Surface CO2 Exchange Dynamics across a Climatic Gradient in McKenzie Valley: Effect of Landforms, Climate and Permafrost
Abstract
:1. Introduction
2. Methods
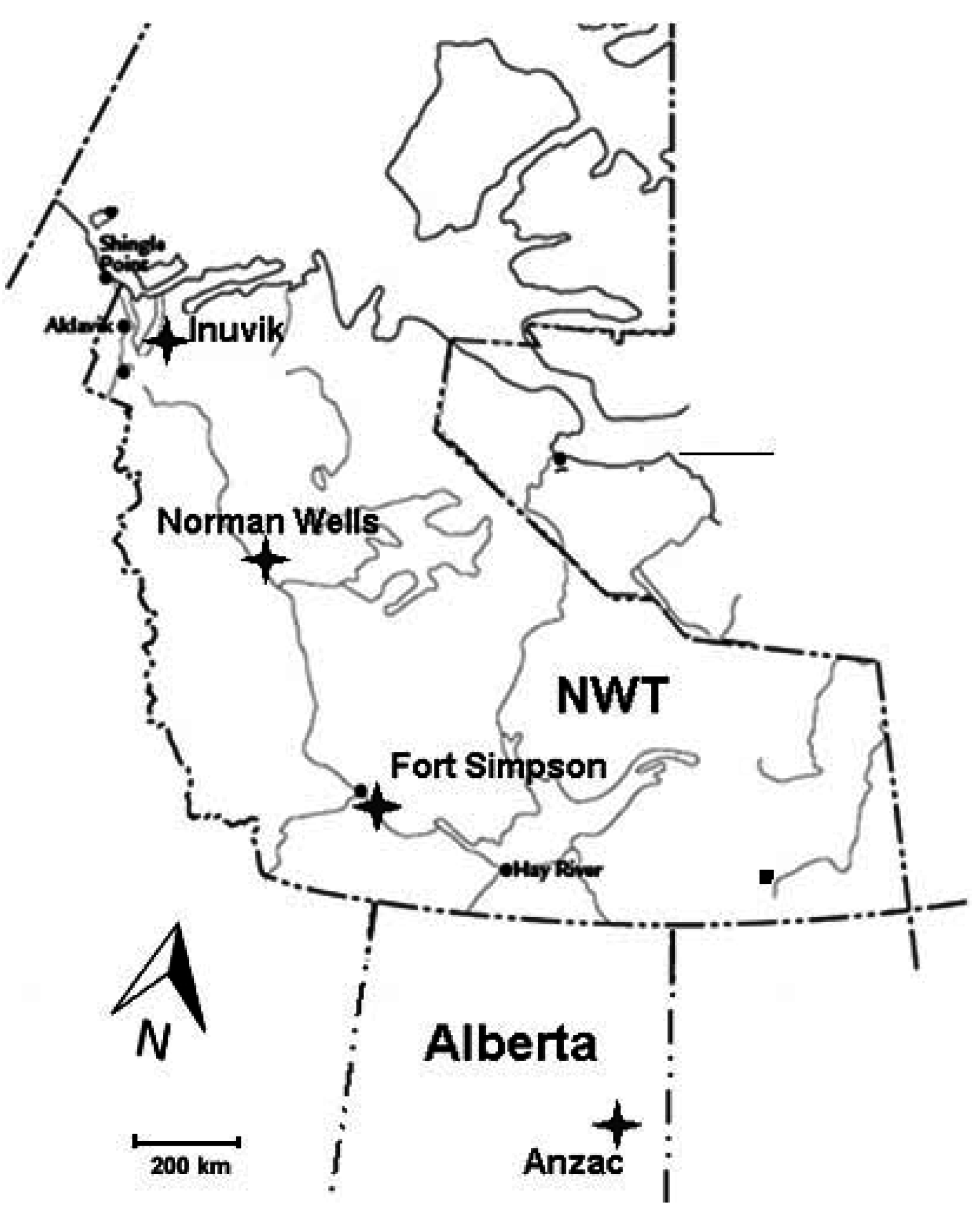
2.1. Site Description
Study Plots Establishment
2.2. Vegetation Description
2.3. Soil Description
2.4. CO2 Flux Measurements
2.4.1. Intra-Site Spatial Variability of CO2 Fluxes
2.4.2. Temporal Variation in CO2 Fluxes
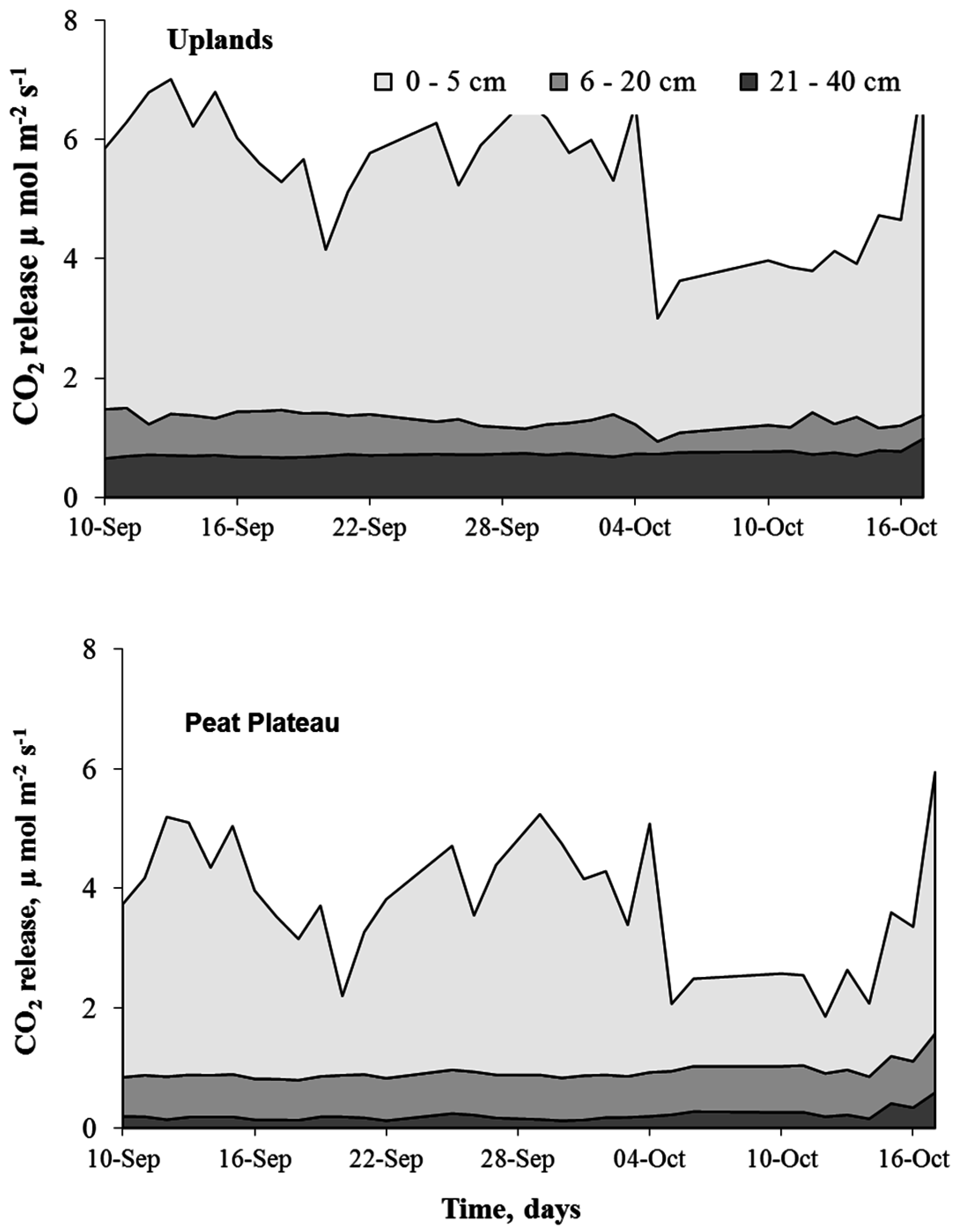
2.4.3. Calculating Soil CO2 Fluxes
2.5. Statistical Analysis
3. Results
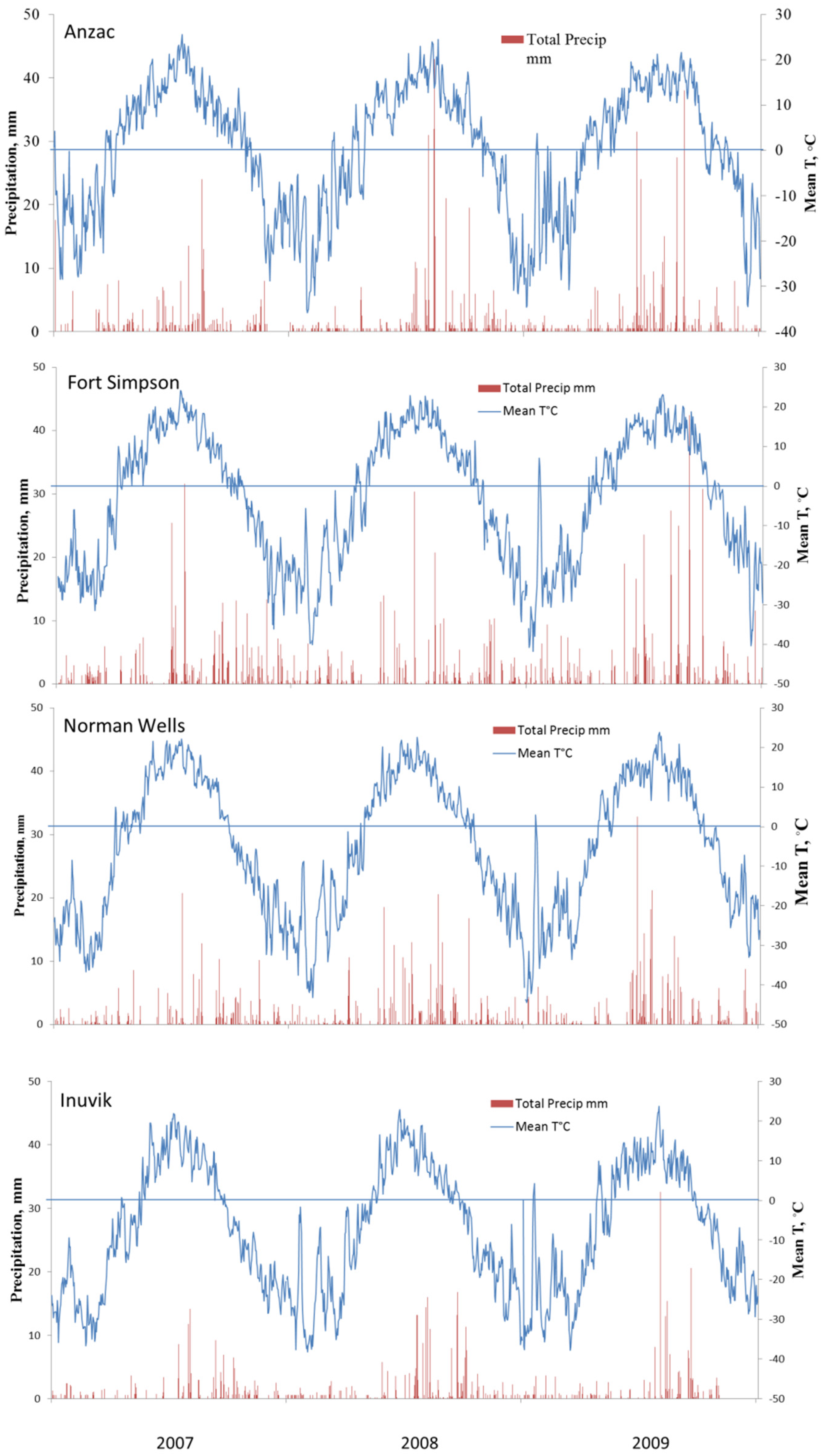
3.1. Weather Monitoring
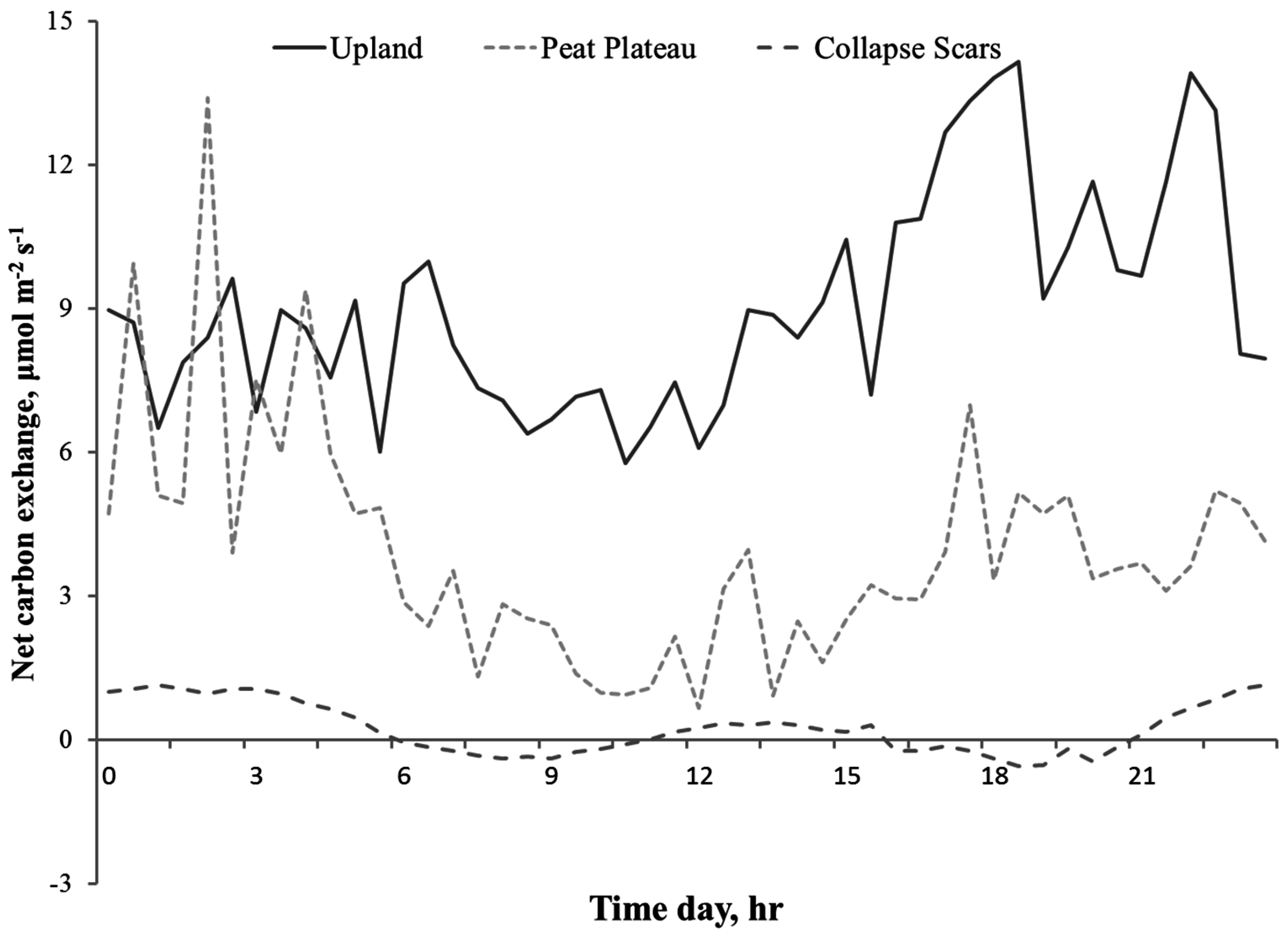
3.2. Diurnal Variations in NCE
3.3. Statistical Model of Surface CO2 Fluxes against Temperature
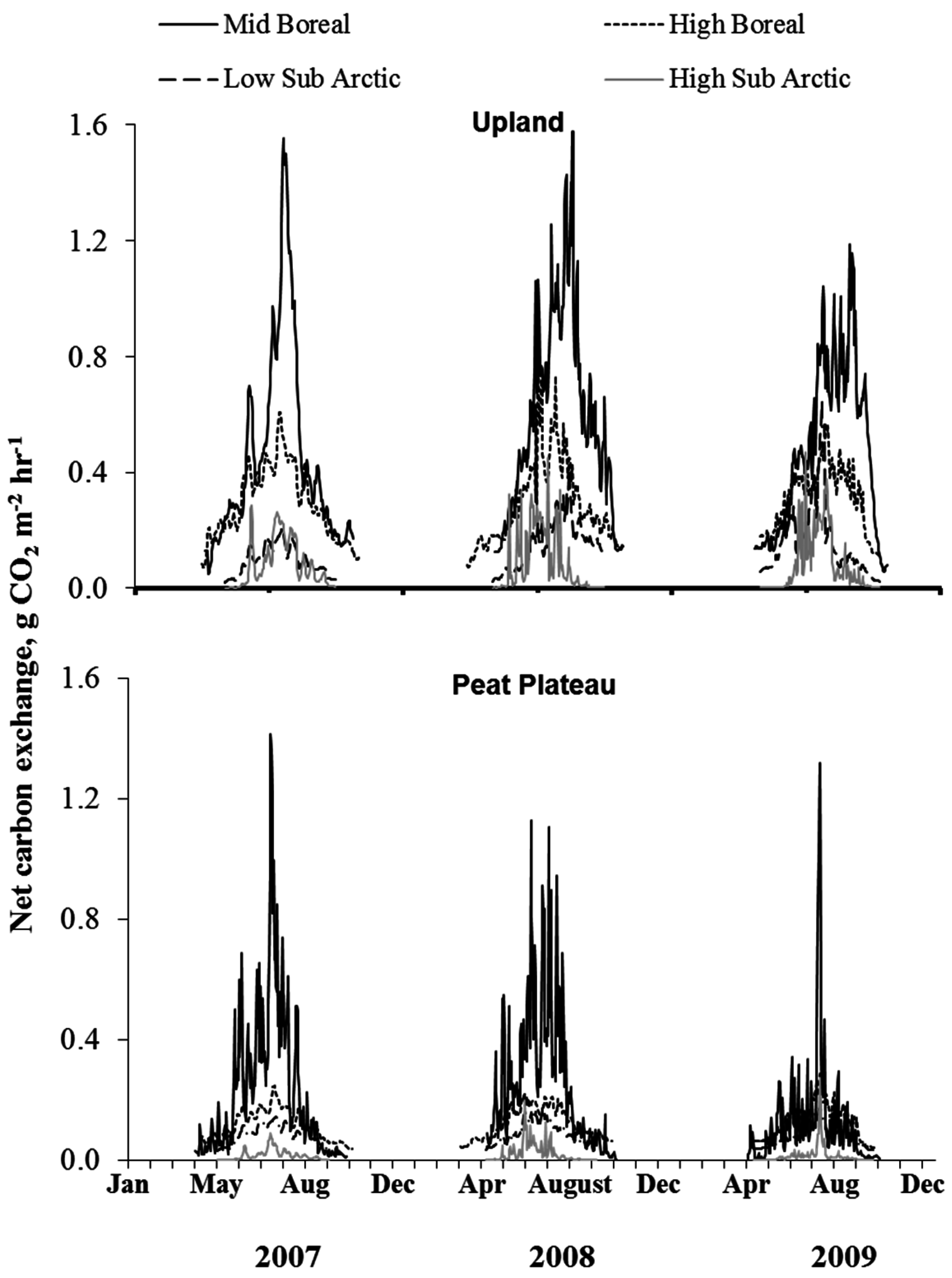
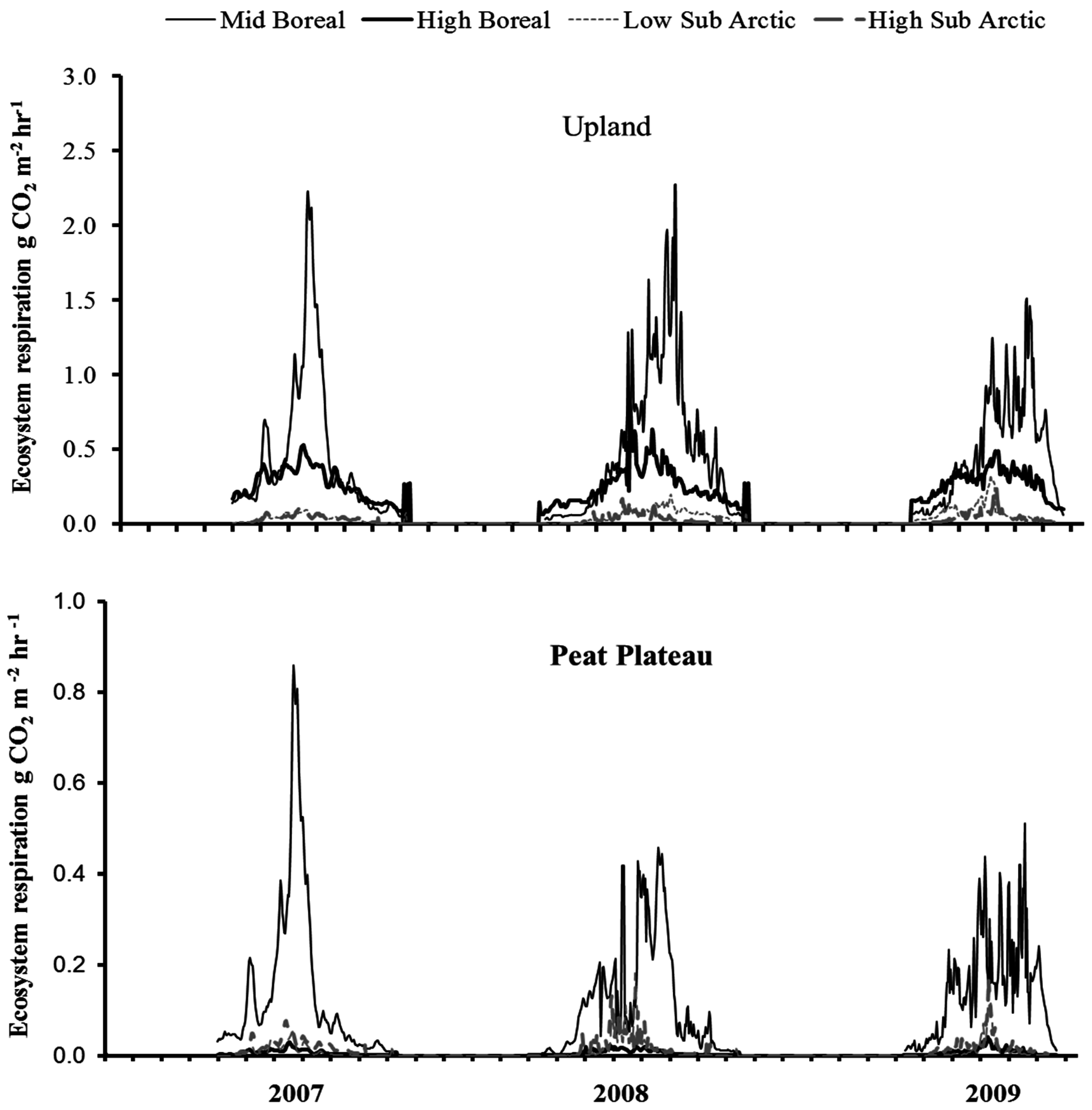
3.4. Time Series of NCE and ER
3.5. Effect of Light on Surface Assimilation Rate
3.6. Annual ER and NCE
4. Discussion
4.1. Spatial Variation in Net Carbon Exchange (NCE)
4.2. Assimilation Rates across Different Landforms
4.3. Spatial Variation in Ecosystem Respiration (ER)
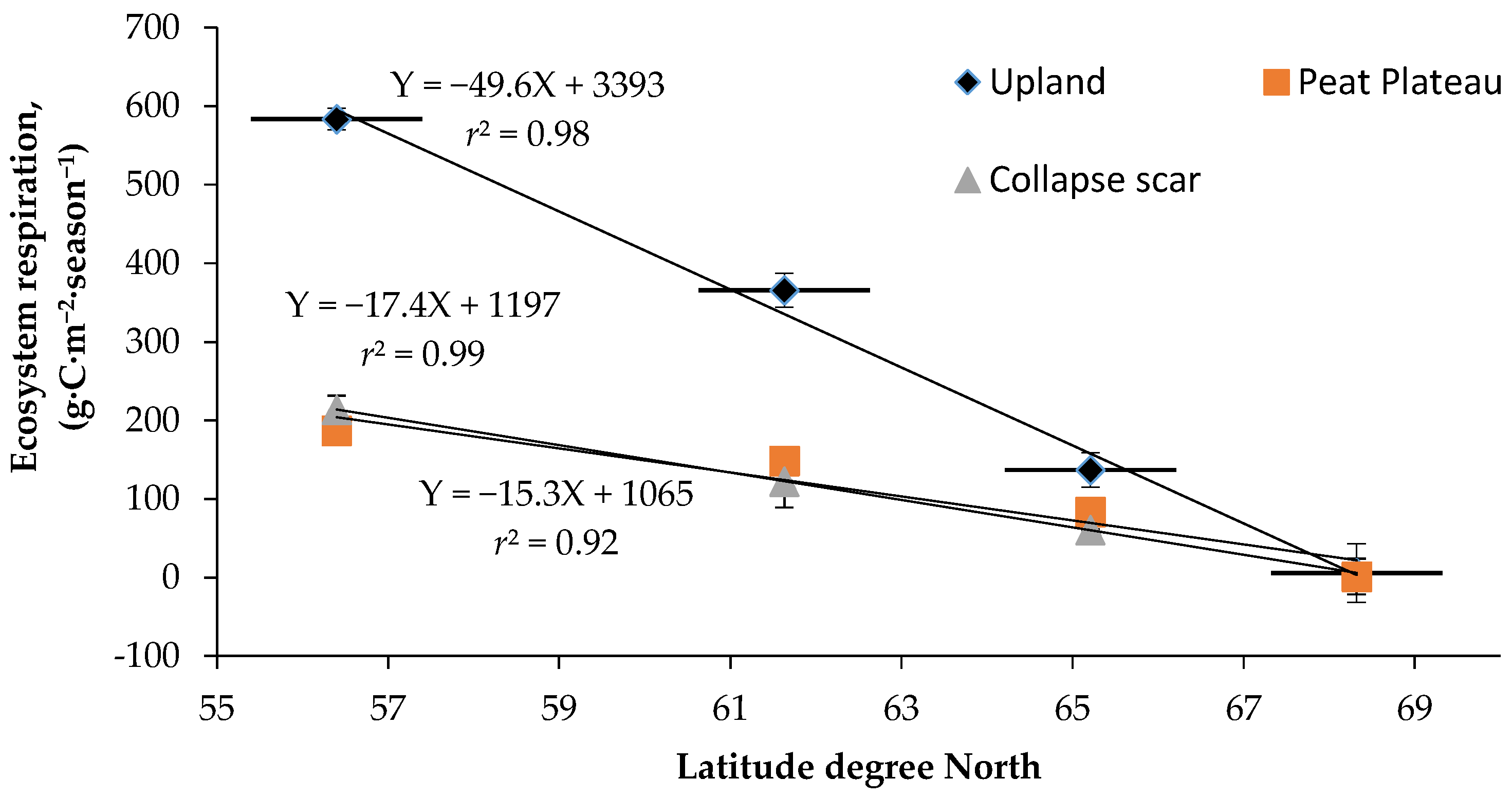
4.4. Quantitative Relationships between NCE and ER and Climate Variables
4.5. Implicatiuons of Changing Climate
