Nutrient Resorption and Phenolics Concentration Associated with Leaf Senescence of the Subtropical Mangrove Aegiceras corniculatum: Implications for Nutrient Conservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Measurement of N and P Concentrations
2.4. Determination of Total Phenolics (TPs), Extractable Condensed Tannins (ECT), Protein-Bound Condensed Tannins (PBCT), and Fiber-Bound Condensed Tannins (FBCT)
2.5. Calculations
2.6. Statistical Analysis
3. Results
3.1. Seasonal Changes of N and P Concentrations, N:P Ratios, and Nutrient Resorption in Mature and Senescent Leaves of A. corniculatum
3.2. Seasonal Changes of Tannins Concentrations in Mature and Senescent Leaves of A. corniculatum
3.3. Seasonal Changes of TPs:N and TCT:N Ratios in Mature and Senescent Leaves of A. corniculatum
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Twilley, R.W.; Lugo, A.E.; Patterson-Zucca, C. Litter production and turnover in basin mangrove forests in southwest Florida. Ecology 1986, 67, 670–683. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: New York, NY, USA, 1986. [Google Scholar]
- Li, M. Nutrient dynamics of a Futian mangrove forest in Shenzhen, South China. Estuar. Coast. Shelf. Sci. 1997, 45, 463–472. [Google Scholar] [CrossRef]
- Odum, W.E.; Heald, E.J. The detritus-based food web of an estuarine mangrove community. In Estuarine Research; Cronin, L.E., Ed.; Academic Press: New York, NY, USA, 1975; pp. 265–286. [Google Scholar]
- Alongi, D.M.; Boto, K.G.; Robertson, A.I. Nitrogen and phosphorus cycles. In Tropical Mangrove Ecosystems; Robertson, A.I., Alongi, D.M., Eds.; American Geographysical Union: Washington, DC, USA, 1992; pp. 251–292. [Google Scholar]
