Both forest stands have been altered by different forest management strategies since the 1850s and by impacts of acid deposition during the 20th century. Former mixed forests were converted into pure spruce monocultures, with current second rotation of Norway spruce in the spruce stand. The beech stand was transformed by acid deposition to broadleaf monoculture during the 1970s and 1980s by induced selective conifer dieback. It is very likely that in the 1850s, similar site conditions prevailed at both stands. Since then, significantly higher soil C and N stocks have been measured in the beech forest compared to the spruce forest. While standing biomass was higher in the beech stand, due to the relatively high tree density combined with the high wood biomass of the 150 year old beech forest, the biomass N stocks were comparable among stands, resulting in a lower C/N ratio in the spruce stand compared to the beech stand.
Although estimated historical N inputs were higher in the spruce stand due to higher pollutant scavenging by spruce needles [
47], current deposition fluxes of N are similar in both forests. On the contrary, DOC throughfall fluxes were higher in the spruce stand. Few differences were measured in the DOC soil solution fluxes in the soil profiles. Nitrogen fluxes were dominated by DON at both stands, with higher NO
3− losses in the beech stand. Soil respiration dynamics were similar for both forest sites, with slightly higher Q
10 in the beech stand compared to the spruce stand. As a result, annual soil respiration was similar at both sites. Higher litterfall C flux in the beech forest caused lower total belowground carbon allocation (TBCA) compared to the spruce stand. The estimated fraction of TBCA that accounted for belowground net primary productivity (BNPP) was similar in both forest ecosystems. Due to the lower TBCA, both gross primary productivity (GPP) and net primary productivity (NPP) were lower in the beech stand compared to the spruce stand. Due to the similar evapotranspiration (ET) in both stands, water use efficiency (WUE) was higher in the spruce stand compared to the beech stand. Higher N fluxes were measured in the beech stand compared to the spruce stand, together with higher susceptibility of inorganic N losses in the beech stand compared to the spruce stand.
4.1. Tree Species Effects on C Cycling
Annual litterfall was higher in the beech stand compared to the spruce stand. Spruce litterfall flux was comparable to other studies on temperate forests of similar age, whereas beech litterfall was higher at our investigated stand [
48,
49]. The relatively high measured litter flux in the beech stand was a result of high crown area and dimensions of beech trees in the plot. Litterfall is an important component of forest ecosystem NPP and nutrient cycling [
50]; thus, it is highly important for forest management strategies to sequester C [
51]. Our results suggest that old growth broadleaf forest could deliver a substantial amount of assimilated C to the forest floor compared with lower inputs in managed forests.
Surprisingly similar fluxes of the DOC from the forest floor were measured in the two forest stands. DOC flux represented 11% of annual litterfall flux in the spruce stand and 6% of annual litterfall flux in the beech stand. This can be interpreted as showing that spruce litter is an important source of soil solution DOC [
52]. As for the forest floor, similar seepage fluxes of DOC were observed in the mineral soil. A significant reduction of DOC flux occurred from top soil to 40 cm depth, by roughly 80%. Whether it is absorbed in the mineral soil or respired needs further research. However, despite comparable fine soil pools in both forest floor and mineral soil, significantly higher C concentration in deeper mineral soil (20–40 cm) caused a significantly higher soil C pool in the beech stand compared to the spruce stand. As we did not observe significantly higher DOC flux from decaying litter in the beech stand compare to the spruce stand, we suggest that higher soil C pool under the beech forest was a result of long-term accumulation of recalcitrant SOM that originated from root and mycorrhizal decomposition [
15,
16]. Simultaneously, higher contents of oxalate-extractable Fe and Al were found in the beech mineral soil [
53], suggesting higher sorption capacity in this stand, favouring C sequestration in the beech stand over the spruce stand. Contrary to previous paired studies (e.g., [
13]), we did find a higher C stock in the forest floor of the beech stand compared to the spruce stand. In an earlier study, we documented partial loss of forest floor C in the spruce stand as a result of recovery from acidification [
54]. In the early 1990s, forest floor C mass was 58 t·ha
−1, comparable to the current beech stand, but it has since declined by 47%. We do not know whether a similar proportional decline occurred at the beech stand due to the lack of available historical data. We can only suggest that relatively high N availability at the beech stand (low soil C/N, some inorganic N leaching) may have stimulated greater stabilization into humus through a combination of chemical reactions and enzyme inhibition [
55,
56], thus preventing SOM loss from the forest floor after acidification retreat.
