Modeling the Boundaries of Plant Ecotones of Mountain Ecosystems
Abstract
:1. Introduction
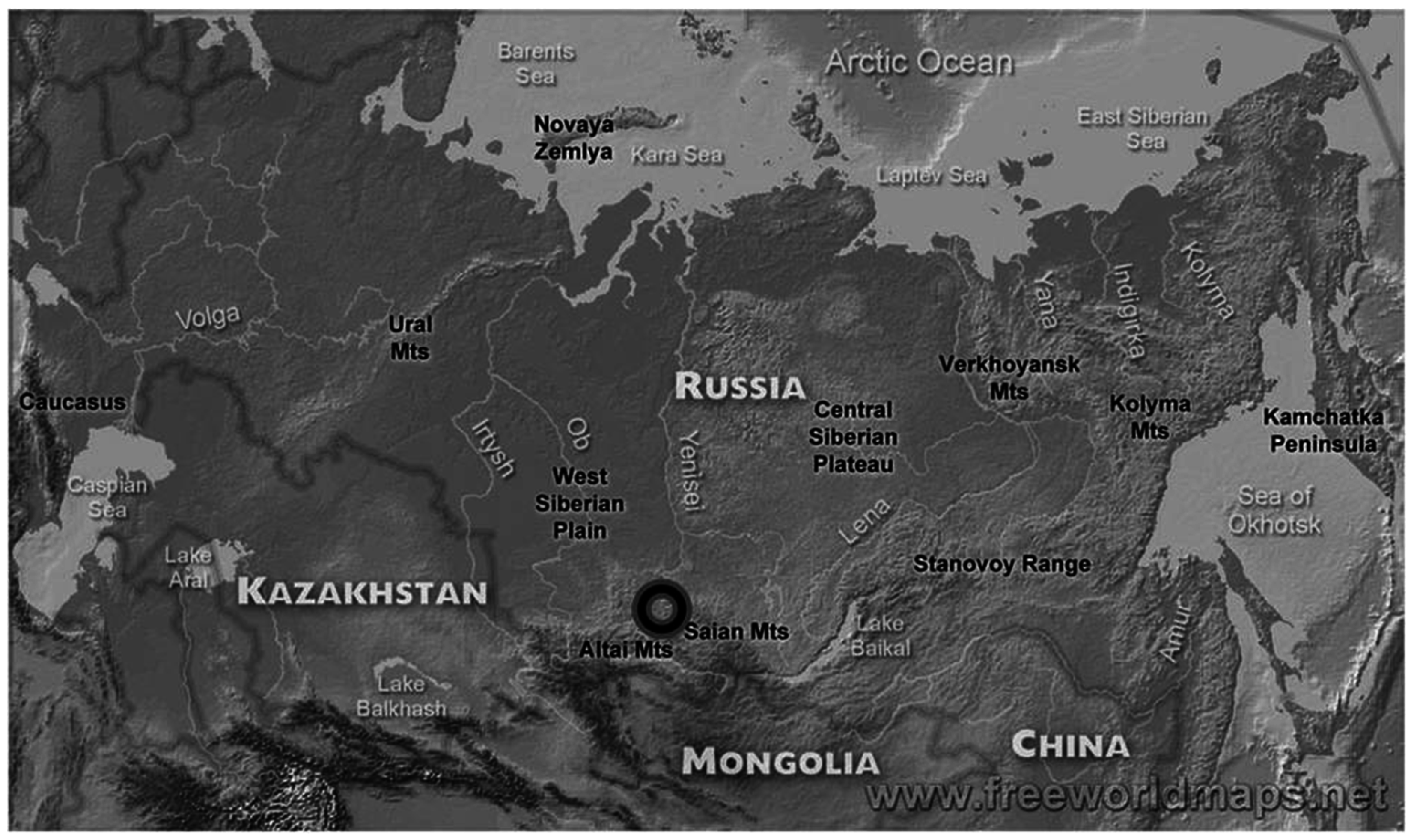
2. Materials and Methods
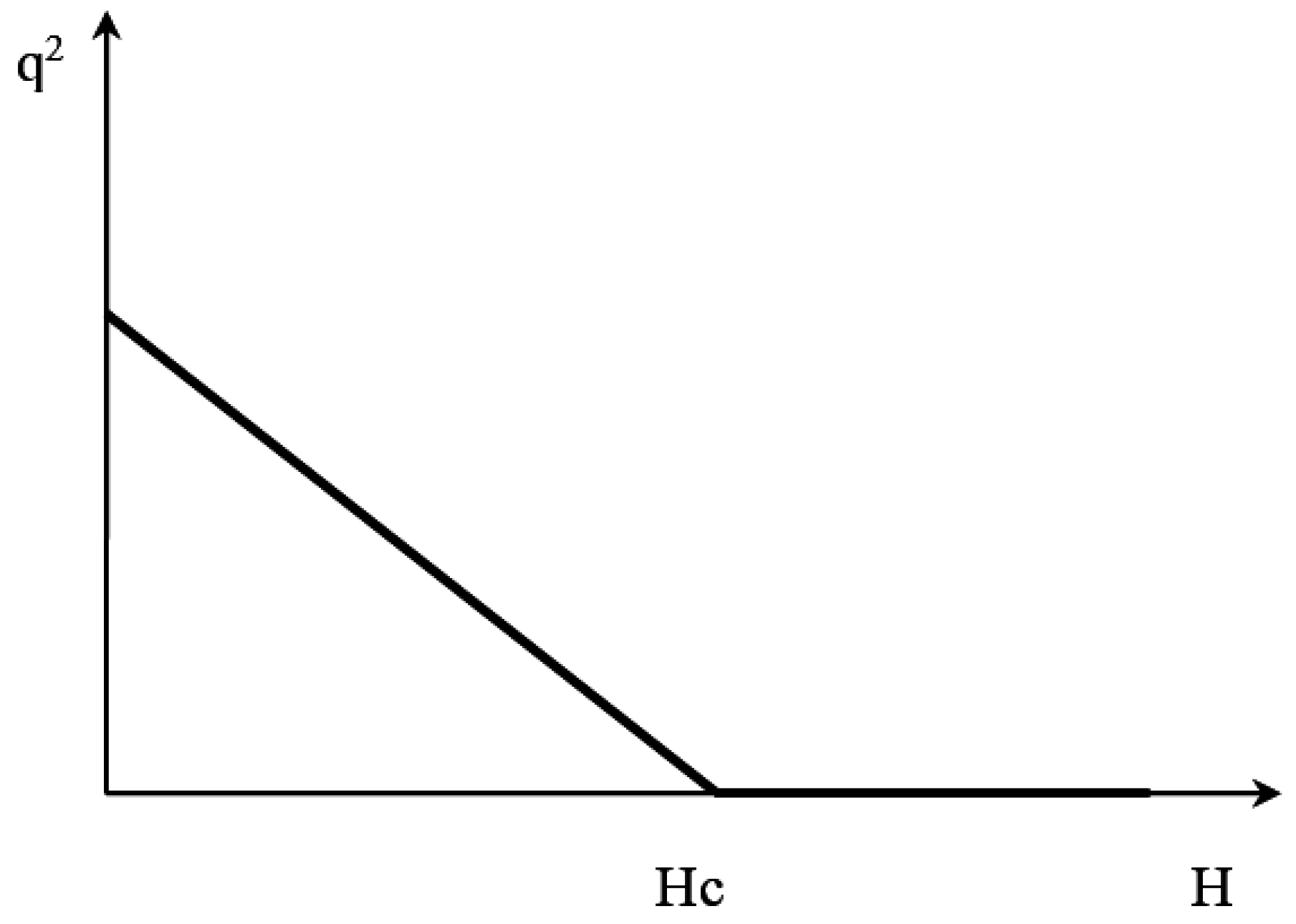
Ecotone Boundary Model
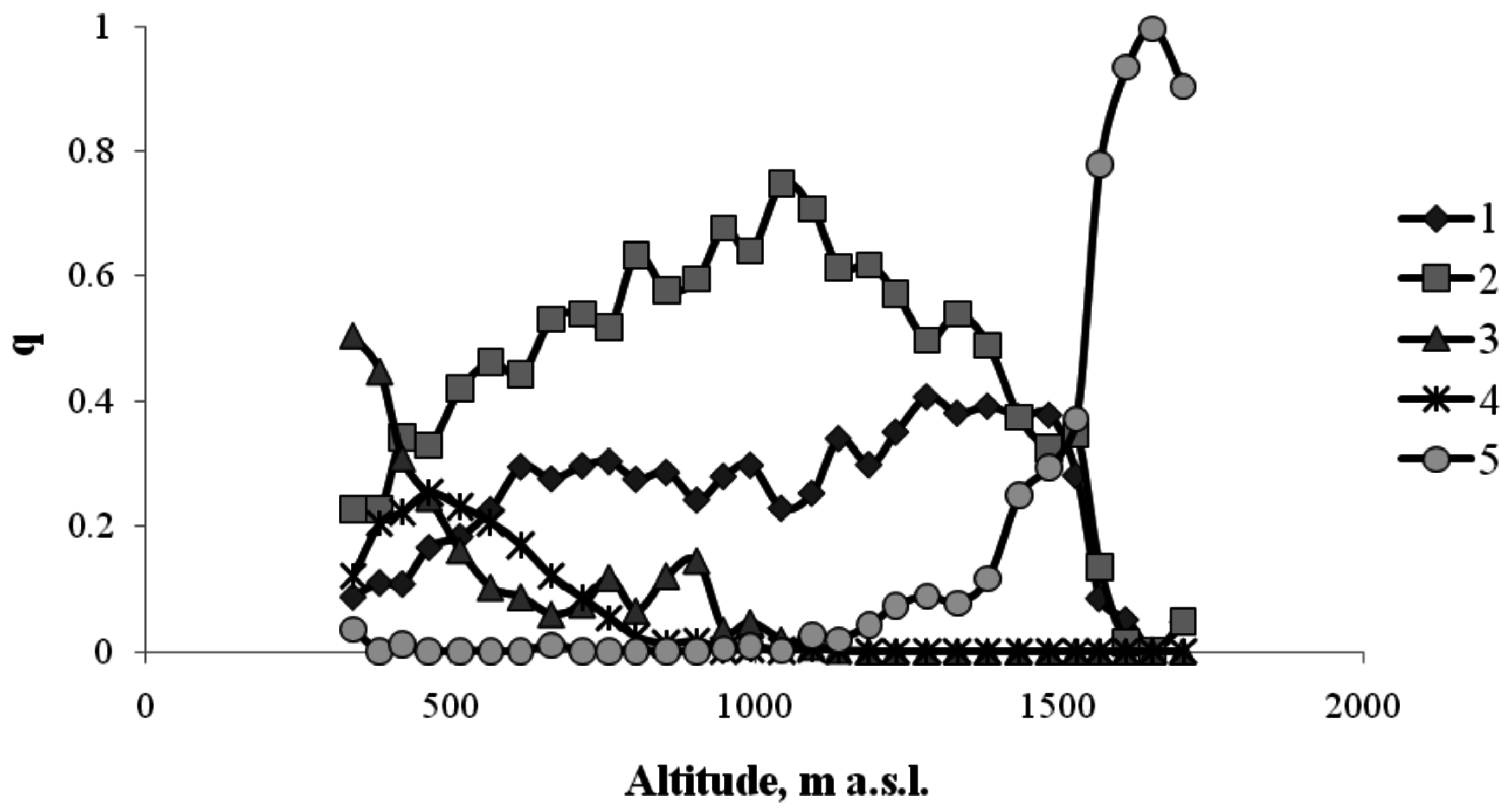
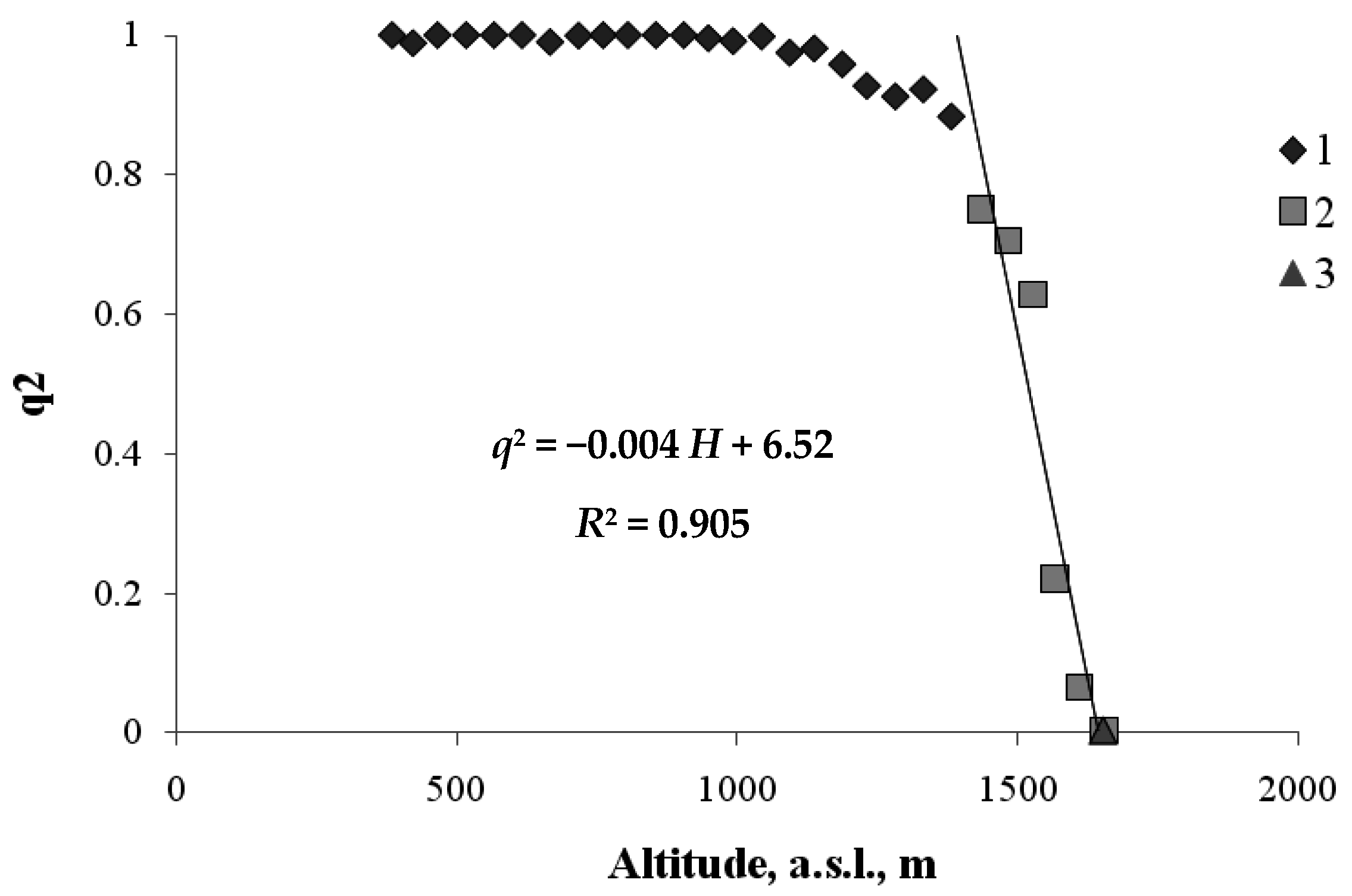
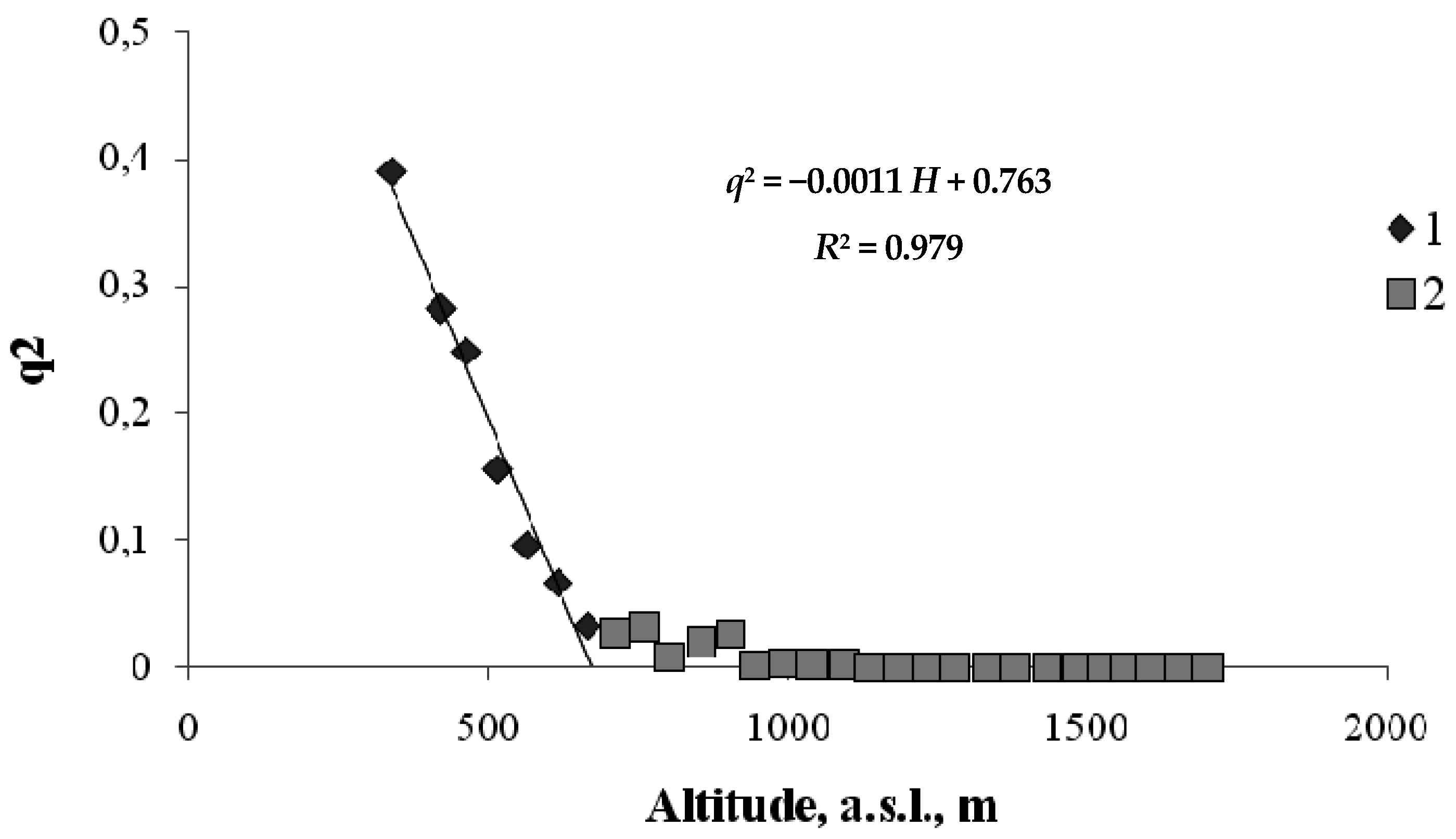
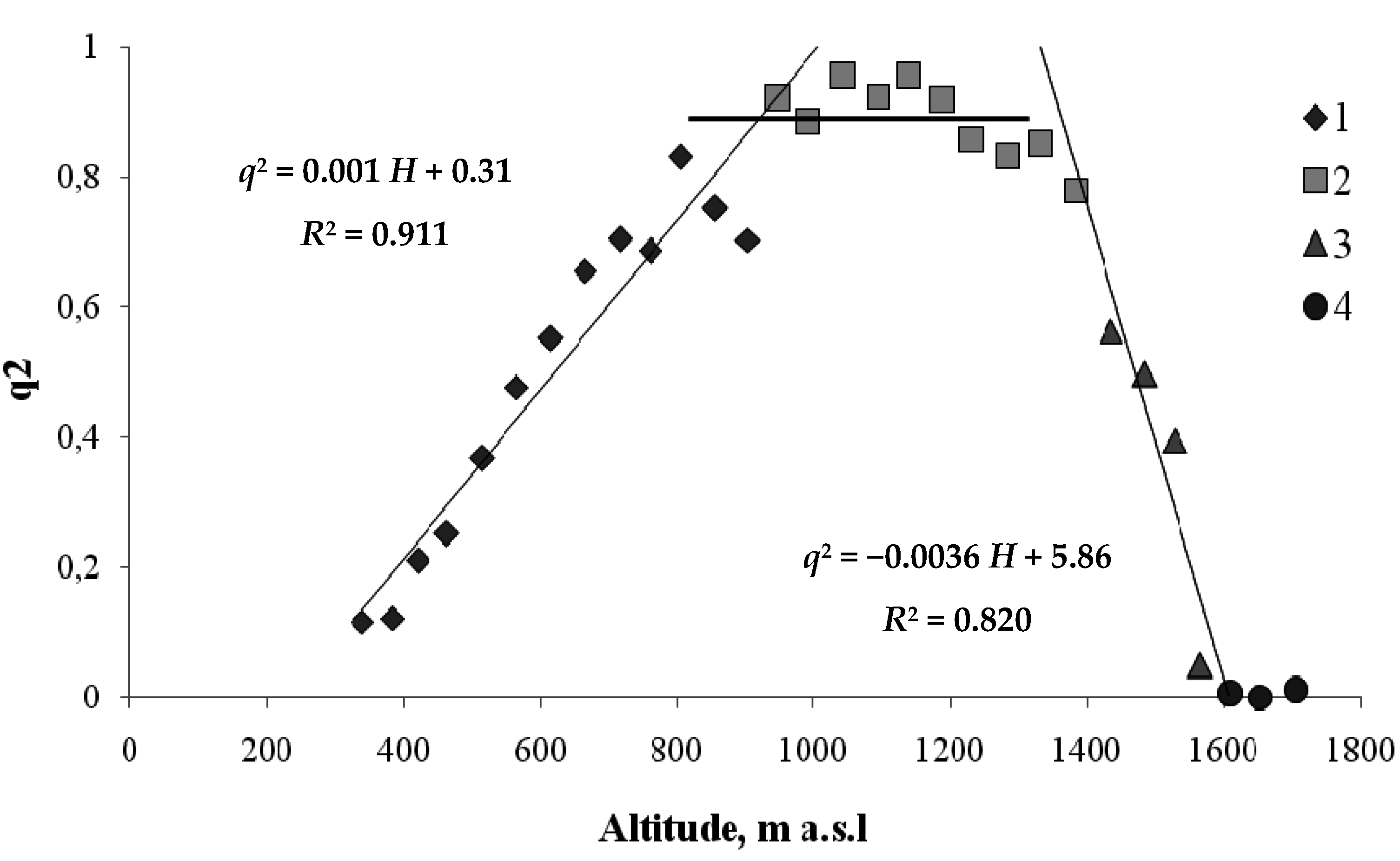
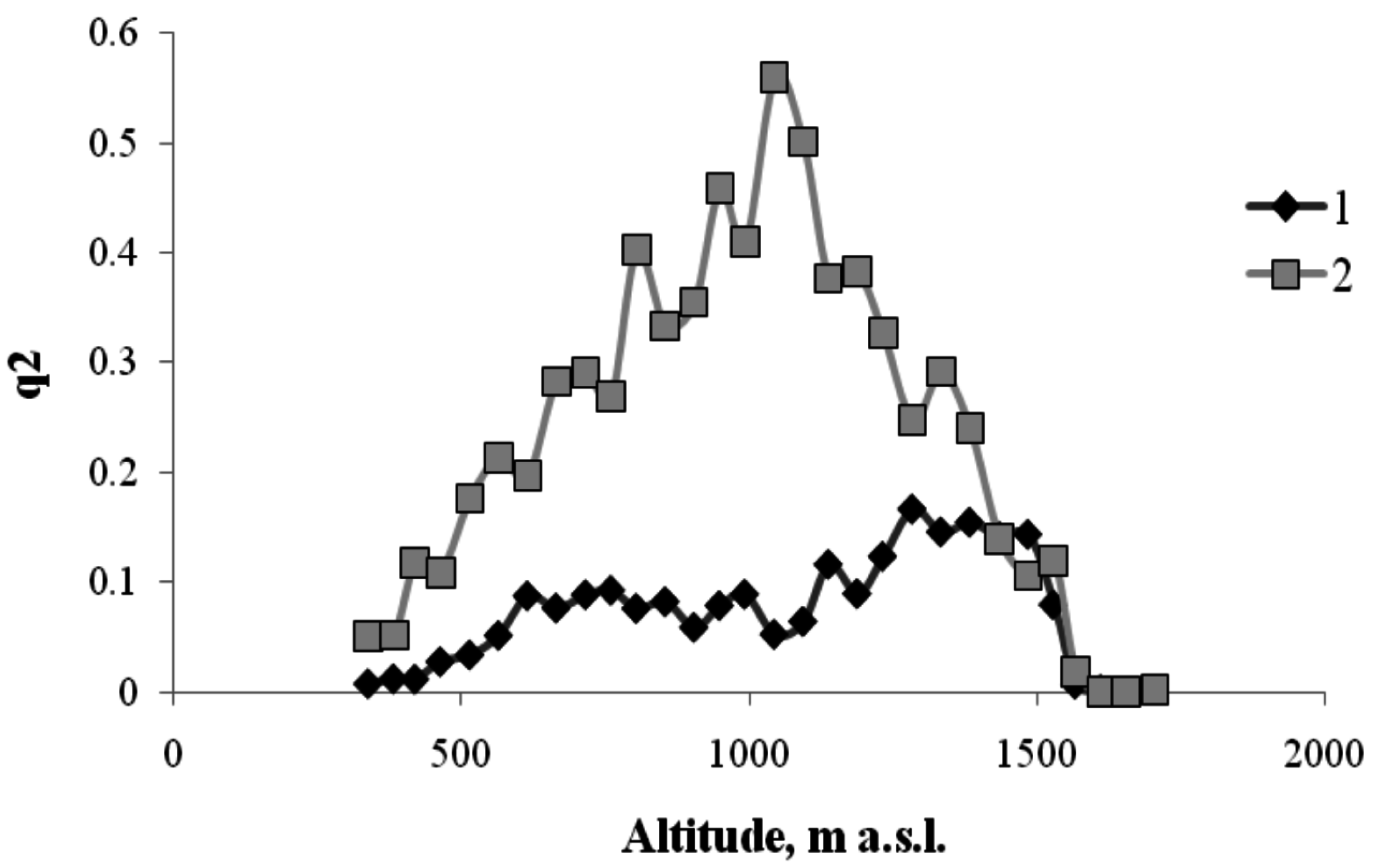
3. Results
4. Discussion
4.1. Interactions between Various Tree Species within the Same Altitudinal Belt
4.2. Climate Factors in the Model
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Odum, E.P. Fundamentals of Ecology; W.B. Saunders Company: Philadelphia, PA, USA, 1953; p. 384. [Google Scholar]
- Whittaker, R.H. Communities and Ecosystems; Macmillan Publishing Co., Inc.: New York, NY, USA, 1975; p. 158. [Google Scholar]
- Auer, S.K.; King, D.I. Ecological and life-history traits explain recent boundary shifts in elevation and latitude of western North American songbirds. Glob. Ecol. Biogeogr. 2014, 23, 867–875. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.M.; Butchart, S.H.M.