1.3 | 1.8 | 1.5 | 1.9 | 1.3 | 1.6 | 1.2 | 1.4 | |||
| Total N (%) | 0.2 | 0.55 | 0.14 | 0.21 | 0.37 | 0.29 | 0.24 | 0.40 | |||
| NH4 (ppm) | 20 | 8 | 7 | 1 | 7 | 9 | 4 | 8 | |||
| NO3 (ppm) | 7 | 9 | 8 | 43 | 5 | 48 | 6 | 65 | |||
| Available P (ppm) | 37 | 34 | 30 | 46 | 4 | 3 | 3 | 3 | |||
| Exchangeable K (ppm) | 104 | 103 | 84 | 163 | 44 | 64 | 48 | 62 | |||
| Exchangeable Mg (ppm) | 285 | 286 | 258 | 368 | 205 | 257 | 252 | 192 | |||
| Exchangeable Ca (ppm) | 3451 | 3549 | 3779 | 3010 | 1163 | 1559 | 1535 | 1010 | |||
| CEC | 15.3 | 15.6 | 16.2 | 14.3 | 6.9 | 9.2 | 8.5 | 5.3 | |||
| K (% BS) | 1.5 | 1.4 | 1.1 | 2.4 | 1.4 | 1.5 | 1.2 | 2.5 | |||
| Mg (% BS) | 13.7 | 13.4 | 11.6 | 18.8 | 21.9 | 20.5 | 21.8 | 26.4 | |||
| Ca (% BS) | 84.8 | 85.2 | 87.2 | 78.7 | 63.6 | 63.5 | 67.9 | 71.1 | |||
| Phenolics (mg g−1 soil) | 0.206 | 0.180 | 0.158 | 0.173 | 0.181 | 0.125 | 0.165 | 0.126 | |||
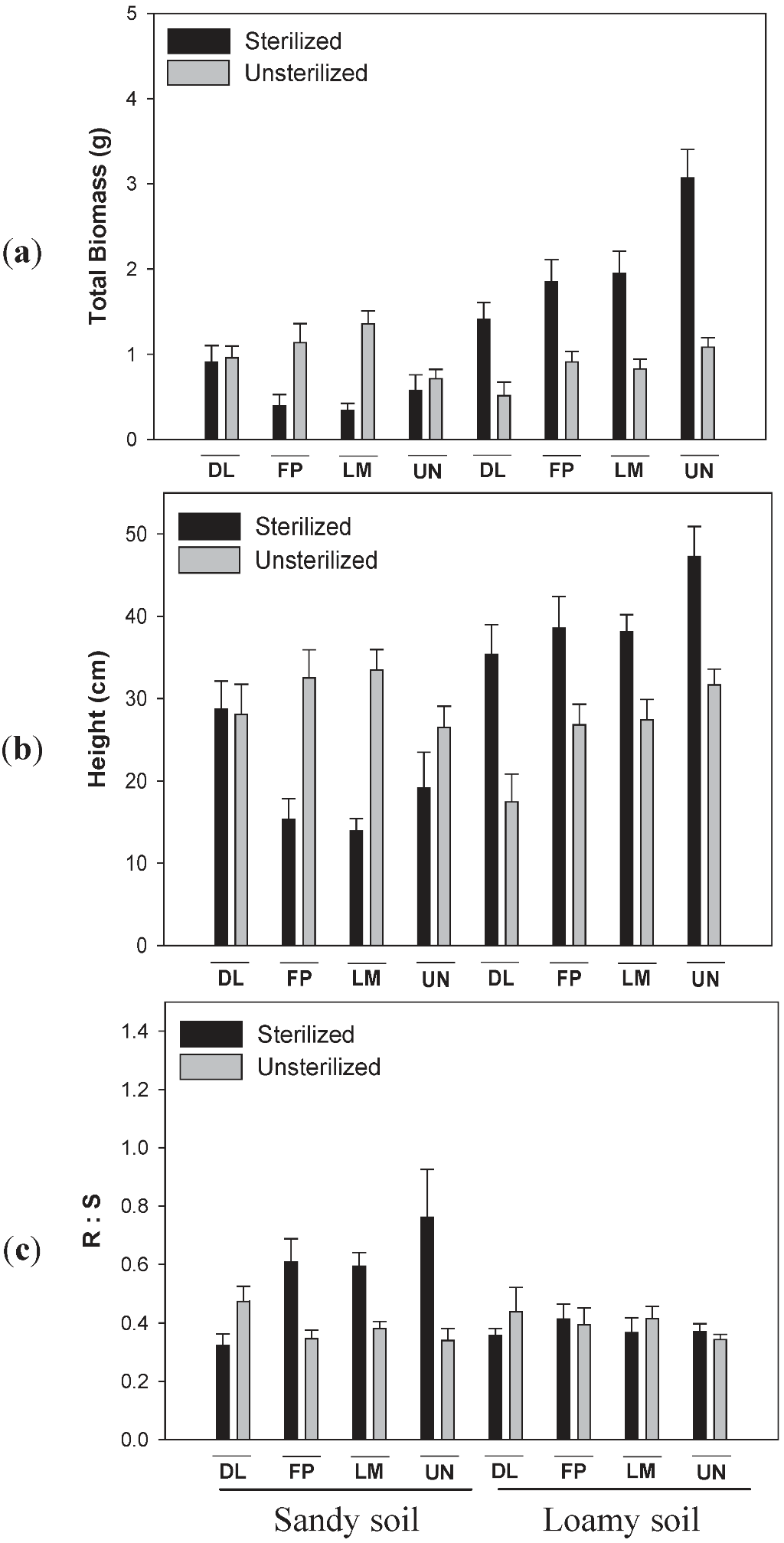
3.2. Effects of Soil Type and Conditioning on Biomass of Lonicera
| Factors | df | Total biomass | Height | Root/Shoot | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Soil Type (T) | 1 | 47.14 | <0.0001 | 23.89 | <0.0001 | 8.65 | 0.004 |
| Condition (C) | 3 | 1.81 | 0.1480 | 0.65 | 0.5874 | 0.58 | 0.6278 |
| T × C | 3 | 8.52 | <0.0001 | 6.19 | 0.0006 | 1.45 | 0.2316 |