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abbott, B.W.; Jones, J.B.J.; Schuur, E.; Chapin, F.; Bowden, W.B.; Bret-Harte, M.S.; Epstein, H.E.; Flannigan, M.D.; Harms, T.K.; Hollingsworth, T.N.; et al. Biomass offsets little or none of permafrost carbon release from soils, streams, and wildfire: An expert assessment. Environ. Res. Lett. 2016, 11, 034014. [Google Scholar] [CrossRef]
- Schuur, E.G.; McGuire, A.D.; Schädel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M.; et al. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Schuur, E.A.; Bockheim, G.J.; Canadell, J.G.; Euskirchen, E.; Field, C.B.; Goryachkin, S.V.; Hagemann, S.; Kuhry, P.; Lafleur, P.M.; Lee, H.; et al. Vulnerability of Permafrost Carbon to Climate Change: Implications for the Global Carbon Cycle. Bioscience 2008, 58, 701–714. [Google Scholar] [CrossRef]
- Hugelius, G.; Strauss, J.; Zubrzycki, S.; Harden, J.W.; Schuur, E.A.G.; Ping, C.-L.; Schirrmeister, L.; Grosse, G.; Michaelson, G.J.; Koven, C.D.; et al. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 2014, 11, 6573–6593. [Google Scholar] [CrossRef] [Green Version]
- Tarnocai, C.; Canadell, J.G.; Schuur, E.A.G.; Kuhry, P.; Mazhitova, G.; Zimov, S.A. Soil organic carbon pools in the northern circumpolar permafrost region. Glob. Biogeochem. Cycles 2009, 23. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Zhuang, Q.; McGuire, A.D.; O’Neill, K.P.; Harden, J.W.; Romanovsky, V.E.; Yarie, J. Modeling soil thermal and carbon dynamics of a fire chronosequence in interior Alaska. J. Geophys. Res. 2003, 107. [Google Scholar] [CrossRef]
