How Biotic Differentiation of Human Impacted Nutrient Poor Deciduous Forests Can Affect the Preservation Status of Mountain Forest Vegetation
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Site and Data Collection
2.2. Data Analysis
3. Results
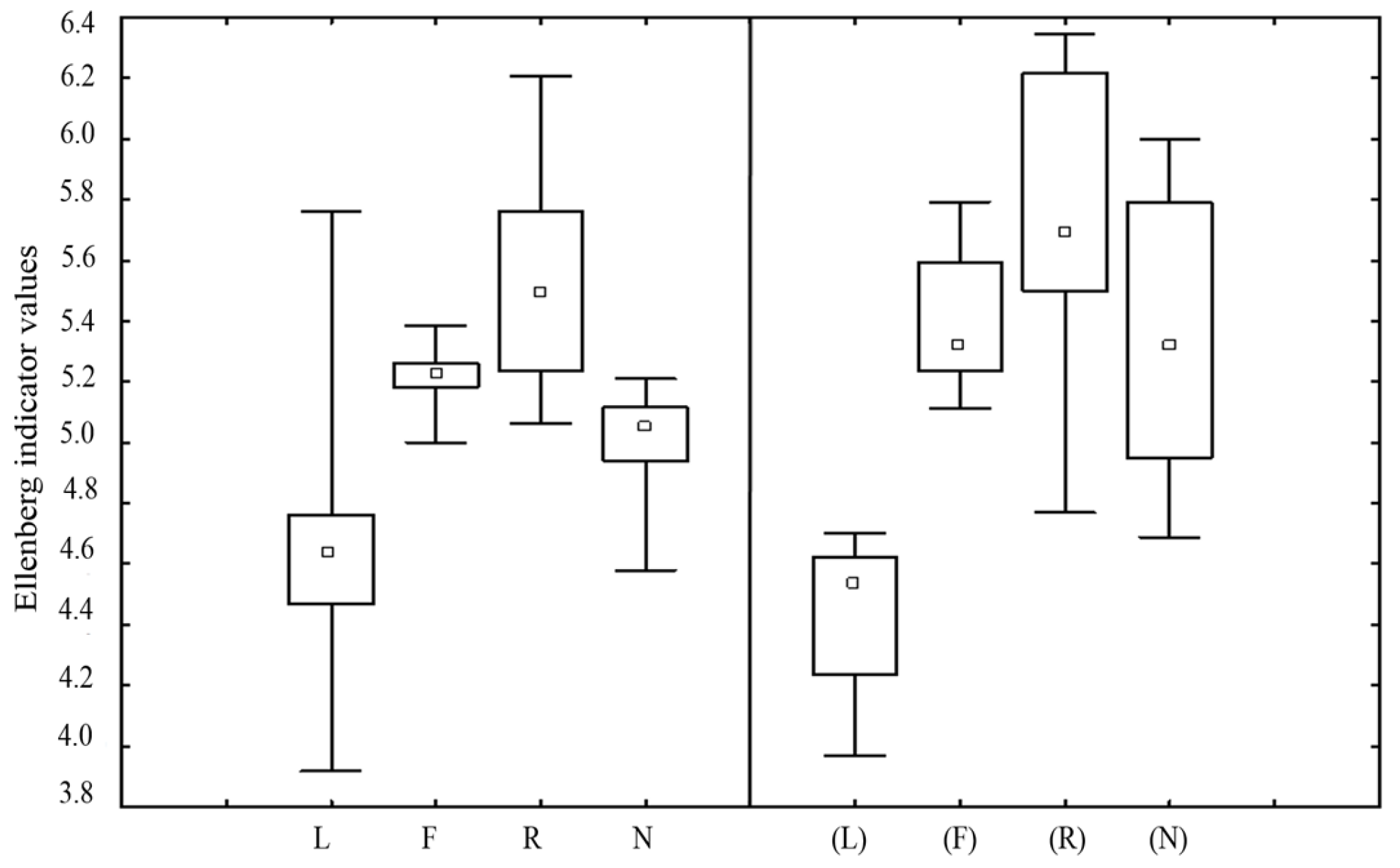
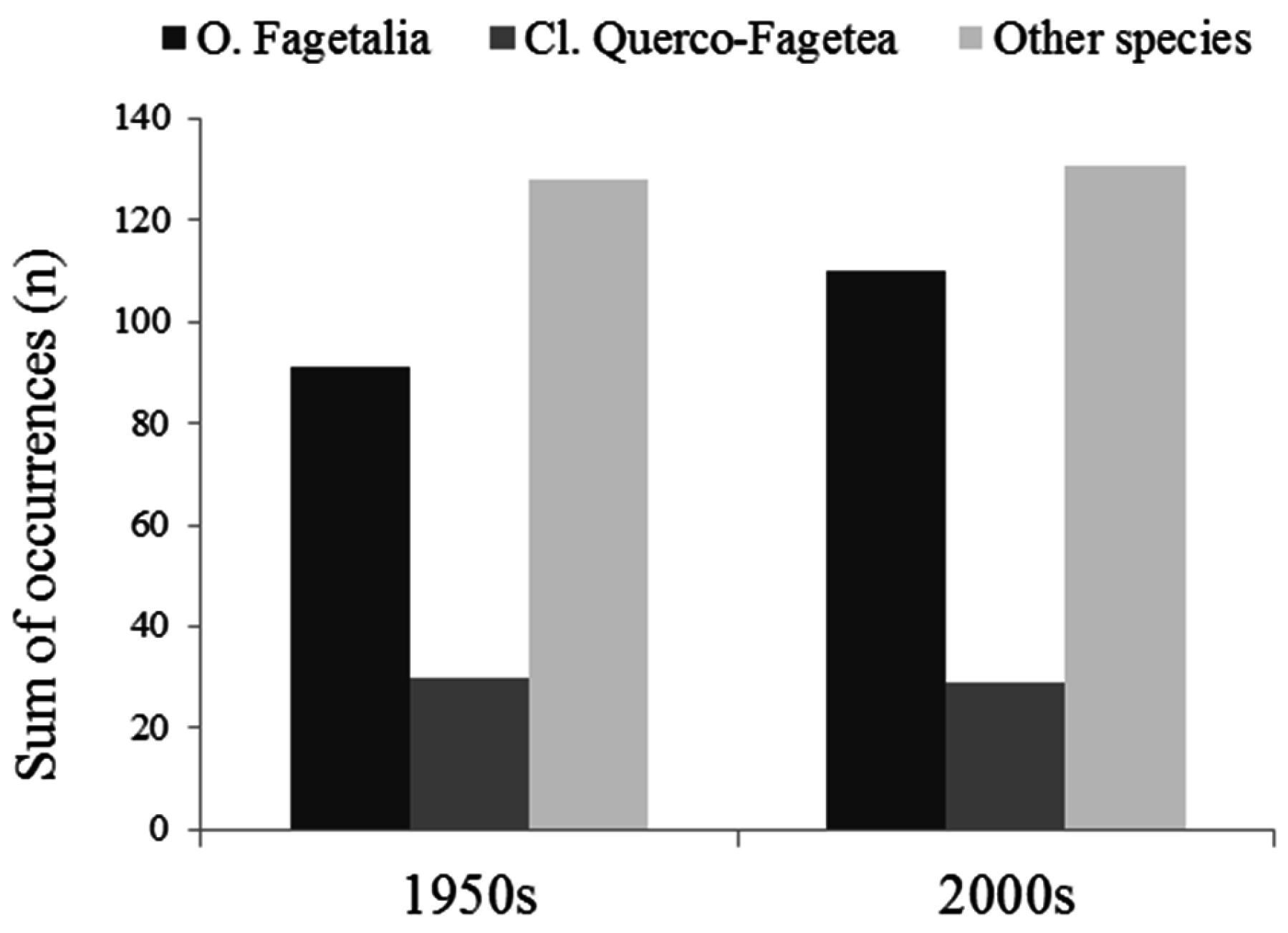
3.1. Changes in Species Abundance and Frequency
3.2. Changes in the Species Composition
3.3. Changes in the Differences between Oak-Hornbeam Forests and Beech Forests
4. Discussion
4.1. Changes in Variability of Species Composition
4.2. Why Did the Increase in Fertility not Lower the Diversity?
4.3. Changes in Distinctiveness between Oak-Hornbeam and Beech Forests
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Chapin, F.S., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.-S.; Nakashizuka, T.; Raffaelli, D.; Schmid, B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human domination of earth’s ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef]
- Sax, D.F.; Gaines, S.D. Species diversity: From global decreases to local increases. Trends Ecol. Evol. 2003, 18, 561–566. [Google Scholar] [CrossRef]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; Van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Olden, J.D.; Rooney, T.P. On defining and quantifying biotic homogenization. Global Ecol. Biogeogr. 2006, 15, 113–120. [Google Scholar] [CrossRef]
- Smart, S.M.; Thompson, K.; Marrs, R.H.; Le Duc, M.G.; Maskell, L.C.; Firbank, L.G. Biotic homogenization and changes in species diversity across human-modified ecosystems. Proc. R. Soc. B 2006, 273, 2659–2665. [Google Scholar] [CrossRef] [PubMed]
- Naaf, T.; Wulf, M. Habitat specialists and generalists drive homogenization and differentiation of temperate forest plant communities at the regional scale. Biol. Conserv. 2010, 143, 848–855. [Google Scholar] [CrossRef]
- Keith, S.A.; Newton, A.C.; Morecroft, M.D.; Bealey, C.E.; Bullock, J.M. Taxonomic homogenization of woodland plant communities over 70 years. Proc. R. Soc. B 2009, 276, 3539–3544. [Google Scholar] [CrossRef] [PubMed]