| Sterilization (S) | 1 | 6.68 | 0.0101 | 0.08 | 0.7730 | 7.01 | 0.0093 |
| T × S | 1 | 87.05 | <0.0001 | 57.86 | <0.0001 | 11.00 | 0.0012 |
| C × S | 3 | 3.60 | 0.0150 | 5.69 | <0.0001 | 5.92 | 0.0009 |
| T × C × S | 3 | 1.57 | 0.2000 | 0.82 | 0.4873 | 2.76 | 0.0457 |
| Error | 112 | - | |||||
| Factors | df | Lonicera maackii | Diervilla lonicera | ||||
|---|---|---|---|---|---|---|---|
| W | F | p | W | F | p | ||
| Time | 6 | 0.047 | 354.94 | <0.0001 | 0.013 | 1252.34 | <0.0001 |
| Time × Soil Type (T) | 6 | 0.692 | 7.92 | <0.0001 | 0.701 | 2.13 | 0.0054 |
| Time × Condition (C) | 6 | 0.778 | 1.56 | 0.0701 | 0.701 | 2.13 | 0.0054 |
| Time × T × C | 18 | 0.816 | 1.25 | 0.2201 | 0.621 | 2.91 | <0.0001 |
| Time × Sterilization (S) | 6 | 0.717 | 7.01 | <0.0001 | 0.451 | 20.47 | <0.0001 |
| Time × T × S | 6 | 0.666 | 8.92 | <0.0001 | 0.810 | 3.94 | 0.0014 |
| Time × C × S | 18 | 0.729 | 1.99 | 0.0101 | 0.548 | 3.76 | <0.0001 |
| Time × T × C × S | 18 | 0.807 | 1.32 | 0.1739 | 0.831 | 1.07 | 0.3819 |
| Error | 106 | - | |||||
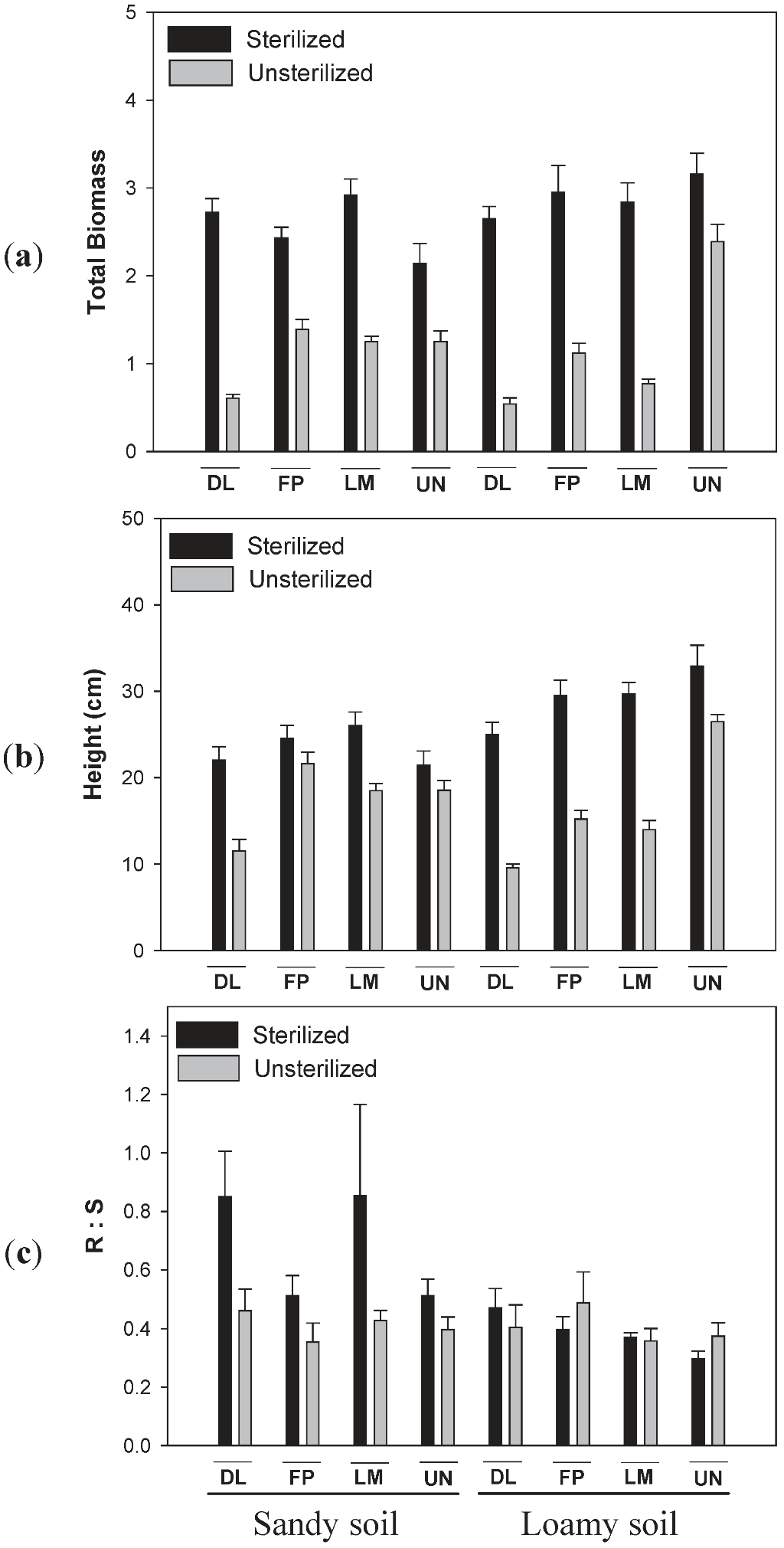
3.3. Effects of Soil Type and Conditioning on Biomass of Diervilla lonicera
| Factors | df | Total biomass | Height | Root/Shoot | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Soil Type (T) | 1 | 2.76 | 0.0998 | 2.99 | 0.0867 | 10.55 | 0.0016 |
| Condition (C) | 3 | 15.73 | <0.0001 | 32.69 | <0.0001 | 2.05 | 0.1112 |