- Kurz, W.A.; Shaw, C.H.; Boisvenue, C.; Stinson, G.; Metsaranta, J.; Leckie, D.; Dyk, A.; Smyth, C.; Neilson, E.T. Carbon in Canada’s boreal forest—A synthesis. Environ. Rev. 2014, 21, 260–292. [Google Scholar] [CrossRef]
- Price, D.T.; Alfaro, R.I.; Brown, K.J.; Flannigan, M.D.; Fleming, R.A.; Hogg, E.H.; Girardin, M.P.; Lakusta, T.; Johnston, M.; McKenney, D.W.; et al. Anticipating the consequences of climate change for Canada’s boreal forest ecosystems. Environ. Rev. 2013, 21, 322–365. [Google Scholar] [CrossRef]
- Harden, J.W.; Koven, C.D.; Ping, C.L.; Hugelius, G.; McGuire, A.D.; Camill, P.; Jorgenson, T.; Kuhry, P.; Michaelson, G.J.; O’Donnell, J.A.; et al. Field information links permafrost carbon to physical vulnerabilities of thawing. Geophys. Res. Lett. 2012, 39, L15704. [Google Scholar] [CrossRef]
- Thie, J. Distribution and Thawing of Permafrost in the Southern Part of the Discontinuous Permafrost Zone in Manitoba. Arctic 1974, 27, 189–200. [Google Scholar] [CrossRef]
- Waddington, J.M.; Roulet, N.T. Carbon balance of a boreal patterned peatland. Glob. Chang. Biol. 2000, 6, 87–98. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 4th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2007; p. 582. [Google Scholar]