- Durak, T.; Durak, R. Vegetation changes in meso- and eutrophic submontane oak–hornbeam forests under long-term high forest management. For. Ecol. Manag. 2015, 354, 206–216. [Google Scholar] [CrossRef]
- Ledig, F.T. Human impacts on genetic diversity in forest ecosystems. Oikos 1992, 63, 87–108. [Google Scholar] [CrossRef]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of European forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- Morris, R.J. Anthropogenic impacts on tropical forest biodiversity: A network tructure and ecosystem functioning perspective. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3709–3718. [Google Scholar] [CrossRef] [PubMed]
- Glatzel, G. The Impact of historic land use and modern forestry on nutrient relations of Central European forest ecosystems. Fertil. Res. 1991, 27, 1–8. [Google Scholar] [CrossRef]
- Bürgi, M.; Gimmi, U. Three objectives of historical ecology: The case of litter collecting in Central European forests. Landsc. Ecol. 2007, 22, 77–87. [Google Scholar] [CrossRef]
- Paudel, S.; Sah, J.P. Effects of different management practices on stand composition and species diversity in subtropical forests in Nepal: Implications of community participation in biodiversity conservation. J. Sustain. For. 2015, 34, 738–760. [Google Scholar] [CrossRef]
- Fleischner, T.L. Ecological costs of livestock grazing in western North America. Conserv. Biol. 1994, 8, 629–644. [Google Scholar] [CrossRef]
- Foster, D.R.; Motzkin, G.; Slater, B. Land-use history as long-term broad-scale disturbance: Regional forest dynamics in Central New England. Ecosystems 1998, 1, 96–119. [Google Scholar] [CrossRef]
- Verheyen, K.; Baeten, L.; De Frenne, P.; Bernhardt-Römermann, M.; Brunet, J.; Cornelis, J.; Decocq, G.; Dierschke, H.; Eriksson, O.; Hédl, R.; Heinken, T.; et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. J. Ecol. 2012, 100, 352–365. [Google Scholar] [CrossRef]