- Wang, W.Q.; Wang, M.; Lin, P. Seasonal changes in element contents in mangrove element retranslocation during leaf senescence. Plant Soil 2003, 252, 187–193. [Google Scholar] [CrossRef]
- Cai, G. Salt content and regulation of mangroves. Ecol. Sci. 1990, 2, 123–127. [Google Scholar]
- Lin, Y.M.; Liu, X.W.; Zhang, H.; Fan, H.Q.; Lin, G.H. Nutrient conservation strategies of a mangrove species Rhizophora stylosa under nutrient limitation. Plant Soil 2010, 326, 469–479. [Google Scholar] [CrossRef]
- Wei, S.D.; Liu, X.W.; Zhang, L.H.; Chen, H.; Zhang, H.; Zhou, H.C.; Lin, Y.M. Seasonal changes of nutrient levels and nutrient resorption in Avicennia marina leaves in Yingluo Bay, China. South. For. 2015, 77, 237–242. [Google Scholar]
- Millard, P.; Neilsen, G.H. The influence of nitrogen supply on the uptake and remobilization of stored N for the seasonal growth of apple trees. Ann. Bot. 1989, 63, 301–309. [Google Scholar]
- Lin, Y.; Sternberg, L.D.L. Nitrogen and phosphorus dynamics and nutrient resorption of Rhizophora mangle leaves in south Florida, USA. Bull. Mar. Sci. 2007, 80, 159–169. [Google Scholar]
- Pugnaire, F.I.; Chapin, F.S. Controls over nutrient resorption from leaves of evergreen Mediterranean species. Ecology 1993, 74, 124–129. [Google Scholar] [CrossRef]
- Feller, I.C. Effects of nutrient enrichment on growth and herbivory of dwarf red mangrove (Rhizophora mangle). Ecol. Monogr. 1995, 65, 477–505. [Google Scholar] [CrossRef]
- Reef, R.; Feller, I.C.; Lovelock, C.E. Nutrition of mangroves. Tree Physiol. 2010, 30, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- McKee, K.L.; Feller, I.C.; Popp, M.; Wanek, W. Mangrove isotopic (δ15N and δ13C) fractionation across a nitrogen vs. phosphorus limitation gradient. Ecology 2002, 83, 1065–1075. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Hernes, P.J.; Benner, R.; Cowie, G.L.; Goni, M.A.; Bergamaschi, B.A.; Hedges, J.I. Tannin diagenesis in mangrove leaves from a tropical estuary: A novel molecular approach. Geochim. Cosmochim. Acta 2001, 65, 3109–3122. [Google Scholar] [CrossRef]