- Drewitt, G.B.; Black, T.A.; Nesic, Z.; Humphreys, E.R.; Jork, E.M.; Swanson, R.; Ethier, G.J.; Griffis, T.; Morgenstern, K. Measuring forest-floor CO2 fluxes in a Douglas-fir forest. Agric. For. Meteorol. 2002, 110, 299–317. [Google Scholar] [CrossRef]
- Green, R.N.; Trowbridge, R.L.; Klinka, K. Towards a taxonomic classification of humus forms. For. Sci. 1993, 39, 1–49. [Google Scholar]
- Halsey, L.A.; Vitt, D.H.; Zoltai, S.C. Disequilibrium response of permafrost in boreal continental western Canada to climate change. Clim. Chang 1995, 30, 57–73. [Google Scholar] [CrossRef]
- Moore, T.R.; Roulet, N.T.; Waddington, J.M. Uncertainty in Predicting the Effect of climatic Change on the Carbon Cycling of Canadian Peatlands. Clim. Chang. 1998, 40, 229–245. [Google Scholar] [CrossRef]
- Kayranli, B.; Scholz, M.; Mustafa, A.; Hedmark, A. Carbon storage and fluxes within freshwater wetlands: A critical review. Wetlands 2010, 30, 111–124. [Google Scholar] [CrossRef]
- Grosse, G.; Harden, J.; Turetsky, M.R.; McGuire, A.D.; Camill, P.; Tarnocai, C.; Frolking, S.; Schuur, E.A.G.; Jorgenson, T.; Marchenko, S.; et al. Vulnerability of high-latitude soil organic carbon in North America to disturbance. J. Geophys. Res. 2011, 116, G00K06. [Google Scholar] [CrossRef]
