Independent Effects of Invasive Shrubs and Deer Herbivory on Plant Community Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Experimental Design and Treatments
2.3. Vegetation Sampling
2.4. Deer Density
2.5. Statistical Analyses
3. Results
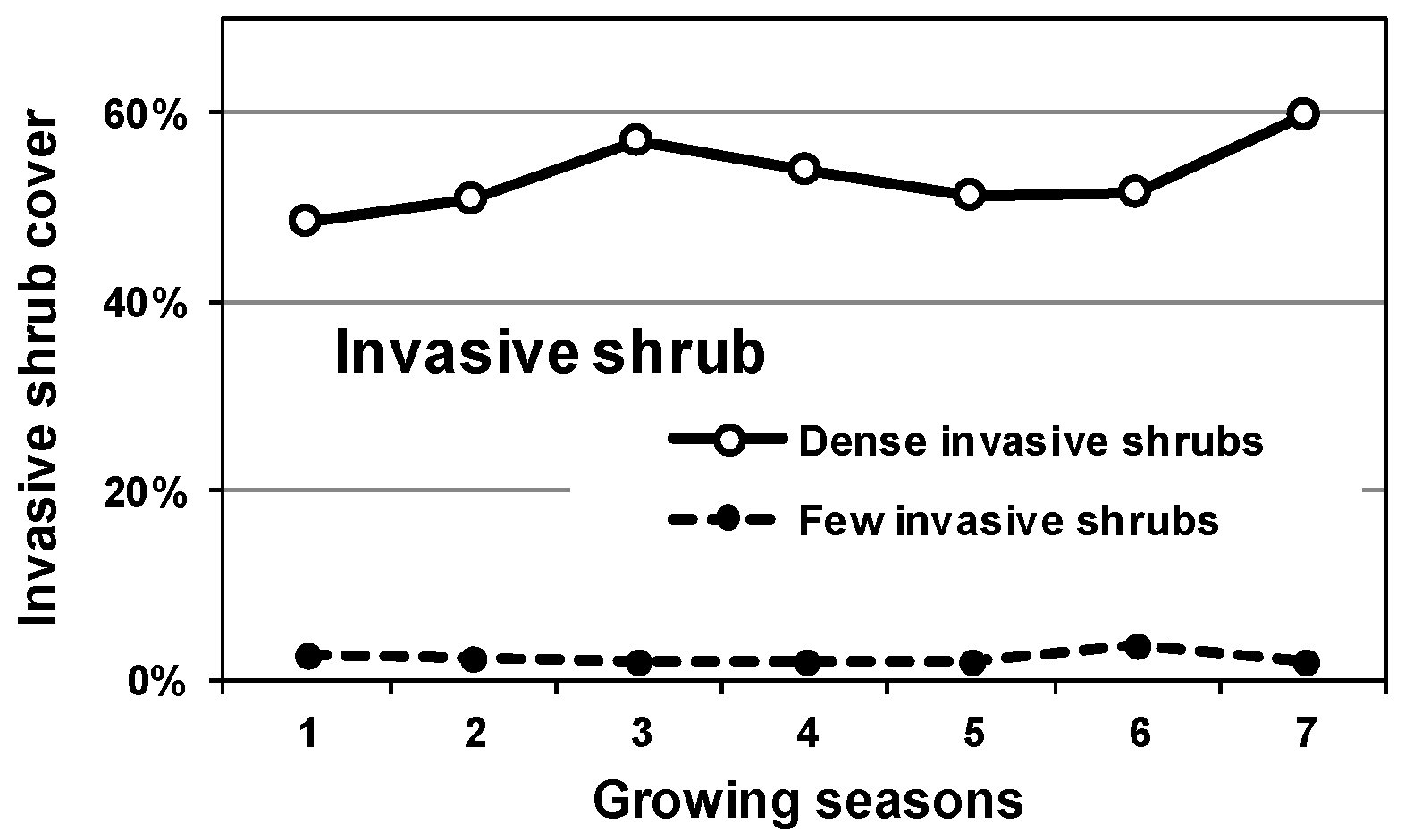
3.1. Pre-Treatment Invasive Cover