- Zucker, W.V. Tannins: Does structure determine function? An ecological perspective. Am. Nat. 1983, 121, 335–365. [Google Scholar] [CrossRef]
- Lin, Y.M.; Liu, J.W.; Xiang, P.; Lin, P.; Ding, Z.H.; Sternberg, L.D.L. Tannins and nitrogen dynamics in mangrove leaves at different age and decay stages (Jiulong River Estuary, China). Hydrobiologia 2007, 583, 285–295. [Google Scholar] [CrossRef]
- Lin, Y.M.; Liu, J.W.; Xiang, P.; Lin, P.; Ye, G.; Sternberg, L.D.L. Tannin dynamics of propagules and leaves of Kandelia candel and Bruguiera gymnorrhiza in the Jiulong River Estuary, Fujian, China. Biogeochemistry 2006, 78, 343–359. [Google Scholar] [CrossRef]
- Serrano, L. Leaching from vegetation of soluble polyphenolic compounds, and their abundance in temporary ponds in the Doñana National Park (SW Spain). Hydrobiologia 1992, 229, 43–50. [Google Scholar] [CrossRef]
- Northup, R.R.; Yu, Z.; Dahlgren, R.A.; Vogt, K.A. Polyphenol control of nitrogen release from pine litter. Nature 1995, 377, 227–229. [Google Scholar] [CrossRef]
- Huang, G.M. Growth characteristics of Spartina alterniflora and its relative competitive ability with Kandelia candle in mangrove areas of the Zhangjiang estuary. Master’s Thesis, Xiamen University, Xiamen, China, 2009. [Google Scholar]
- Chen, H.; Lu, W.; Yan, G.; Yang, S.; Lin, G. Typhoons exert signifificant but differential impacts on net ecosystem carbon exchange of subtropical mangrove forests in China. Biogeosciences 2014, 11, 5323–5333. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice, 2nd ed.; The International Rice Research Institute: Manila, Philippines, 1972; pp. 7–9, 36–38. [Google Scholar]