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akcakaya, H.R.; et al. Assessing species vulnerability to climate change. Nat. Clim. Chang. 2015, 5, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Feagan, R. The place of food: Mapping out the ‘local’ in local food systems. Prog. Hum. Geogr. 2007, 31, 23–42. [Google Scholar] [CrossRef]
- Kelly, A.E.; Goulden, M.L. Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 11823–11826. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Gugout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.; Agudo, R. The future of species under climate change: Resilience or decline? Science 2013, 341, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.-R.; Beizner, S.; Burga, C.A. Trends in the upward shift of alpine plants. J. Veg. Sci. 2005, 16, 541–548. [Google Scholar] [CrossRef]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate effects on mountain plants. Nature 1994, 369, 448. [Google Scholar] [CrossRef] [PubMed]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, A.; Williams, J.W. Climatic and biotic velocities for woody taxa distributions over the last 16,000 years in eastern North America. Ecol. Lett. 2013, 16, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Kosanic, A.; Anderson, K.; Frure, C.H.; Harrison, S. Regional vegetation change and implications for local conservation: An example from West Cornwall (United Kingdom). Glob. Ecol. Concerv. 2015, 4, 405–413. [Google Scholar] [CrossRef]
- Prach, K.; Rauch, O. On filter effects of ecotones. Ecologia (CSFR) 1992, 11, 293–298. [Google Scholar]
- Janik, J. Ecotone and ecocline: Two questenable concepts in ecology. Ecologia (CSFR) 1992, 11, 243–250. [Google Scholar]
- Hickey, M.B.C.; Doran, B. A review of the efficiency of buffer strips for the maintenance and enhancement of riparian ecosystems. Water Qual. Res. J. Can. 2004, 39, 311–317. [Google Scholar]
- Chen, X.-W. Modeling the effects of global climate change at the ecotone of boreal larch forest and temperate forest in Northeast China. Clim. Chang. 2002, 55, 77–97. [Google Scholar] [CrossRef]
- Peters, D.P.C.; Gosz, J.R.; Pockman, W.T.; Small, E.E.; Parmenter, R.R.; Collins, S.L.; Muldavin, E. Integrating patch and boundary dynamics to understand and predict biotic transitions at multiple scales. Landsc. Ecol. 2006, 21, 19–33. [Google Scholar] [CrossRef]