- Durak, T.; Holeksa, J. Biotic homogenisation and differentiation along a habitat gradient resulting from the ageing of managed beech stands. For. Ecol. Manag. 2015, 351, 47–56. [Google Scholar] [CrossRef]
- Rooney, T.P.; Olden, J.D.; Leach, M.K.; Rogers, D.A. Biotic homogenization and conservation prioritization. Biol. Conserv. 2007, 134, 447–450. [Google Scholar] [CrossRef]
- Bohn, U.; Gollub, G.; Hettwer, C.; Neuhäuslová, Z.; Raus, Th.; Schlüter, H.; Weber, H. Karte Der Natürlichen Vegetation Europas. Map of the Natural Vegetation of Europe, Maßstab/Scale 1:2.500.000, Interaktive/Interactive CD-ROM - Erläuterungstext, Legende, Karten / Explanatory Text, Legend, Maps; Landwirtschaftsverlag: Münster, Germany, 2004. [Google Scholar]
- Matuszkiewicz, W. Przewodnik Do Oznaczania Zbiorowisk Roślinnych Polski; Scientific Publishers PWN: Warszawa, Poland, 2001. [Google Scholar]
- Adamczyk, B.; Zarzycki, K. Gleby bieszczadzkich zbiorowisk leśnych. Acta Agrar. Silv. Ser. Silv. 1963, 3, 133–175. [Google Scholar]
- Kubijowicz, W. Życie Pasterskie W Beskidach Wschodnich; Prace Instytutu Geograficznego UJ 5: Kraków, Poland, 1926. [Google Scholar]
- Connell, J.H. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Skiba, S.; Drewnik, M. Soil Map of the Polish Carpathian Mountains. Rocz. Bieszcz. 2003, 11, 15–20. [Google Scholar]
- Nowosad, M. Outlines of climate of the Bieszczady National Park and its buffer zone in the light of previous studies. Rocz. Bieszcz. 1995, 4, 163–183. [Google Scholar]
- Zarzycki, K. Lasy Bieszczadów Zachodnich. Acta Agrar. Silv. Ser. Silv. 1963, 3, 1–132. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge Der Vegetationskunde; Springer-Verlag: Vienna, Austria; New York, NY, USA, 1964. [Google Scholar]
- Fischer, M.; Stöcklin, J. Local extinctions of plants in remnants of extensively used calcareous grasslands 1950–1985. Conserv. Biol. 1997, 11, 727–737. [Google Scholar] [CrossRef]
- Durak, T. Changes in vegetation of fertile Carpathian beech forests with perennial honesty Lunaria rediviva based on analysis of the herb layer (Słonne Mountains, Eastern Carpathians). Sylwan 2011, 155, 120–128. [Google Scholar]