3.2. Deer Density
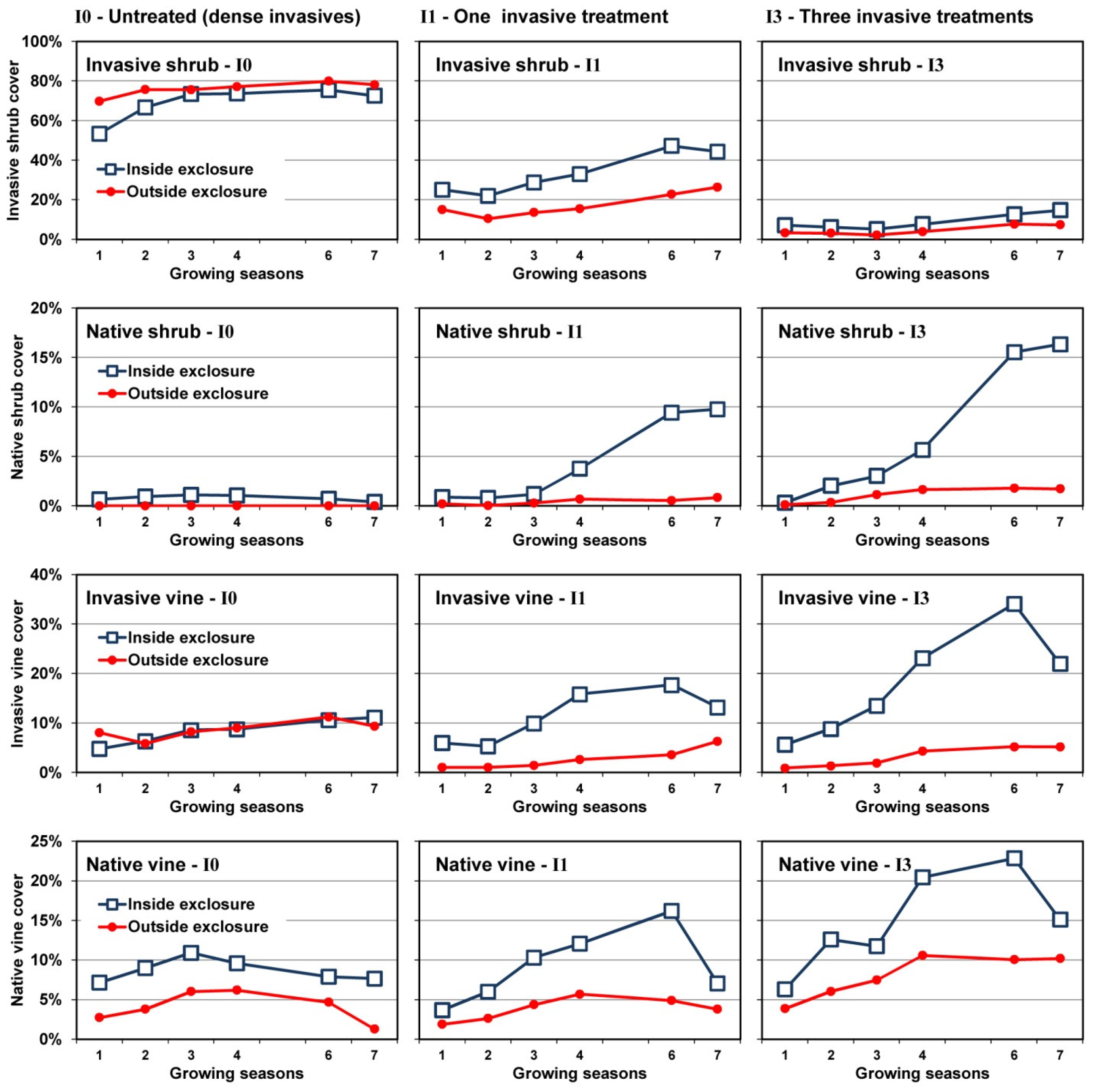
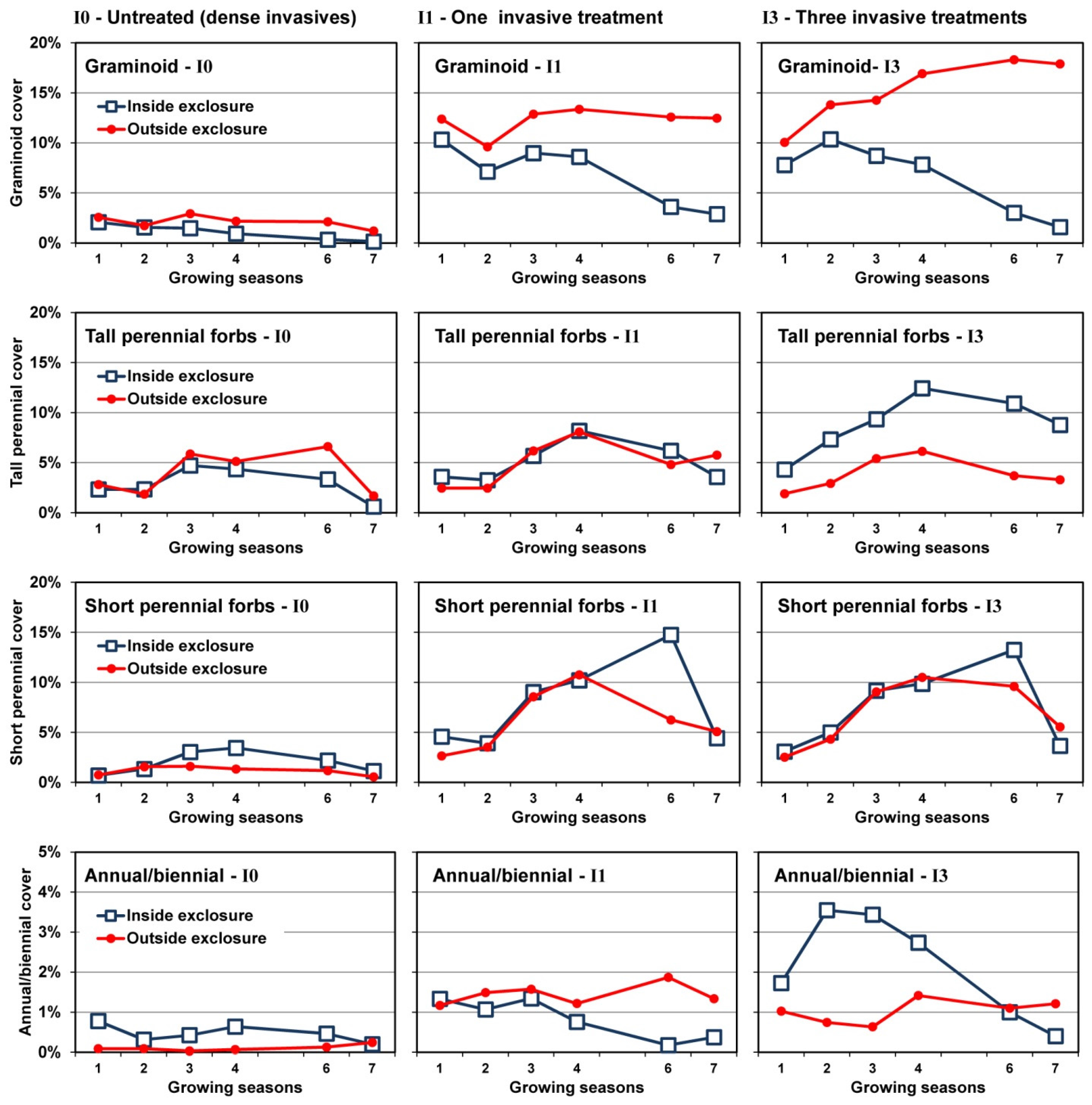
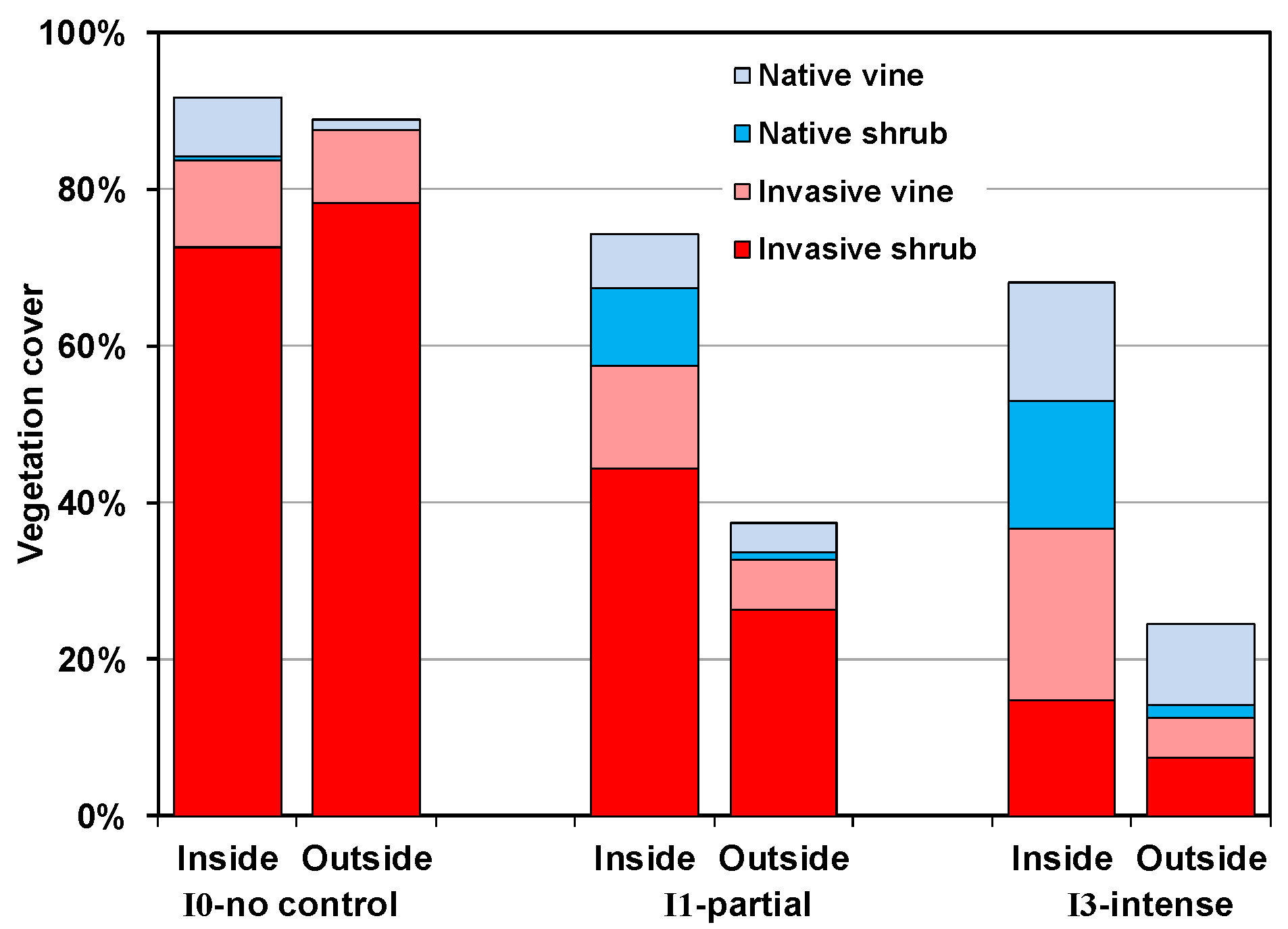
3.3. Changes in Cover over Time
3.4. Plant Community Composition
4. Discussion
4.1. Invasive Shrub Thickets
4.2. Deer Herbivory
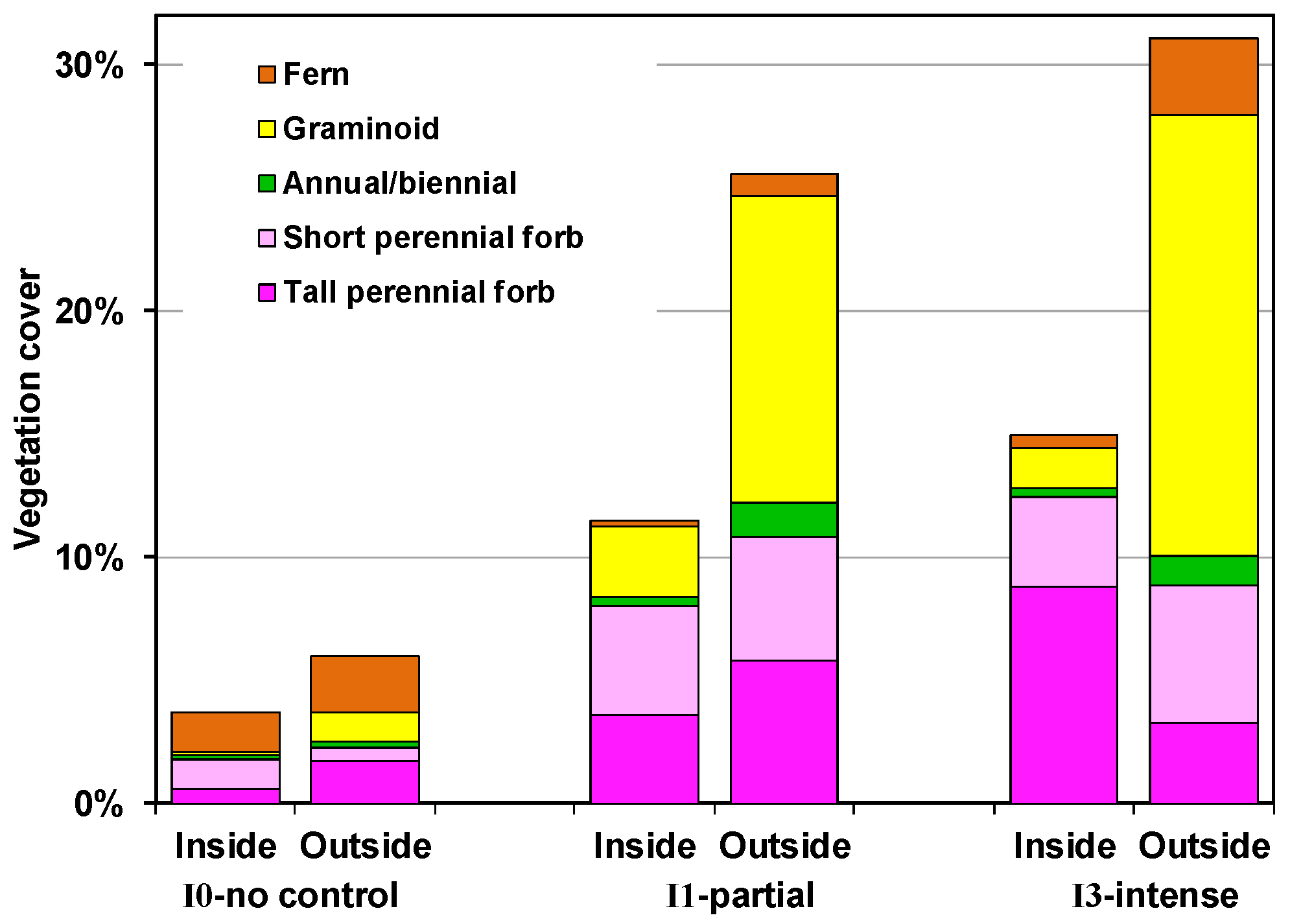
4.2.1. Outside Exclosures
4.2.2. Inside Exclosures
5. Conclusions
- (1)
- Neither invasive shrub control nor deer exclusion by themselves will restore native plant communities; restoration will require both stressors to be addressed.
- (2)
- Plant community composition remained largely stable for a seven year period where recalcitrant invasive shrubs were not treated, even where deer were excluded. As none of the guilds showed evidence of directional change over the course of the study without controlling invasive shrubs, we believe it is likely these communities will persist for at least another decade, if not longer.
- (3)
- Both mowing (I1) and intensive control (I3) of well-established invasive shrub thickets will not lead to reestablishment of native shrubs if they are not already present or can be recruited from the seedbank, as was the case with Rubus in the current study.