- Fortin, M.-J. Edge detection algorithms for two-dimensional ecological data. Ecology 1994, 75, 956–965. [Google Scholar] [CrossRef]
- Jacquez, G.M.; Maruca, S.; Fortin, M.J. From fields to objects: A review of geographic boundary analysis. J. Geogr. Syst. 2000, 2, 221–241. [Google Scholar] [CrossRef]
- Fisher, P.; Arnot, C.; Wadsworth, R.; Wellens, J. Detecting change in vague interpretations of landscapes. Ecol. Inform. 2006, 1, 163–178. [Google Scholar] [CrossRef]
- Hufkens, K.; Ceulemans, R.; Scheunders, P. Estimating the ecotone width in patchy ecotones using a sigmoid wave approach. Ecol. Inform. 2008, 3, 97–104. [Google Scholar] [CrossRef]
- Hufkens, K.; Scheunders, P.; Ceulemans, R. Ecotones: Methodology and definitions revisited. Ecol. Res. 2009, 24, 977–986. [Google Scholar] [CrossRef]
- Hobbs, R.J. Remote sensing of spatial and temporal dynamics of vegetation. In Remote Sensing of Biosphere Functioning; Hobbs, R.J., Mooney, H.A., Eds.; Springer: New York, NY, USA, 1990; pp. 203–219. [Google Scholar]
- Csillag, F.; Kabos, S. Wavelets, boundaries, and the spatial analysis of landscape pattern. Ecoscience 2002, 9, 177–190. [Google Scholar] [CrossRef]