- Nanjing Institute of Soil Science. Physical and Chemical Analysis of Soils; Science and Technology Press: Shanghai, China, 1978; pp. 1–320. [Google Scholar]
- Graham, H.D. Stabilization of the Prussian blue color in the determination of polyphenols. J. Agric. Food Chem. 1992, 40, 801–805. [Google Scholar] [CrossRef]
- Terrill, T.H.; Rowan, A.M.; Douglas, G.B.; Barry, T.N. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. J. Sci. Food Agric. 1992, 58, 321–329. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrients in senesced leaves: Keys to the search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Rejmánková, E. Nutrient resorption in wetland macrophytes: Comparison across several regions of different nutrient status. New Phytol. 2005, 167, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 1998. [Google Scholar]
- Aerts, R.J.; Barry, T.N.; McNabb, W.C. Polyphenols and agriculture: Beneficial effects of proanthocyanidins forages. Agr. Ecosyst. Environ. 1999, 75, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.H.; Ye, G.F.; Lin, Y.M.; Zhou, H.C.; Zeng, Q. Seasonal changes in tannin and nitrogen contents of Casuarina equisetifolia branchlets. J. Zhejiang Uni. Sci. B 2009, 10, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.F.; Zhang, S.J.; Zhang, L.H.; Lin, Y.M.; Wei, S.D.; Liao, M.M.; Lin, G.H. Age-related changes in nutrient resorption patterns and tannin concentration of Casuarina equisetifolia plantations. J. Trop. For. Sci. 2012, 24, 546–556. [Google Scholar]
- Aerts, R.; Chapin, F.S. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Kobe, R.K.; Lepczyk, C.A.; Iyer, M. Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 2005, 86, 2780–2792. [Google Scholar] [CrossRef]