- Kopecký, M.; Macek, M. Vegetation resurvey is robust to plot location uncertainty. Divers. Distrib. 2015, 21, 322–330. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 2001, 26, 32–46. [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulissen, D. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot. 1992, 18, 1–248. [Google Scholar]
- Van Calster, H.; Baeten, L.; De Schrijver, A.; De Keersmaeker, L.; Register, J.E.; Verheyen, K.; Hermy, M. Management driven changes (1967–2005) in soil acidity and the understorey plant community following conversion of a coppice-with-standards forest. For. Ecol. Manag. 2007, 241, 258–271. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 2005, 80, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Rooney, T.P.; Waller, D.M. Direct and indirect effects of white-tailed deer in forest ecosystems. For. Ecol. Manag. 2003, 181, 165–176. [Google Scholar] [CrossRef]
- Decocq, G.; Aubert, M.; Dupont, F.; Bardat, J.; Wattez-Franger, A.; Saguez, R.; De Foucault, B.; Alard, D.; Delelis-Dusollier, A. Silviculture-driven vegetation change in a European temperate deciduous forest. Ann. For. Sci. 2005, 62, 313–323. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed]
- Duprè, C.; Stevens, C.J.; Ranke, T.; Bleeker, A.; Peppler-Lisbach, C.; Gowing, D.J.G.; Dise, N.B.; Dorland, E.; Bobbink, R.; Diekmann, M. Changes in species richness and composition in European acidic grasslands over the past 70 years: The contribution of cumulative atmospheric nitrogen deposition. Glob. Chang. Biol. 2010, 16, 344–357. [Google Scholar] [CrossRef]
- Gilliam, F.S. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J. Ecol. 2006, 94, 1176–1191. [Google Scholar] [CrossRef]
- Grodzińska, K.; Szarek-Łukaszewska, G. Polish mountain forests: past, present and future. Environ. Pollut. 1997, 98, 369–374. [Google Scholar] [CrossRef]
- Główny Inspektorat Ochrony Środowiska (Chief Inspectorate of Environmental Protection). Available online: http://www.gios.gov.pl/chemizm2010/index.html (accessed on 5 June 2016).