- Nystrom, M.; Holmgren, J.; Olsson, H. Prediction of tree biomass in the forest–tundra ecotone using airborne laser scanning. Remote Sens. Environ. 2012, 123, 271–279. [Google Scholar] [CrossRef]
- Heiskanen, J. Estimating aboveground tree biomass and leaf area index in a mountain birch forest using ASTER satellite data. Int. J. Remote Sens. 2006, 27, 1135–1158. [Google Scholar] [CrossRef]
- Hill, R.A.; Granica, K.; Smith, G.M.; Schardt, M. Representation of an alpine treeline ecotone in SPOT 5 HRG data. Remote Sens. Environ. 2007, 110, 458–467. [Google Scholar] [CrossRef]
- Ranson, K.J.; Sun, G.; Kharuk, V.I.; Kovacs, K. Assessing tundra-taiga boundary with multi-sensor satellite data. Remote Sens. Environ. 2004, 93, 283–295. [Google Scholar] [CrossRef]
- Weiss, D.J.; Walsh, S.J. Remote sensing of mountain environments. Geogr. Compass 2009, 3, 1–21. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xu, M.; Adams, J.; Wang, X.C. Can Landsat imagery detect tree line dynamics? Int. J. Remote Sens. 2009, 30, 1327–1340. [Google Scholar] [CrossRef]