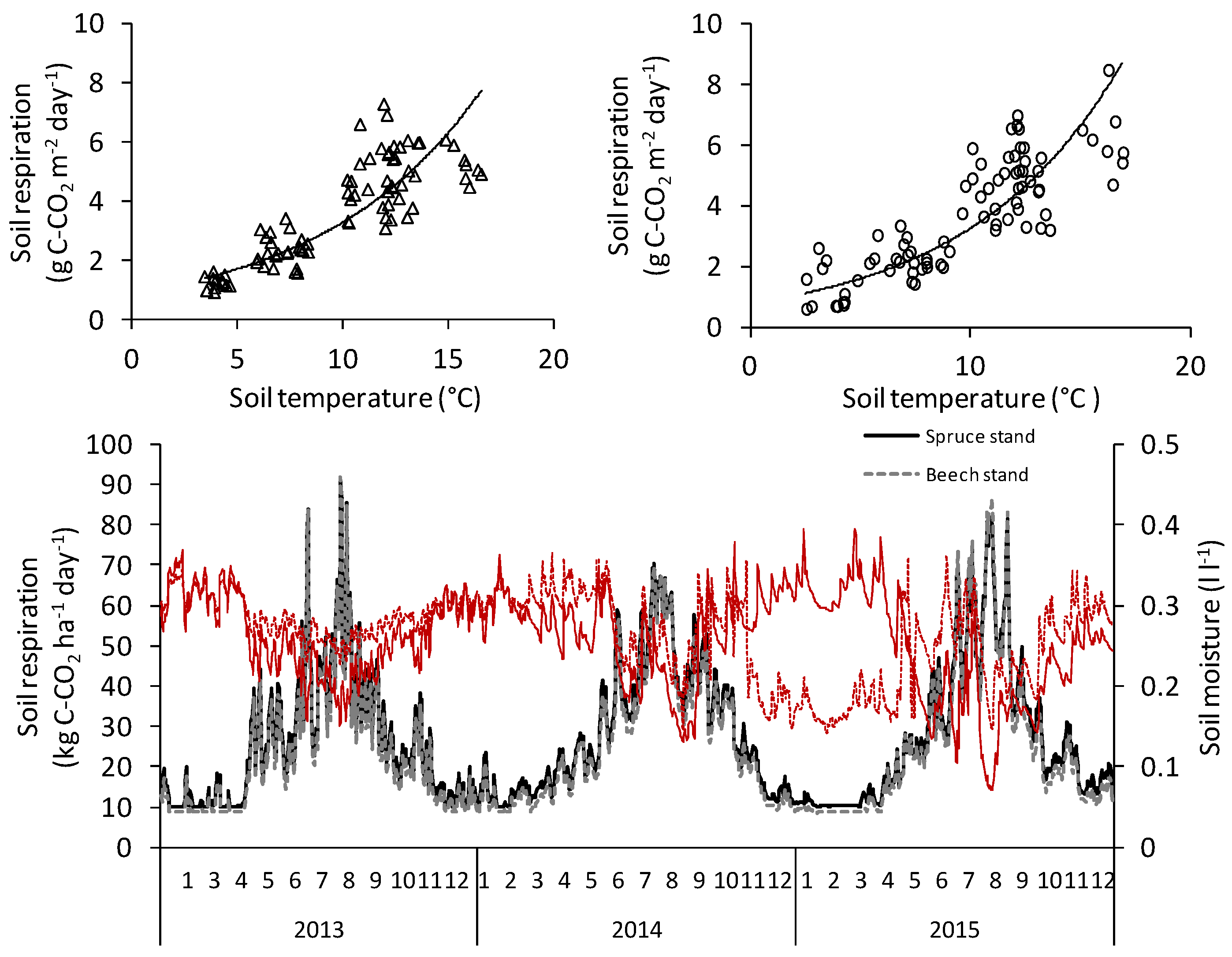
Soil temperature explained 78% and 72% of temporal variation in soil respiration in the spruce and beech stands, respectively. Soil moisture did not provide additional explanatory power in multiple regression analysis, suggesting low moisture stress in our mountainous forest ecosystems. Q
10 was slightly higher in the beech stand (4.2) compared to the spruce stand (3.7), but both values were close to the Q
10 values reported for other temperate ecosystems [
23,
41,
57]. Based on our calculations, potential respiration at 0 °C was higher in the spruce (0.89 g·m
−2·day
−1 of C) than in the beech stand (0.78 g·m
−2·day
−1 of C) and similar at 10 °C. Thus, annual CO
2 soil efflux was higher in the spruce stand compared to the beech stand, due to the calculated higher soil respiration in winter and spring periods.