- Liblik, L.; Moore, T.R.; Bubier, J.L.; Robinson, S.D. Methane emissions from wetlands in the discontinuous permafrost zone: Fort Simpson, NWT, Canada. Glob. Biogeochem. Cycles 1997, 11, 485–494. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Wieder, R.K.; Vitt, D.H. Boreal peatland C fluxes under varying permafrost regimes. Soil Biol. Biochem. 2002, 34, 907–912. [Google Scholar] [CrossRef]
- Prater, J.L.; Chanton, J.P.; Whiting, G.J. Variation in methane production pathways associated with permafrost decomposition in collapse scar bogs of Alberta, Canada. Glob. Biogeochem. Cycle 2007, 21, GB4004. [Google Scholar] [CrossRef]
- Flanagan, L.B.; Syed, K.H. Simulation of both photosynthesis and respiration in response to warmer and drier conditions in a boreal peatland ecosystem. Glob. Chang. Biol. 2011, 17, 2271–2872. [Google Scholar] [CrossRef]
- Elberling, B.; Michelsen, A.; Schädel, C.; Schuur, E.A.G.; Christiansen, H.H.; Berg, L.; Tamstorf, M.P.; Sigsgaard, C. Long-term CO2 production following permafrost thaw. Nat. Clim. Chang. 2013, 3, 890–894. [Google Scholar] [CrossRef]
- Skinner, W.; Maxwell, B. Climatic Patterns, Trends and Scenarios in the Arctic. In Proceedings of the Sixth Biennial AES/DIAND Meeting on Northern Climate & Mid-Study Workshop of the Mackenzie Basin Impact Study, Yellowknife, NT, Canada, 10–14 April 1994; pp. 125–137.