| T × C | 3 | 15.58 | <0.0001 | 13.74 | <0.0001 | 1.52 | 0.2127 |
| Sterilization (S) | 1 | 426.27 | <0.0001 | 222.11 | <0.0001 | 6.59 | 0.0117 |
| T × S | 1 | 3.02 | 0.0854 | 26.75 | <0.0001 | 9.01 | 0.0033 |
| C × S | 3 | 20.87 | <0.0001 | 16.52 | <0.0001 | 1.18 | 0.3197 |
| T × C × S | 3 | 2.53 | 0.0614 | 2.59 | 0.0564 | 0.04 | 0.9881 |
| Error | 106 | - | |||||
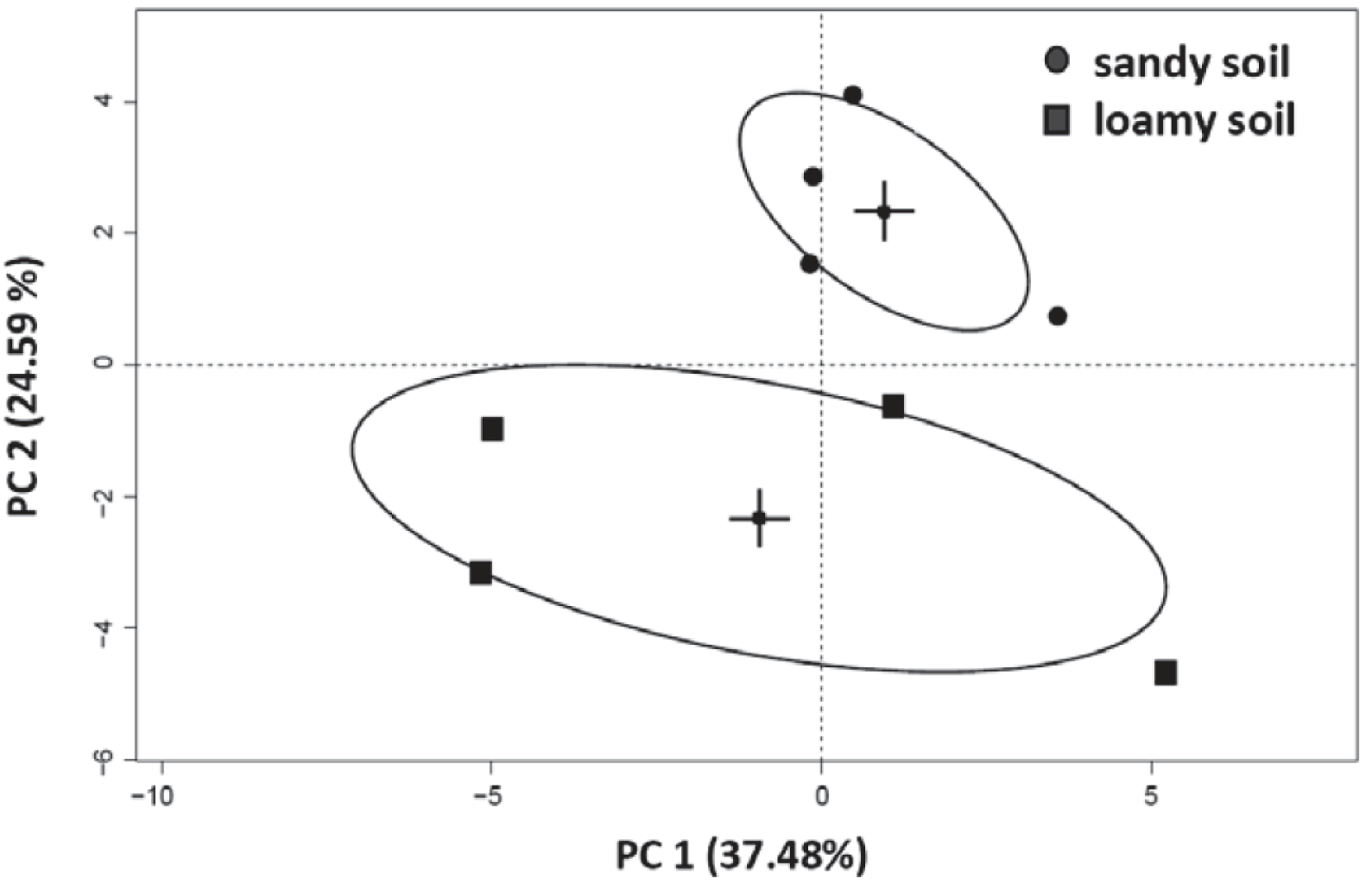
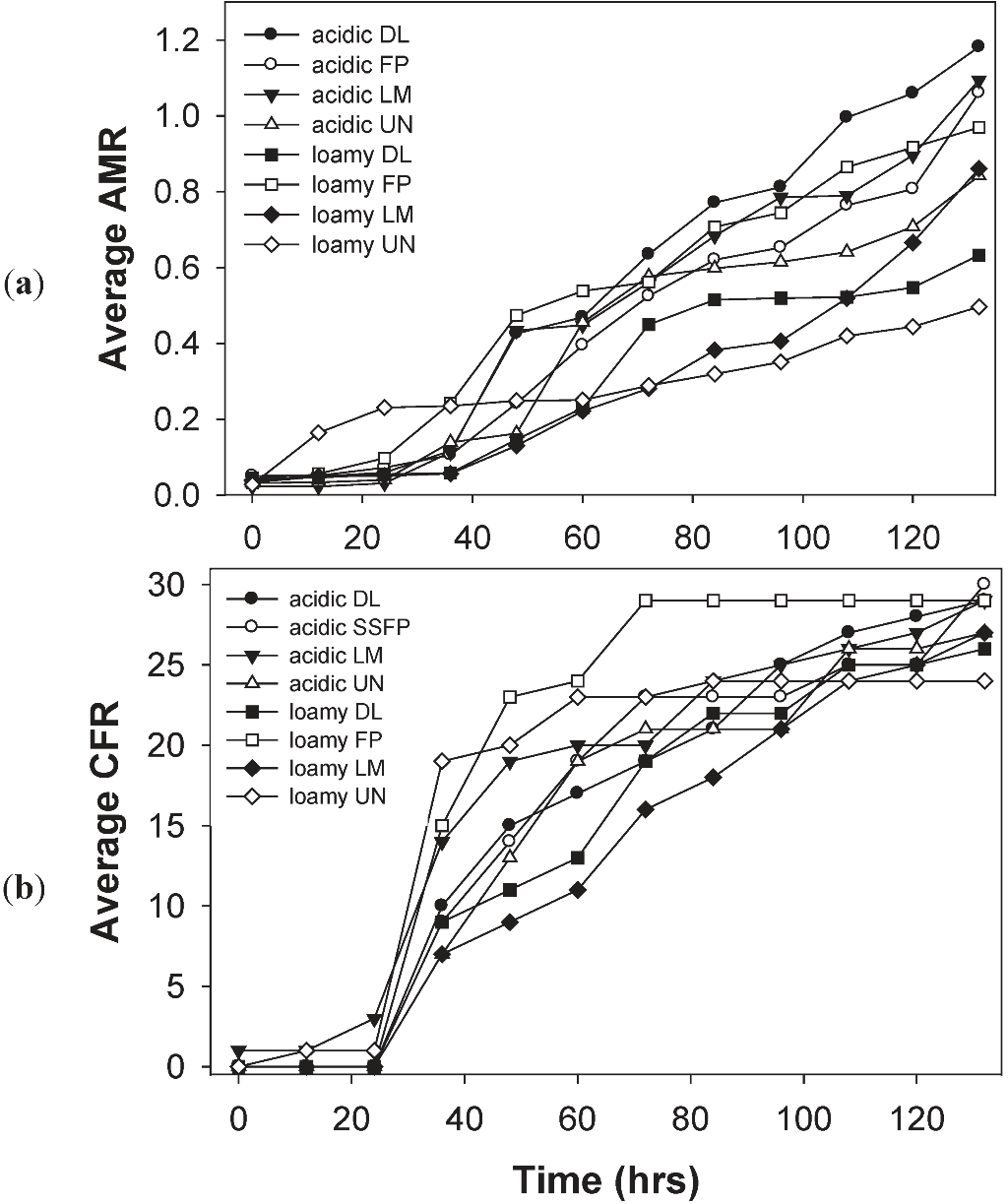
3.4. Effects of Soil Type and Conditioning on the Soil Microbial Community
4. Conclusions
4.1. Effects of Soil Type and Sterilization on Plant-Soil Feedbacks
4.2. Effects of Soil Type and Sterilization on Root/Shoot Ratios
4.3. Conspecific versus Heterospecific Feedbacks and Allelopathy
5. Future Research
Acknowledgments
Conflict of Interest
References
- Klironomos, J. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 2002, 417, 67–70. [Google Scholar] [CrossRef]
- Agrawal, A. Enemy release? An experiment with congeneric plant pairs and diverse above- and below-ground enemies. Ecology 2005, 86, 2979–2989. [Google Scholar] [CrossRef]
- Hawkes, C.; Wren, I.; Herman, D.; Fireston, M. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol. Lett. 2005, 8, 976. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. A potential source of information for screening and monitoring the impact of exotic plants on ecosystems. Biol. Invasions 2006, 8, 1511–1521. [Google Scholar] [CrossRef]