- Polikarpov, N.P.; Chebakova, N.M.; Nazimova, D.I. Klimat i Gornyye Lesa Yuzhnoy Sibiri (Climate and Mountain Forests in South Siberia); Novosibirsk: Nauka, Russia, 1986; p. 225. (In Russian) [Google Scholar]
- Soukhovolsky, V.G.; Ovchinnikova, T.M.; Baboy, S.D. Altitudinal zonation of tree species in the Sayan Mountains: A model of ecological second-order phase transitions. Zhurnal Obshchey Biologii 2014, 75, 38–47. (In Russian) [Google Scholar]
- Landau, L.D.; Lifshitz, E.M. Statistical Physics; Pergamon Press: Oxford, UK, 1980; pp. 501–535. [Google Scholar]
- Bruce, A.D.; Cowley, R.A. Structural Phase Transitions; Taylor & Francis: London, UK, 1981; pp. 97–125. [Google Scholar]
- Ricklefs, R.E.; Miller, G.L. Ecology; W.H. Freeman & Co.: New York, NY, USA, 2000; p. 822. [Google Scholar]
- Matala, J.; Ojansuu, R.; Peltola, H.; Raitio, H.; Kellomaki, S. Modelling the response of tree growth to temperature and CO2 elevation as related to the fertility and current temperature sum of a site. Ecol. Model. 2006, 199, 39–52. [Google Scholar] [CrossRef]