- Jassal, R.; Black, A.; Novak, M.; Morgenstern, K.; Nesic, Z.; Gaumont-Guay, D. Relationship between soil CO2 concentrations and forest-floor CO2 effluxes. Agric. For. Meteorol. 2005, 130, 176–192. [Google Scholar]
- Millington, R.J.; Quirk, J.M. Transport in Porous Media; Elsevier: Amsterdam, The Netherlands, 1960; pp. 97–106. [Google Scholar]
- Sallam, A.; Jury, W.A.; Letey, J. Measurement of gas diffusion coefficient under relatively low air-filled porosity. Soil Sci. Am. J. 1984, 48, 3–6. [Google Scholar] [CrossRef]
- SAS Institute Inc. The SAS System for Windows, 9th ed.; SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
- Lee, X.; Wu, H.J.; Sigler, J.; Oishi, C.; Siccama, T. Rapid and transient response of soil respiration to rain. Glob. Chang. Biol. 2004, 10, 1017–1026. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 597–582. [Google Scholar] [CrossRef] [PubMed]
- Bronson, D.R.; Gower, S.T.; Tanner, M.; Linder, M.; van Herk, I. Response of soil surface CO2 flux in a boreal forest to ecosystem warming. Glob. Chang. Biol. 2008, 14, 856–867. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Davidson, E.A.; Jassens, I.A. Temperature sensitivity of soil carbon decomposition and feedback to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Schuur, E.A.G.; Abbott, B.W.; Bowden, W.B.; Brovkin, V.; Camill, P.; Canadell, J.G.; Chanton, J.P.; Chapin, F.S., III; Christensen, T.R.; Ciais, P.; et al. Expert assessment of vulnerability of permafrost carbon to climate change. Clim. Chang. 2013, 119, 359–374. [Google Scholar] [CrossRef]
- Clymo, R.S. The limits to peat bog growth. Philos. Trans. Soc. R. Ser. B 1984, 3113, 605–654. [Google Scholar] [CrossRef]
- Wickland, K.P.; Striegl, R.G.; Neff, J.C.; Sachs, T. Effects of permafrost melting on CO2 and CH4 exchange of a poorly drained black spruce lowland. J. Geophys. Res. 2006, 111, G02011. [Google Scholar] [CrossRef]
- Strachan, I.B.; Pelletier, L.; Bonneville, M.-C. Interannual variability in water table depth controls net ecosystem carbon dioxide exchange in a boreal peatland. Biogeochemistry 2016, 127, 99–111. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.D.; Enomoto, H.; Kushida, K.; Kondoh, M.; Uchida, M. Latitudinal distribution of soil CO2 efflux and temperature along the Dalton Highway, Alaska. Polar Sci. 2013, 7, 162–173. [Google Scholar] [CrossRef]
- Aurela, M.; Laurila, T.; Tuovinen, J.-P. The timing of snow melt controls the annual CO2 balance in a subarctic fen. Geophys. Res. Lett. 2004, 31, LI6119. [Google Scholar] [CrossRef]
- Bisbee, K.E.; Gower, S.T.; Norman, J.M.; Nordheim, E.V. Environmental controls on ground cover species composition and productivity in a boreal black spruce forest. Oecologia 2001, 129, 261–270. [Google Scholar] [CrossRef]
- Yuan, W.P.; Luo, Y.Q.; Richardson, A.D.; Oren, R.; Luyssaert, S.; Janssens, I.A.; Ceulemans, R.; Zhou, X.H.; Grunwald, T.; Aubinet, M.; et al. Latitudinal patterns of magnitude and interannual variability in net ecosystem exchange regulated by biological and environmental variables. Glob. Chang. Biol. 2009, 15, 2905–2920. [Google Scholar] [CrossRef]
- Wang, C.; Bond-Lamberty, B.; Gower, S.T. Carbon distribution of a well- and poorly-drained black spruce fire chronosequence. Glob. Chang. Biol. 2003, 9, 1–14. [Google Scholar] [CrossRef]
- Aurela, M.; Lohila, A.; Tuovinen, J.-P.; Hatakka, J.; Riutta, T.; Laurila, T. Carbon dioxide exchange on a northern boreal fen. Boreal Environ. Res. 2009, 14, 699–710. [Google Scholar]
- Griffis, T.J.; Rouse, W.R.; Waddington, J.M. Interannual variability of net ecosystem CO2 exchange at a subarctic fen. Glob. Biogeochem. Cycles 2000, 14, 1109–1121. [Google Scholar] [CrossRef]
- Lafleur, P.M.; Humphrey, E.R. Spring warming and carbon dioxide exchange over low Arctic tundra in central Canada. Glob. Chang. Biol. 2007, 14, 740–756. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Oechel, W.C.; Zulueta, R.C.; Hastings, S.J. Effects of climate variability on carbon sequestration among adjacent wet sedge tundra and moist tussock tundra ecosystems. J. Geophys. Res. 2006, 111, G03014. [Google Scholar] [CrossRef]
- Groendahl, L.; Friborg, T.; Soegaard, H. Temperature and snowmelt controls on interannual variability in carbon dioxide exchange in the high Arctic. Theor. Appl. Climatol. 2007, 88, 111–125. [Google Scholar] [CrossRef]
- Humphreys, E.R.; Lafleur, P.M. Does earlier snowmelt lead to greater CO2 sequestration in two low Arctic tundra ecosystems? Geophys. Res. Lett. 2011, 38, L09703. [Google Scholar] [CrossRef]
- Tuba, Z.; Csintalan, Z.; Proctor, M.C.F. Photosynthetic Responses of a Moss, Tortula ruralis, ssp. ruralis, and the Lichens Cladonia convoluta and C. furcata to Water Deficit and Short Periods of Desiccation, and Their Ecophysiological Significance: A Baseline Study at Present-Day CO2 Concentration. New Phytol. 1996, 133, 353–361. [Google Scholar] [CrossRef]
- Lund, M.; Lafleur, P.M.; Roulet, N.T.; Lindroth, A.; Christensen, T.R.; Aurela, M.; Chojnicki, B.H.; Flanagan, L.B.; Humphreys, E.R.; Laurila, T.; et al. Variability in exchange of CO2 across 12 northern peatland and tundra sites. Glob. Chang. Biol. 2010, 16, 2436–2448. [Google Scholar] [CrossRef]