- Wright, I.J.; Westoby, M. Nutrient concentration, resorption and lifespan: Leaf traits of Australian sclerophyll species. Funct. Ecol. 2003, 17, 10–19. [Google Scholar] [CrossRef]
- Chapin, F.S.; Kedrowski, R.A. Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 1983, 64, 376–391. [Google Scholar] [CrossRef]
- Lal, C.B.; Annapurna, C.; Raghubanshi, A.S.; Singh, J.S. Effect of leaf habit and soil type on nutrient resorption and conservation in woody species of a dry tropical environment. Can. J. Bot. 2001, 79, 1066–1075. [Google Scholar]
- Cai, Z.; Bongers, F. Contrasting nitrogen and phosphorus resorption efficiencies in trees and lianas from a tropical montane rain forest in Xishuangbanna, south-west China. J. Trop. Ecol. 2007, 23, 115–118. [Google Scholar] [CrossRef]
- Güsewell, S. Nutrient resorption of wetland graminoids is related to the type of nutrient limitation. Funct. Ecol. 2005, 19, 344–354. [Google Scholar] [CrossRef]
- Feeny, P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 1970, 51, 565–581. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Teklay, T. Seasonal dynamics in the concentrations of macronutrients and organic constituents in green and senesced leaves of three agroforestry species in southern Ethiopia. Plant Soil 2004, 267, 297–307. [Google Scholar] [CrossRef]
- Mafongoya, P.L.; Giller, K.E.; Palm, C.A. Decomposition and nitrogen release patterns of tree prunings and litter. Agrofor. Syst. 1998, 38, 77–97. [Google Scholar] [CrossRef]
- Constantinides, M.; Fownes, J.H. Nitrogen mineralization from leaves and litter of tropical plants: Relationship to nitrogen, lignin and soluble polyphenol concentrations. Soil Biol. Biochem. 1994, 26, 49–55. [Google Scholar] [CrossRef]