- (4)
- Recovery of both native shrubs and native forbs increases with intensity of invasive shrub control treatments provided plants are protected from deer herbivory. Without controlling deer herbivory, recovery of native graminoids, and probably ferns, will increase with intensity of invasive shrub control treatments.
- (5)
- Because both invasive vines and an invasive grass also increased following invasive shrub control where deer herbivory was excluded, managers will need to plan for this possibility.
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Latin | Species | Occurrence | Cover |
|---|---|---|---|
| Non-native species | |||
| Shrub | |||
| Berberis thunbergii DC. | Japanese barberry * | 884 | 33.6% + 1.1% |
| Berberis vulgaris L. | Common barberry | 4 | 30.9% + 8.8% |
| Rosa multiflora Thunb. | Multiflora rose * | 434 | 17.6% + 1.2% |
| Rubus phoenicolasius Maxim. | Wine raspberry * | 149 | 6.4% + 0.7% |
| Vine | |||
| Celastrus orbiculatus Thunb. | Oriental bittersweet * | 668 | 12.7% + 0.8% |
| Lonicera japonica Thunb. | Japanese honeysuckle * | 251 | 21.7% + 1.3% |
| Graminoid | |||
| Microstegium vimineum (Trin.) A. Camus | Japanese stilt grass * | 15 | 10.9% + 3.8% |
| Tall perennial forb | |||
| Coronilla varia L. | Crown vetch | 2 | t |
| Cynanchum louiseae Kartesz & Gandhi | Louise’s (black) swallow-wort * | 1 | t |
| Solanum dulcamara L. | Climbing nightshade | 1 | t |
| Trifolium pratense L. | Red clover | 1 | t |
| Short perennial forb | |||
| Allium canadense L. | Meadow garlic | 1 | 2.0% + % |
| Commelina communis L. | Asiatic dayflower | 1 | t |
| Plantago major L. | Common plantain | 4 | 2.8% + 1.4% |
| Prunella vulgaris L. | Common selfheal | 1 | 3.0% + % |
| Taraxacum officinale F.H. Wigg | Common dandelion | 40 | 1.1% + 0.2% |
| Veronica officinalis L. | Common speedwell | 82 | 2.5% + 0.3% |
| Annual/biennial | |||
| Alliaria petiolata (M. Bieb.) C&G | Garlic mustard * | 40 | 3.4% + 0.7% |
| Polygonum caespitosum Blume, nom. inq. | Oriental lady’s thumb | 75 | 3.3% + 0.6% |
| Verbascum thapsus L. | Common mullein | 17 | 1.9% + 0.5% |
| Origin uncertain | |||
| Tall perennial forb | |||
| Mentha spp. L. | Unknown mint | 23 | 2.6% + 1.3% |
| Short perennial forb | |||
| Polygonum spp. L. | Smartweed | 2 | 1.0% + 0.5% |
| Unknown | Unknown forb | 3 | 5.3% + 0.3% |
| Annual/biennial | |||
| Galium spp. L. | Bedstraw | 279 | 3.8% + 0.3% |
| Native species | |||
| Shrub | |||
| Rubus allegheniensis Porter | Allegheny blackberry | 7 | 4.3% + 2.1% |
| Rubus spp. L. | Blackberry/raspberry | 293 | 12.0% + 1.0% |
| Vine | |||
| Mitchella repens L. | Partidgeberry | 162 | 2.8% + 0.2% |
| Parthenocissus quinquefolia (L.) Planch. | Virginia creeper | 719 | 6.1% + 0.3% |
| Potentilla spp. L. | Cinquefoil | 122 | 4.4% + 0.6% |
| Rubus hispidus L. | Bristly dewberry | 206 | 7.0% + 0.6% |
| Smilax rotundifolia L. | Common greenbrier | 42 | 3.3% + 0.6% |
| Toxicodendron radicans (L.) Kuntze | Eastern poison ivy | 452 | 3.3% + 0.3% |