- McNeil, P.; Waddington, J.M. Moisture controls on Sphagnum growth and CO2 exchange on a cut-over bog. J. Appl. Ecol. 2003, 40, 354–367. [Google Scholar] [CrossRef]
- Tuittila, E.-S.; Vasander, H.; Luine, J. Sensitivity of C sequestration in reintroduced Sphagnum to water-level variation in a cutaway peatland. Restor. Ecol. 2004, 12, 483–493. [Google Scholar] [CrossRef]
- Moore, T.R.; Bubier, J.L.; Bledzki, L. Litter decomposition in temperate peatland ecosystems: The effect of substrate and site. Ecosystems 2007, 10, 949–963. [Google Scholar] [CrossRef]
- Euskirchen, E.S.; Edgar, C.W.; Turetsky, M.R.; Waldrop, M.P.; Harden, J.W. Differential response of carbon fluxes to climate in three peatland ecosystems that vary in the presence and stability of permafrost. J. Geophys. Res. Biogeosci. 2014, 119, 1576–1595. [Google Scholar] [CrossRef]
- Gaumont-Guay, D.; Black, T.A.; Barr, A.G.; Jassal, R.S.; Nesic, Z. Biophysical controls on rhizospheric and heterotrophic components of soil respiration in a boreal black spruce stand. Tree Physiol. 2008, 28, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Gaumont-Guay, D.; Black, T.A.; Griffis, T.J.; Barr, A.G.; Jassal, R.S.; Nesic, Z. Interpreting the dependence of soil respiration on soil temperature and water content in a boreal aspen stand. Agric. For. Meteorol. 2006a, 140, 220–235. [Google Scholar] [CrossRef]
- Bergeron, O.; Margolis, H.A.; Coursolle, C. Forest floor carbon exchange of a boreal black spruce forest in eastern North America. Biogeosciences, 2009, 6, 1849–1864. [Google Scholar] [CrossRef]
- Tang, J.W.; Dennis, D.B.; Qi, Y.; Xu, L.K. Assessing soil CO2 efflux using continuous measurement of CO2 profiles in soils with small solid-state sensors. Agric. For. Meteo 2003, 118, 207–220. [Google Scholar] [CrossRef]
- Gaumont-Guay, D.; Black, T.A.; Griffis, T.J.; Barr, A.G.; Jassal, R.A.; Nesic, Z. Influence of temperature and drought on seasonal and interannual variations of soil, bole and ecosystem respiration in a boreal aspen stand. Agr. Forest Meteorol. 2006, 140, 203–219. [Google Scholar] [CrossRef]
- Helfter, C.; Campbell, C.; Dinsmore, K.J.; Drewer, J.; Coyle, M.; Anderson, M.; Skiba, U.; Nemitz, E.; Billett, M.F.; Sutton, M.A. Drivers of long-term variability in CO2 net ecosystem exchange in a temperate peatland. Biogeosciences 2015, 12, 1799–1811. [Google Scholar] [CrossRef]
- Raich, J.W.; Potter, C.S.; Bhagawati, D. Interannual variability in global soil respiration, 1980–94. Glob. Chang. Biol. 2002, 8, 800–812. [Google Scholar] [CrossRef]
- Rodeghiero, M.; Cescatti, A. Main determinants of forest soil respiration along an elevation/temperature gradient in the Italian Alps. Glob. Chang. Biol. 2005, 11, 1024–1041. [Google Scholar] [CrossRef]
- Lavigne, M.B.; Ryan, M.G.; Anderson, D.E.; Baldocchi, D.D.; Crill, P.M.; Fitzjarrald, D.R.; Goulden, M.L.; Gower, S.T.; Massheder, J.M.; McCaughey, J.H.; et al. Comparing nocturnal eddy covariance measurements to estimates of ecosystem respiration made by scaling chamber measurements at six coniferous boreal sites. J. Geophys. Res. 1997, 102, 28977–28985. [Google Scholar] [CrossRef]
- Rayment, M.B.; Jarvis, P.G. Temporal and spatial variation of soil CO2 efflux in a Canadian boreal forest. Soil Biol. Biochem. 2000, 32, 35–45. [Google Scholar] [CrossRef]
- Xu, M.; Qi, Y. Soil-surface CO2 efflux and its spatial and temporal variations in a young ponderosa pine plantation in northern California. Glob. Chang. Biol. 2001, 7, 667–677. [Google Scholar] [CrossRef]
- Peichl, M.; Öquist, M.; Löfvenius, M.O.; Ilstedt, U.; Sagerfors, J.; Grelle, A.; Lindroth, A.; Nilsson, M.B. A 12-year record reveals pre-growing season temperature and water table level threshold effects on the net carbon dioxide exchange in a boreal fen. Environ. Res. Lett. 2014, 9, 055006. [Google Scholar] [CrossRef]
- Startsev, N.A.; Lieffers, V.J. Emission of nitrogen gas, nitrous oxide, and carbon dioxide on rehydration of dry feathermosses. Soil Sci. Soc. Am. J. 2007, 71, 214–218. [Google Scholar] [CrossRef]
- Bubier, J.L.; Crill, P.M.; Moore, T.R.; Savage, K.; Varner, R.K. Seasonal patterns and controls on net ecosystem CO2 exchange in a boreal peatland complex. Glob. Biogeochem. Cycles 1998, 12, 703–714. [Google Scholar] [CrossRef]
- Lu, X.; Fan, J.; Yan, Y.; Wang, X. Responses of Soil CO2 Fluxes to Short-Term Experimental Warming in Alpine Steppe Ecosystem, Northern Tibet. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Yavitt, J.B.; Lang, G.E.; Wieder, R.K. Control of carbon mineralization to CH4 and CO2 in anaerobic, Sphagnum- derived peat from Big Run Bog. West Virginia. Biogeochemisty 1987, 4, 141–157. [Google Scholar] [CrossRef]
- Winston, G.C.; Sundquist, E.T.; Stephens, B.B.; Trumbore, S.E. Winter CO2 fluxes in a boreal forest. J. Geophys. Res. 1997, 102, 795–804. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada. Adjusted and Homogenized Canadian Climate Data (AHCCD). Available online: http://www.ec.gc.ca/dccha-ahccd/ (accessed on 14 November 2016).