- Kuhajek, J.M.; Payton, I.J.; Monks, A. The impact of defoliation on the foliar chemistry of southern rata (Metrosideros umbellata). N. Z. J. Ecol. 2006, 30, 237–249. [Google Scholar]
- Mansfield, J.L.; Curtis, P.S.; Zak, D.R.; Pregitzer, K.S. Genotypic variation for condensed tannin production in trembling aspen (Populus tremuloides, Salicaceae) under elevated CO2 and in high-and low-fertility soil. Am. J. Bot. 1999, 86, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.P.; Chapin, F.S., III; Klein, D.R. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef]
- Kandil, F.E.; Grace, M.H.; Seigler, D.S.; Cheeseman, J.M. Polyphenolics in Rhizophora mangle L. leaves and their changes during leaf development and senescence. Trees 2004, 18, 518–528. [Google Scholar] [CrossRef]
- Maie, N.; Behrens, A.; Knicker, H.; Kogel-Knabner, I. Changes in the structure and protein binding ability of condensed tannins during decomposition of fresh needles and leaves. Soil Biol. Biochem. 2003, 35, 577–589. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems—A review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Zasoski, R.J.; Dahlgren, R.A. Fertility and pH effects on polyphenol and condensed tannin concentrations in foliage and roots. Plant Soil 2004, 262, 95–109. [Google Scholar] [CrossRef]
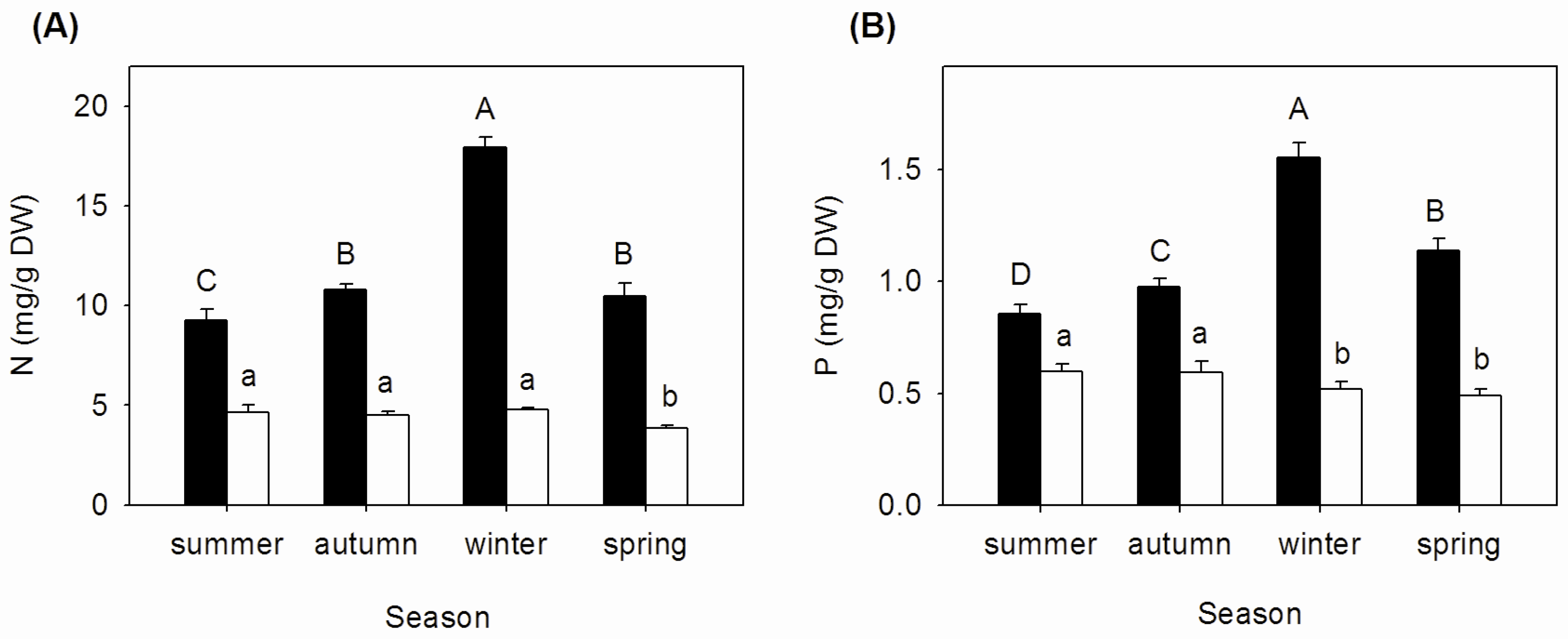
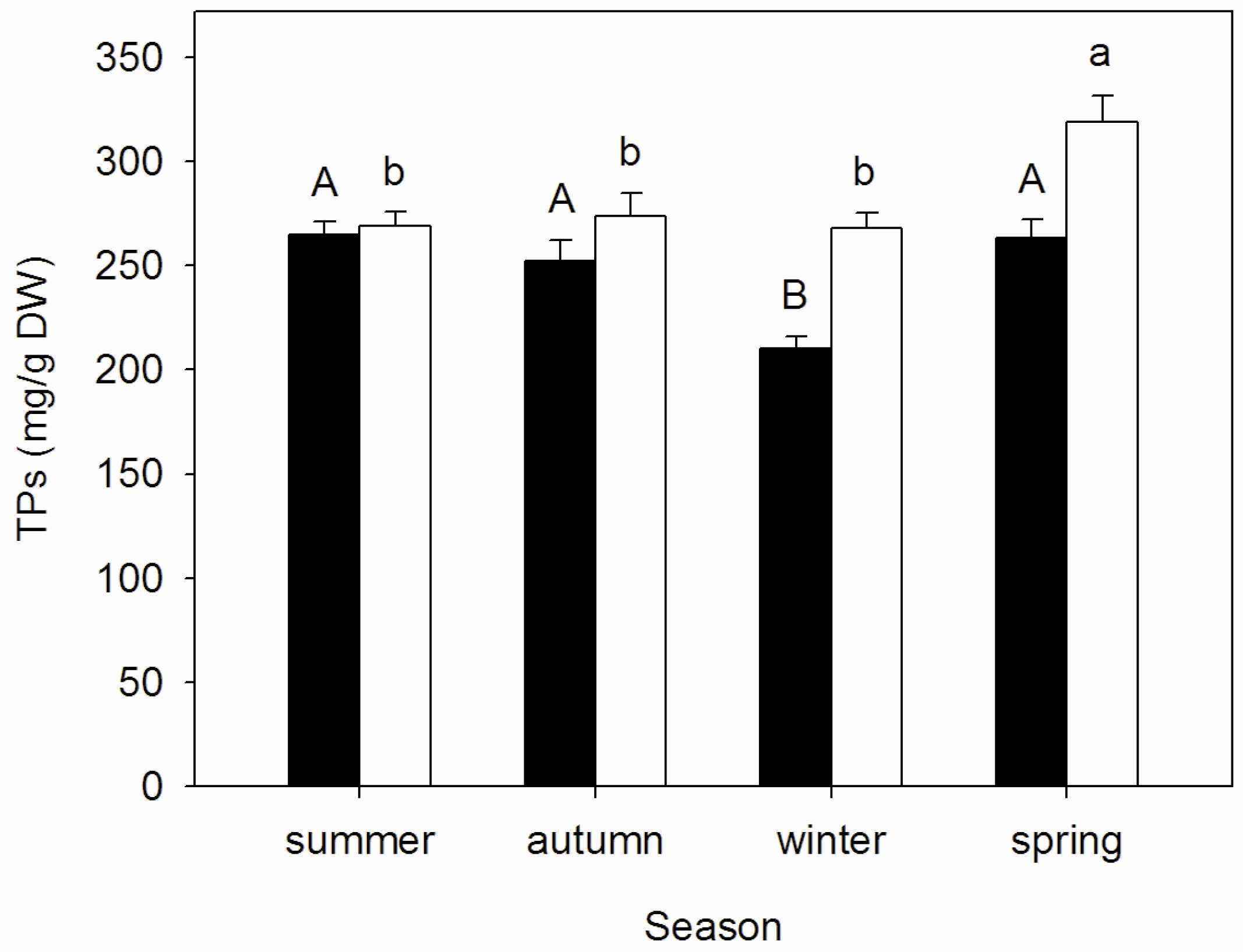
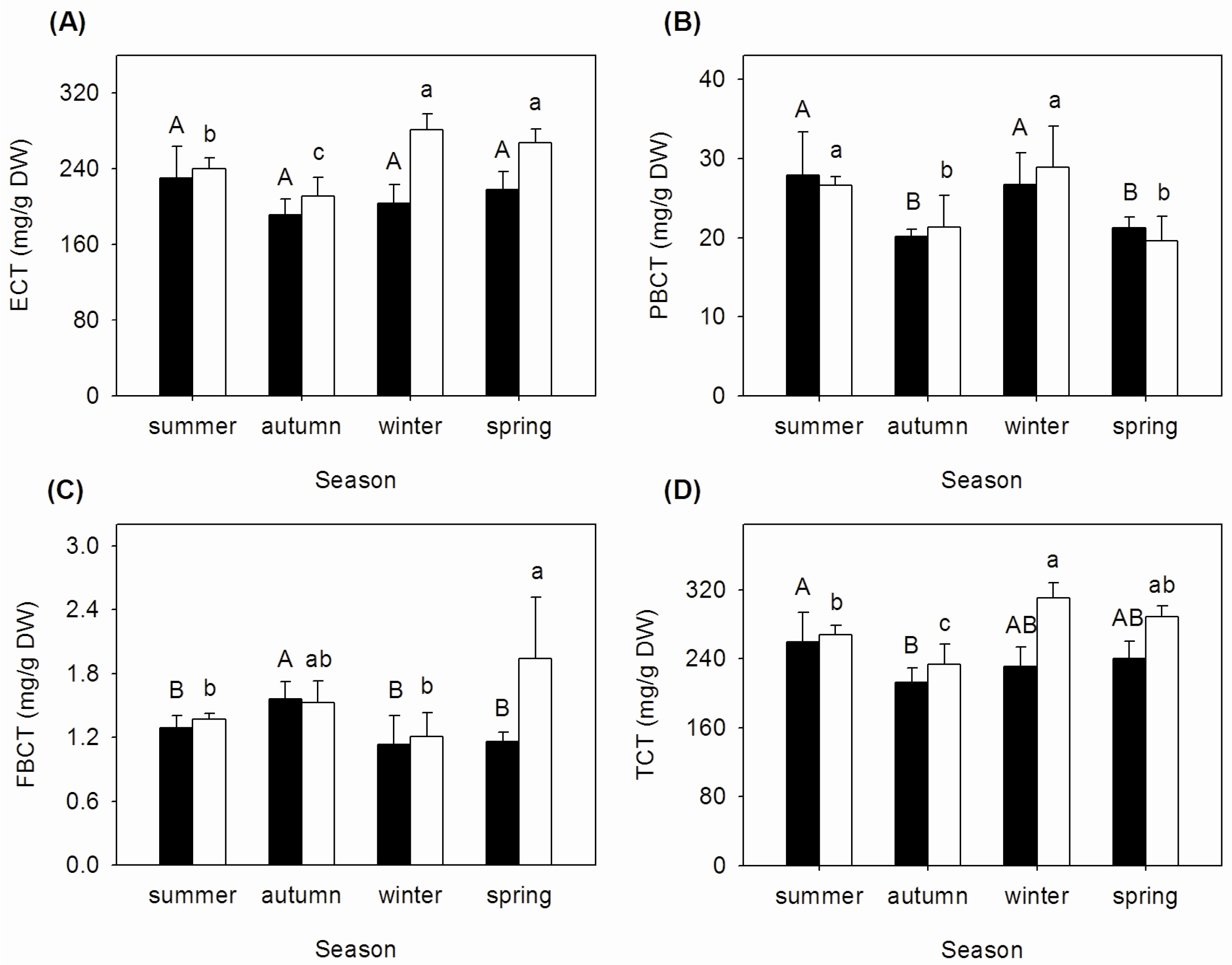
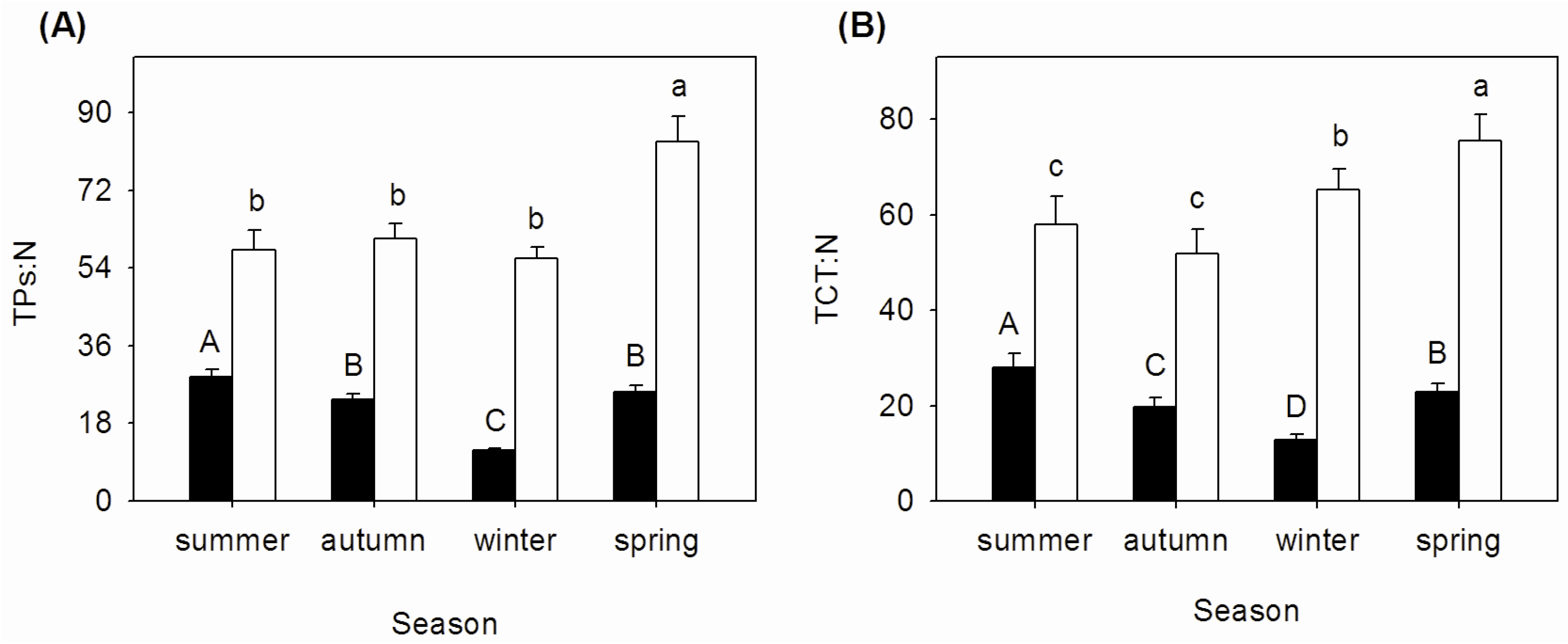
| Season | Nm:Pm | NRE (%) | PRE (%) | NRP (%) | PRP (%) |
|---|---|---|---|---|---|
| Summer | 10.90 ± 1.13 a | 49.87 ± 4.39 d | 30.01 ± 4.43 d | 0.46 ± 0.04 a | 0.06 ± 0.00 a |