One of the assumptions when calculating TBCA is that the total soil C pool is in a steady state. This assumption might be violated, given the significant decline of forest floor C in the spruce stand noted above [
54]. It is difficult to predict whether the spruce soil is currently in a steady state; thus, further calculation might be biased by the annual net loss of C from soil. Moreover, comparison with published relationships between soil respiration and litterfall presented in [
42,
58] suggested higher soil CO
2 efflux compared to annual C in litterfall in the spruce stand. The soil respiration rate of the beech stand is in line with similar published global measurements. Based on our data, 42% and 39% of TBCA goes to the BNPP in the spruce and beech stand, respectively. This is less than published results from the Rocky Mountains by McDowell and others [
59], but within the range of BNPP:TBCA ratios (0.26–0.53) published by Litton and Giardina [
60]. Based on our calculation, 27% and 34% of TBCA (spruce and beech, respectively) goes to the fine roots, mycorrhizal production and root exudates. Our estimates of fine root production and root respiration are based on literature review data [
44,
45] with the same partitioning of NPP applied to both stands. Thus, the presented results are more or less indicative. Nevertheless, our estimates are close to a published review by Nadelhoffer and Raich [
61], who estimated that approx. 33% of TBCA goes to fine root production.
Calculated ANPP and BNPP were used to calculate autotrophic respiration of both belowground and aboveground components [
45] to constrain GPP of both stands. Despite similar ANPP, lower BNPP at the beech stand caused lower GPP compared to the spruce stand. However, both GPP and NPP were lower in the old beech forest ecosystem compared to the younger spruce forest, suggesting continuous decline of both GPP and
Rtotal in aging forests [
62,
63]. Similar evapotranspiration (ET) for both stands and lower GPP for the beech stand resulted in lower WUE for the beech stand compared to the spruce stand.
4.2. Tree Species Effects on N Cycling
In the spruce throughfall, higher DON and lower NH
4 fluxes were measured compared to beech throughfall. Our results are in line with the study of Kopacek and others [
64], showing the importance of the Norway spruce canopy in chemical N transformation of precipitation. On average, beech stemflow accounted for roughly 10% of total N input to the forest floor by precipitation. Despite similar current N throughfall fluxes, estimated historical N deposition to the spruce forest was higher compared with the beech stand [
32], suggesting higher cumulative N deposition flux in the spruce plantation compared to the beech forest. Litterfall N flux was higher in the beech stand compared to the spruce stand; however, a lower C/N litterfall ratio was measured in the spruce litter (42) compared to beech litter (52). Combined precipitation and litterfall fluxes yielded higher N flux in the forest floor in the beech stand compared to the spruce stand. Dissolved losses of N from the forest floor accounted for 16% of annual N litterfall in both stands. Forest floor N losses were dominated by DON, with higher inorganic N leaching (mainly as NO
3−) in the beech stand compared to the spruce stand. Nevertheless, long-term measurements at the spruce stand revealed cessation of NO
3− leaching following reduction of acid deposition [
33]. Currently, N seepage losses in the 30 cm soil depth account for ca. 10% and 23% of precipitation inputs in the spruce and beech forests, respectively.
Annual forest requirements to build up canopy foliage accounted for 45 kg·ha
−1·year
−1 of N in the spruce stand and for 116 kg·ha
−1·year
−1 of N in the beech stand. Those values are, with respect to the beech stand, much higher than annual N recycling through aboveground litter production and precipitation deposition. Thus, retranslocation of N from senescing foliage is probably crucial for N retention in the beech stand. We may hypothesise that retranslocation of N before the onset of litterfall prevents the ecosystem from leaching higher amounts of this limiting nutrient, as enhanced SOM mineralization and nitrification in the period of low plant N demand can attribute to the mineral N losses from the ecosystem. Calculated N
uptake from soil was, due to N translocation, similar in both forest stands. Moreover, calculated potential N soil requirements were higher compared to N inputs via throughfall and litterfall in the spruce stand and comparable in the beech stand. This suggests more tightly constrained N cycling in the spruce stand compared to the beech stand. Higher susceptibility of the beech stand to inorganic N soil leaching could be due to this less constrained N cycling. However, high gross N mineralization and nitrification rates in old growth forests [
65,
66] can lead to N losses when availability of labile C declines. Thus, N leaching in an old growth beech stand can be a result of C limitation coupled with a higher N availability due to the higher total N return in litter [
50].