- Stinson, K.; Campbell, S.; Powell, J.; Wolfe, B.; Callaway, R.; Thelen, G.; Hallett, S.; Prata, D.; Klironimos, J. Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol. 2006, 4, e140:1–e140:5. [Google Scholar]
- Cipollini, K.; McClain, G.; Cipollini, D. Separating above- and belowground effects of Alliaria petiolata and Lonicera maackii on the performance of Impatiens capensis. Am. Midl. Nat. 2008, 160, 117–128. [Google Scholar] [CrossRef]
- Cipollini, K.; Schradin, K. Guilty in the court of public opinion: Testing presumptive impacts and allelopathic potential of Ranunculus ficaria. Am. Midl. Nat. 2002, 166, 63–74. [Google Scholar] [CrossRef]
- Callaway, R.; Aschehoug, E. Invasive plant versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef]
- Callaway, R.; Thelen, G.; Rodriguez, A.; Holben, W. Soil biota and exotic plant invasion. Nature 2004, 427, 731–733. [Google Scholar] [CrossRef]
- Mangan, S.A. Specificity between 91 Neotropical tree seedlings and their fungal mutualists leads to plant-soil feedback. Ecology 2010, 91, 2594–2603. [Google Scholar] [CrossRef]
- Kueffer, C.; Schumacher, E.; Fleishmann, K.; Edwards, P.; Dietz, H. Strong below-ground competition shapes tree regeneration in invasive Cinnamomum verum forests. J. Ecol. 2007, 95, 273–282. [Google Scholar] [CrossRef]