- Sistla, S.A.; Moore, J.C.; Simpson, R.T.; Gough, L.; Shaver, G.R.; Schimel, J.P. Long-term warming restructures Arctic tundra without changing net soil carbon storage. Nature 2013, 497, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Quinton, W.L.; Hayashi, M.; Chasmer, L.E. Permafrost-thaw-induced land-cover change in the Canadian subarctic: Implications for water resources. Hydrol. Process. 2013, 25, 152–158. [Google Scholar] [CrossRef]
- Baltzer, J.L.; Veness, T.; Chasmer, L.E.; Sniderhan, A.E.; Quinton, W.L. Forests on thawing permafrost: Fragmentation, edge effects, and net forest loss. Glob. Chang. Biol. 2014, 20, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, S.D.; Pastor, J.; Dewey, B.; Weltzin, J.F.; Updegraff, K. Rapid carbon response of peatlands to climate change. Ecology 2008, 89, 3041–3048. [Google Scholar] [CrossRef]


| Latitude (°) | Longitude (°) | Ecozone | Permafrost status | Mean Annual Air Temperature (°C) | Growing Degree Days above 0 °C, (Days) | Mean Annual Snow Pack, (cm) | Mean Annual Precipitation, (mm) | |
|---|---|---|---|---|---|---|---|---|
| Anzac (AZ) | 56.40 | −111.03 | Mid-boreal | Isolated patches (0%–10%) | 1.49 | 2251 | 32 | 464 |
| Fort Simpson (FS) | 61.63 | −121.40 | High-boreal | Sporadic discontinuous (10%–50%) | −2.17 | 2064 | 54 | 361 |
| Norman Wells (NW) | 65.21 | −127.01 | Low Subarctic | Extensive discontinuous (50%–90%) | −4.89 | 1817 | 48 | 318 |
| Inuvik (IN) | 68.32 | −133.43 | High Subarctic | Continuous (90%–100%) | −6.9 | 1295 | 60 | 259 |
| Site | Location | a (g·CO2·m−2·h−1) | b (°C−1) | R2 |
|---|---|---|---|---|
| Mid-boreal | Upland | 0.128 | 0.17 | 0.91 |
| Peat Plateau | 0.018 | 0.28 | 0.75 | |
| Collapse scar | 0.072 | 0.08 | 0.79 | |
| High-boreal | Upland | 0.151 | 0.09 | 0.84 |
| Peat Plateau | 0.048 | 0.11 | 0.91 | |
| Collapse scar | 0.012 | 0.15 | 0.82 | |
| Low Subarctic | Upland | 0.035 | 0.15 | 0.82 |
| Peat Plateau | 0.034 | 0.11 | 0.76 | |
| Collapse scar | 0.047 | 0.11 | 0.68 | |
| High Subarctic | Upland | 0.023 | 0.16 | 0.97 |
| Peat Plateau | 0.010 | 0.27 | 0.87 | |
| Collapse scar | 0.011 | 0.356 | 0.82 |
| Site | Location | a (g·CO2·m−2·h−1) | b (°C−1) | R2 |
|---|---|---|---|---|
| Mid-boreal | Upland | 0.061 | 0.251 | 0.68 |
| Peat Plateau | 0.010 | 0.339 | 0.83 | |
| High-boreal | Upland | 0.146 | 0.080 | 0.63 |
| Peat Plateau | 0.001 | 0.216 | 0.91 | |
| Low Subarctic | Upland | 0.008 | 0.208 | 0.65 |
| Peat Plateau | 0.001 | 0.519 | 0.58 | |
| High Subarctic | Upland | 0.010 | 0.183 | 0.56 |
| Peat Plateau | 0.002 | 0.299 | 0.46 |
| Site | Uplands | Peat Plateau | Collapse Scar |
|---|---|---|---|
| Mid-boreal | 5.38 | 11.49 | 3.97 |
| High-boreal | 3.41 | 11.36 | 3.28 |
| Low Subarctic | 3.16 | 5.45 | 2.18 |
| High Subarctic | 2.86 | 5.53 | 1.22 |
| Site | Location | A′ | b′ | R2 |
|---|---|---|---|---|
| Mid boreal | Upland | 0.41 | 0.15 | 0.57 |
| Peat Plateau | 0.45 | 0.17 | 0.76 | |
| Collapse Scar | 1.27 | 0.26 | 0.81 | |
| High boreal | Upland | 0.21 | 0.07 | 0.71 |
| Peat Plateau | 0.20 | 1.14 | 0.55 | |
| Collapse Scar | 0.17 | 0.53 | 0.67 | |
| Low Subarctic | Upland | 0.18 | 0.93 | 0.69 |
| Peat Plateau | 0.11 | 0.61 | 0.93 | |
| Collapse Scar | 0.15 | 0.75 | 0.74 | |
| High Subarctic | Upland | 0.16 | 0.64 | 0.66 |
| Peat Plateau | 0.02 | 0.03 | 0.71 | |
| Collapse Scar | 0.38 | 0.12 | 0.87 |
| Site | Upland | Peat Plateau | Collapse Scar |
|---|---|---|---|
| Low boreal | 82.6 ± 4.01 Aa | 139.2 ± 12.4 Ba | 211.3 ± 9.68 Ca |
| High boreal | 62.0 ± 6.58 Ab | 92.0 ± 8.92 Bb | 198.0 ± 5.24 Ca |
| Low Subarctic | 56.7 ± 3.74 Ac | 63.3 ± 7.69 Ac | 127.4 ± 3.92 Cb |
| High Subarctic | 38.12 ± 4.62 Ad | 54.6 ± 8.16 Bc | 86.5 ± 6.15 Cc |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Startsev, N.; Bhatti, J.S.; Jassal, R.S. Surface CO2 Exchange Dynamics across a Climatic Gradient in McKenzie Valley: Effect of Landforms, Climate and Permafrost. Forests 2016, 7, 279. https://doi.org/10.3390/f7110279
Startsev N, Bhatti JS, Jassal RS. Surface CO2 Exchange Dynamics across a Climatic Gradient in McKenzie Valley: Effect of Landforms, Climate and Permafrost. Forests. 2016; 7(11):279. https://doi.org/10.3390/f7110279
Chicago/Turabian StyleStartsev, Natalia, Jagtar S. Bhatti, and Rachhpal S. Jassal. 2016. "Surface CO2 Exchange Dynamics across a Climatic Gradient in McKenzie Valley: Effect of Landforms, Climate and Permafrost" Forests 7, no. 11: 279. https://doi.org/10.3390/f7110279