- Dzwonko, Z.; Gawronski, S. Effect of litter removal on species richness and acidification of a mixed oak-pine woodland. Biol. Conserv. 2002, 106, 389–398. [Google Scholar] [CrossRef]
- Vild, O.; Kalwij, J.M.; Hédl, R. Effects of simulated historical tree litter raking on the understorey vegetation in a central European forest. Appl. Veg. Sci. 2015, 18, 569–578. [Google Scholar] [CrossRef]
- Augusto, L.; Jacques, R.; Binkley, D.; Andreas, R. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar] [CrossRef]
- Jacob, M.; Weland, N.; Platner, C.; Schaefer, M.; Leuschner, C.; Thomas, F.M. Nutrient release from decomposing leaf litter of temperate deciduous forest trees along a gradient of increasing tree species diversity. Soil Biol. Biochem. 2009, 41, 2122–2130. [Google Scholar] [CrossRef]
- Grodzińska, K.; Godzik, B.; Frączek, W.; Badea, O.; Oszlányi, J.; Postelnicu, D.; Shparyk, Y. Vegetation of the selected forest stands and land use in the Carpathian Mountains. Environ. Pollut. 2004, 130, 17–32. [Google Scholar] [CrossRef] [PubMed]


| Axis 1 | Axis 2 | |||
|---|---|---|---|---|
| Spearman's rho correlation coefficients | p | Spearman's rho correlation coefficients | p | |
| LL | 0.81 | <0.0001 | 0.07 | 0.775 |
| LH | 0.19 | 0.446 | −0.38 | 0.117 |
| FL | −0.21 | 0.409 | −0.49 | 0.039 |
| FH | 0.04 | 0.867 | 0.03 | 0.920 |
| RL | −0.42 | 0.087 | -0.58 | 0.010 |
| RH | 0.74 | <0.001 | 0.24 | 0.332 |
| NL | 0.15 | 0.557 | −0.55 | 0.017 |
| NH | 0.48 | 0.042 | 0.22 | 0.374 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durak, T.; Durak, R. How Biotic Differentiation of Human Impacted Nutrient Poor Deciduous Forests Can Affect the Preservation Status of Mountain Forest Vegetation. Forests 2016, 7, 241. https://doi.org/10.3390/f7100241
Durak T, Durak R. How Biotic Differentiation of Human Impacted Nutrient Poor Deciduous Forests Can Affect the Preservation Status of Mountain Forest Vegetation. Forests. 2016; 7(10):241. https://doi.org/10.3390/f7100241
Chicago/Turabian StyleDurak, Tomasz, and Roma Durak. 2016. "How Biotic Differentiation of Human Impacted Nutrient Poor Deciduous Forests Can Affect the Preservation Status of Mountain Forest Vegetation" Forests 7, no. 10: 241. https://doi.org/10.3390/f7100241
APA StyleDurak, T., & Durak, R. (2016). How Biotic Differentiation of Human Impacted Nutrient Poor Deciduous Forests Can Affect the Preservation Status of Mountain Forest Vegetation. Forests, 7(10), 241. https://doi.org/10.3390/f7100241