- Mangan, S.; Schnitzer, A.; Herre, E.; Keenan, M.; Valencia, M.; Sanchez, E.; Bever, J. Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 2010, 466, 752–755. [Google Scholar] [CrossRef]
- Beckstead, J.; Parker, I. Invasiveness of Ammophila arenaria: Release from soil-borne pathogens? J. Ecol. 2003, 84, 2824–2831. [Google Scholar] [CrossRef]
- Reinhart, K.; Callaway, R. Soil biota facilitate exotic Acer invasions in Europe and North America. Ecol. Appl. 2004, 14, 1737–1745. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Effects of exotic plant invasions onsoil nutrient cycling processes. Ecosystems 2003, 6, 503–523. [Google Scholar] [CrossRef]
- Rodgers, V.; Wolfe, B.; Werden, L.; Finzi, A. The invasive species Alliaria petiolata (garlic mustard) increases soil nutrient availability in northern hardwood-conifer forests. Oecologia 2008, 157, 459–471. [Google Scholar] [CrossRef]
- Cipollini, D.; Dorning, M. Direct and indirect effects of conditioned soils and tissue extracts of the invasive shrub, Lonicera maackii, on plant performance. Castanea 2008, 73, 166–176. [Google Scholar] [CrossRef]
- Cipollini, D.; Stevenson, R.; Cipollini, K. Contrasting direct and indirect effects of allelochemicals from two invasive plants on the performance of a non-mycorrhizal plant. Int. J. Plant Sci. 2008, 169, 371–375. [Google Scholar] [CrossRef]