- Chmura, D.J.; Anderson, P.D.; Howe, G.T.; Harrington, C.A.; Halofsky, J.E.; Peterson, D.L.; Shaw, D.C.; St. Clair, J.B. Forest responses to climate change in the northwestern United States: Ecophysiological foundations for adaptive management. For. Ecol. Manag. 2011, 261, 1121–1142. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate change and forest disturbances. Bioscience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Briceno-Elizondo, E.; Garcia-Gonzalo, J.; Peltola, H.; Matala, J.; Kellomaki, S. Sensitivity of growth of Scots pine, Norway spruce and silver birch to climate change and forest management in boreal conditions. For. Ecol. Manag. 2006, 232, 152–167. [Google Scholar] [CrossRef]
- Bergh, J.; Freeman, M.; Sigurdsson, B.; Kellomäki, S.; Laitinen, K.; Niinistö, S.; Peltola, H.; Linder, S. Modelling the short-term effects of climate change on the productivity of selected tree species in Nordic countries. For. Ecol. Manag. 2003, 183, 327–340. [Google Scholar] [CrossRef]
- Condés, S.; García-Robredo, F. An empirical mixed model to quantify climate influence on the growth of Pinus halepensis Mill. stands in South-Eastern Spain. For. Ecol. Manag. 2012, 284, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Favier, C.; Chave, J.; Fabing, A.; Schwartz, D.; Dubois, M. Modelling forest–savanna mosaic dynamics in man-influenced environments: Effects of fire, climate and soil heterogeneity. Ecol. Model. 2004, 171, 85–102. [Google Scholar] [CrossRef]
- Loehle, C.; LeBlanc, D. Model-based assessments of climate change effects on forests: A critical review. Ecol. Model. 1996, 90, 1–31. [Google Scholar] [CrossRef]
| Slope | Critical Heights * | Ecotone | |
|---|---|---|---|
| Hardwood–Conifer | Conifer–Meadow | ||
| South-facing | LB | 420 | 1157 |
| UB | 600 | 1610 | |
| ∆Н | 180 | 453 | |
| North-facing | LB | 308 | 1380 |
| UB | 694 | 1627 | |
| ∆Н | 386 | 247 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, Y.; Soukhovolsky, V. Modeling the Boundaries of Plant Ecotones of Mountain Ecosystems. Forests 2016, 7, 271. https://doi.org/10.3390/f7110271
Ivanova Y, Soukhovolsky V. Modeling the Boundaries of Plant Ecotones of Mountain Ecosystems. Forests. 2016; 7(11):271. https://doi.org/10.3390/f7110271
Chicago/Turabian StyleIvanova, Yulia, and Vlad Soukhovolsky. 2016. "Modeling the Boundaries of Plant Ecotones of Mountain Ecosystems" Forests 7, no. 11: 271. https://doi.org/10.3390/f7110271