| Autumn | 11.09 ± 0.41 a | 58.18 ± 2.33 c | 38.84 ± 5.94 c | 0.45 ± 0.02 a | 0.06 ± 0.00 a |
| Winter | 11.55 ± 0.55 a | 73.39 ± 1.06 a | 66.51 ± 2.37 a | 0.48 ± 0.01 a | 0.05 ± 0.00 b |
| Spring | 9.23 ± 0.92 b | 63.36 ± 2.03 b | 57.08 ± 3.48 b | 0.38 ± 0.02 b | 0.05 ± 0.00 b |
| Correlation | F | R | p |
|---|---|---|---|
| Nm-Pm | 108.070 | 0.926 | <0.001 |
| Ns-Ps | 5.765 | 0.493 | 0.027 |
| Nm-NRE | 55.731 | 0.869 | <0.001 |
| Ns-NRE | 0.117 | −0.080 | 0.737 |
| Pm-PRE | 91.214 | 0.914 | <0.001 |
| Ps-PRE | 32.805 | −0.804 | <0.001 |
| NRE-PRE | 82.361 | 0.906 | <0.001 |
| Correlation | F | R | p |
|---|---|---|---|
| TPsm-Nm | 122.338 | −0.934 | <0.001 |
| TPss-Ns | 34.399 | −0.810 | <0.001 |
| TPsm-Pm | 56.098 | −0.870 | <0.001 |
| TPss-Ps | 11.631 | −0.627 | 0.003 |
| TPsm-Nm:Pm | 3.940 | −0.424 | 0.063 |
| TPss-Nm:Pm | 12.143 | −0.635 | 0.003 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Xu, B.; Wei, S.; Zhang, L.; Zhou, H.; Lin, Y. Nutrient Resorption and Phenolics Concentration Associated with Leaf Senescence of the Subtropical Mangrove Aegiceras corniculatum: Implications for Nutrient Conservation. Forests 2016, 7, 290. https://doi.org/10.3390/f7110290
Chen H, Xu B, Wei S, Zhang L, Zhou H, Lin Y. Nutrient Resorption and Phenolics Concentration Associated with Leaf Senescence of the Subtropical Mangrove Aegiceras corniculatum: Implications for Nutrient Conservation. Forests. 2016; 7(11):290. https://doi.org/10.3390/f7110290
Chicago/Turabian StyleChen, Hui, Benbo Xu, Shudong Wei, Lihua Zhang, Haichao Zhou, and Yiming Lin. 2016. "Nutrient Resorption and Phenolics Concentration Associated with Leaf Senescence of the Subtropical Mangrove Aegiceras corniculatum: Implications for Nutrient Conservation" Forests 7, no. 11: 290. https://doi.org/10.3390/f7110290