- Inderjit. Soil microorganisms: An important determinant of allelopathic activity. Plant Soil 2005, 274, 227–236. [CrossRef]
- Kulmatiski, A.; Beard, K.; Stevens, J.; Cobbold, S. Plant-soil feedbacks: A meta-analytical review. Ecol. Lett. 2006, 11, 980–992. [Google Scholar]
- Dakshini, K.M.M. Inderjit. Allelopathic effect of Pluchea lanceolata (Asteraceae) on characteristics of four soils and tomato and mustard growth. Am. J. Bot. 1994, 81, 799–804. [Google Scholar] [CrossRef]
- Callaway, R.M.; Cipollini, D.; Barto, K.; Thelen, G.; Hallett, S.; Prati, D.; Stinson, K.; Klironomos, J. Novel Weapons: Invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 2008, 89, 1043–1055. [Google Scholar] [CrossRef]
- Pollock, J.; Kogan, L.; Thorpe, A.S.; Holben, W. (±)-Catechin, a root exudate of the invasive Centaurea Stoebe Lam. (spotted knapweed) exhibits bacteriostatic activity against multiple soil bacterial populations. J. Chem. Ecol. 2011, 37, 1044–1053. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Dorning, M.; Cipollini, D. Leaf extracts of the invasive shrub, Lonicera maackii inhibit seed germination of three herbs with no autotoxic effects. Plant Ecol. 2006, 184, 287–296. [Google Scholar] [CrossRef]
- Cipollini, D.; Stevenson, R.; Enright, S.; Eyles, A.; Bonello, P. Phenolic metabolites in leaves of the invasive shrub, Lonicera maackii, and their potential phytotoxic and anti-herbivore effects. J. Chem. Ecol. 2008, 34, 144–152. [Google Scholar] [CrossRef]
- Hudson, B.D. Soil organic matter and available water capacity. J. Soil Water Conserv. 1994, 49, 189–194. [Google Scholar]
- Hutchinson, T.F.; Vankat, J.L. Invasibility and effect of Amur honeysuckle in southwestern Ohio. Conserv. Biol. 1997, 11, 1117–1124. [Google Scholar]
- Collier, M.H.; Vankat, J.L.; Hughes, M.R. Diminished plant richness and abundance below Lonicera maackii, an invasive shrub. Am. Midl. Nat. 2011, 147, 60–71. [Google Scholar]
- US Department of Agriculture, the PLANTS Database. Available online: http://plants.usda.gov (accessed on 8 October 2012).
- Gee, G.; Bauder, J. Particle-size analysis. In Methods of Soil Analysis. Part 1, 2nd; Klute, A.S., Ed.; ASA (American Society of Agronomy) and SSSA (Soil Science Society of America): Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Ohio Department of Natural Resources, Division of Soil and Water Resources–Soil Regions. Available online: http://www.dnr.state.oh.us/ (accessed on 8 October 2012).
- US Department of Agriculture Soil Conservation Service, Soil Survey of Scioto County, Ohio. Available online: http://soildatamart.nrcs.usda.gov/ (accessed on 8 October 2012).
- Jacobs, B. Evolution of fruit and seed characters in the Diervilla and Lonicera clades (Caprifoliaceae, Dipsacales). Ann. Bot. 2009, 104, 253–276. [Google Scholar] [CrossRef]
- Trevors, J. Sterilization and inhibition of microbial activity in soil. J. Microbiol. Method 1996, 26, 53–59. [Google Scholar] [CrossRef]
- Wolf, D.C.; Dao, T.H.; Scott, H.D.; Lavy, T.L. Influence of sterilization methods on selected soil microbiological, physical and chemical properties. J. Environ. Qual. 1989, 18, 39–44. [Google Scholar]
- Hidayati, S.; Baskin, J.; Baskin, C. Dormancy-breaking and germination requirements for seeds of four Lonicera species (Caprifoliaceae) with underdeveloped spatulate embryos. Seed Sci. Res. 2000, 10, 459–469. [Google Scholar]
- Scharfy, D. Invasion of Solidago gigantea in contrasting experimental plant communities: Effects on soil microbes, nutrients and plant-soil feedbacks. J. Ecol. 2010, 98, 1379–1388. [Google Scholar] [CrossRef]
- Stefanowicz, A. The Biolog Plates technique as a tool in ecological studies of microbial communities. Pol. J. Environ. Stud. 2006, 15, 669–676. [Google Scholar]
- Hitzl, W.; Ranggera, A.; Sharma, S.; Insam, H. Separation power of 95 substrates of the biology system determined in various soils. FEMS Microbiol. Ecol. 1997, 22, 167–174. [Google Scholar] [CrossRef]
- McCewan, R.; Arthur, M.; Alverson, S. Throughfall chemistry and soil nutrient effects of the invasive shrub Lonicera maackii in deciduous forests. Am. Midl. Nat. 2012, 168, 43–55. [Google Scholar]
- Kardol, P.; Bezemer, T.; van der Putten, W. Temporal variation in plant-soil feedback controls succession. Ecol. Lett. 2006, 9, 1080–1088. [Google Scholar] [CrossRef]
- Troelstra, S.; Wagenaar, R.; Smant, W.; Peters, B. Interpretation of bioassays in the study of interactions between soil organisms and plants: Involvement of nutrient factors. New Phytol. 2001, 150, 697–706. [Google Scholar] [CrossRef]
- Beest, M.; Stevens, N.; Olff, H.; van der Putten, W.H. Plant-soil feedback induces shifts in biomass allocation in the invasive plant Chromolaena odorata. J. Ecol. 2009, 97, 1281–1290. [Google Scholar] [CrossRef]
- Luken, J.O.; Kuddes, L.M.; Tholemeier, T.C.; Haller, D.M. Comparative responses of Lonicera maackii (Amur Honeysuckle) and Lindera benzoin (Spicebush) to increased light. Am. Midl. Nat. 1997, 138, 331–343. [Google Scholar] [CrossRef]
- Luken, J.O. Population structure and biomass allocation of naturalized shrub Lonicera maackii (Rupr.) Maxim. in forest and open habitats. Am. Midl. Nat. 1988, 119, 258–267. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef]
- Flamini, G.; Braca, A.; Cioni, P.J.; Morelli, I. Three new flavonoids and other constituents from Lonicera implexa. J. Nat. Prod. 1997, 60, 449–452. [Google Scholar] [CrossRef]
- Daskalaki, D.; Kefi, G.; Kotsiou, K.; Tasioula-Margari, M. Evaluation of phenolic compounds degradation in virgin olive oil during storage and heating. J. Food Nutr. 2009, 48, 31–41. [Google Scholar]
- Cipollini, D.; Wang, Q.; Whitehill, J.G.A.; Powell, J.; Bonello, P.; Herms, D. Distinguishing defensive characteristics in the phloem of ash species resistant and susceptible to the Emerald Ash Borer. J. Chem. Ecol. 2011, 37, 450–459. [Google Scholar] [CrossRef]
- Csiszár, Á. Allelopathic effects of invasive woody plant species in Hungary. Acta Silv. Lignaria Hung. 2009, 5, 9–17. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schradin, K.; Cipollini, D. The Sign and Strength of Plant-Soil Feedback for the Invasive Shrub, Lonicera maackii, Varies in Different Soils. Forests 2012, 3, 903-922. https://doi.org/10.3390/f3040903
Schradin K, Cipollini D. The Sign and Strength of Plant-Soil Feedback for the Invasive Shrub, Lonicera maackii, Varies in Different Soils. Forests. 2012; 3(4):903-922. https://doi.org/10.3390/f3040903
Chicago/Turabian StyleSchradin, Kelly, and Don Cipollini. 2012. "The Sign and Strength of Plant-Soil Feedback for the Invasive Shrub, Lonicera maackii, Varies in Different Soils" Forests 3, no. 4: 903-922. https://doi.org/10.3390/f3040903