| Vitis spp. L. | Grape | 406 | 3.9% + 0.4% |
| Graminoid | |||
| Cyperaceae | Unknown sedge | 784 | 12.4% + 0.7% |
| Poaceae | Unknown grass | 13 | 11.6% + 4.7% |
| Tall perennial forb | |||
| Actaea pachypoda Elliott | White baneberry | 74 | 1.9% + 0.2% |
| Ageratina altissima (L.) R.M. King & H. Rob. | White snakeroot | 2 | t |
| Amphicarpaea bracteata (L.) Fernald | Hogpeanut | 136 | 4.0% + 0.4% |
| Arisaema triphyllum (L.) Schott | Jack-in-the-pulpit | 407 | 3.7% + 0.2% |
| Asclepias syriaca L. | Common milkweed | 5 | 2.1% + 1.1% |
| Boehmeria cylindrica (L.) Sw. | False nettle | 4 | 1.5% + 0.6% |
| Circaea lutetiana L. | Enchanter’s nightshade | 164 | 6.0% + 0.6% |
| Desmodium paniculatum (L.) DC. | Panicledleaf ticktrefoil | 3 | 2.3% + 1.3% |
| Eupatorium dubium Willd. ex Poir. | Eastern joe-pye weed | 1 | 3.0% + % |
| Euphorbia corollata L. | Flowering spurge | 1 | t |
| Eurybia divericata (L.) G.L. Nesom. | White wood aster | 297 | 4.9% + 0.3% |
| Eurybia spp. (Cass.) Cass. | Unknown aster | 24 | 4.3% + 1.1% |
| Euthamia tenuifolia (Pursh) Nutt. | Slender fragrant goldenrod | 7 | 2.5% + 0.7% |
| Galium asprellum Michx. | Rough bedstraw | 1 | t |
| Galium circaezans Michx. | White wild licorice | 1 | t |
| Galium lanceolatum Torr. | Lance-leaved wild licorice | 56 | 2.5% + 0.4% |
| Geranium maculatum L. | Wild geranium | 9 | 1.7% + 0.4% |
| Geum canadense Jacq. | White avens | 11 | 1.4% + 0.3% |
| Geum virginianum L. | Rough avens | 14 | 1.6% + 0.4% |
| Hypericum ascyron L. | Great St. Johnswort | 1 | 11.8% + % |
| Lysimachia quadrifolia Sims | Whorled loosestrife | 6 | t |
| Maianthemum racemosum (L.) Link | False Solomon’s seal | 90 | 1.7% + 0.2% |
| Medeola virginiana L. | Indian cucumber root | 11 | 1.7% + 0.3% |
| Penstemon digitalis Nutt. ex Sims | Talus slope penstemon | 1 | t |
| Phryma leptostachya L. | American lopseed | 15 | 1.4% + 0.4% |
| Phytolacca americana L. | Pokeweed | 32 | 3.2% + 0.7% |
| Polygonatum biflorum (Walter) Elliott | Great Solomon’s seal | 16 | 4.8% + 1.4% |
| Polygonatum pubescens (Willd.) Pursh | Hairy Solomon’s seal | 58 | 2.7% + 0.4% |
| Polygonum scandens L. | Climbing false buckwheat | 1 | t |
| Polygonum virginianum L. | Virginia jumpseed | 1 | t |
| Prenanthes altissima L. | Tall white lettuce | 33 | 2.5% + 0.6% |
| Prenanthes trifoliolata (Cass.) Fernald | Gall-of-the-earth | 14 | 1.8% + 0.8% |
| Ranunculus recurvatus Poir. | Hooked crowfoot | 10 | 2.0% + 0.5% |
| Smilax herbacea L. | Smooth carrionflower | 4 | 1.5% + 0.6% |
| Solidago caesia L. | Bluestem goldenrod | 218 | 3.9% + 0.2% |
| Solidago hispida Muhl. ex Willd. | Hairy goldenrod | 1 | t |
| Solidago rugosa Mill. | Rough-stemmed goldenrod | 135 | 3.5% + 0.4% |
| Solidago spp. L. | Goldenrod | 110 | 3.3% + 0.4% |
| Symphyotrichum lateriflorum (L.) Löve & Löve | Calico aster | 16 | 1.7% + 0.4% |
| Symplocarpus foetidus (L.) Salisb. ex Barton | Skunk cabbage | 2 | t |
| Urtica dioica L. | Stinging nettle | 1 | 1.0% + % |
| Short perennial forb | |||
| Allium canadense L. | Wild garlic | 7 | 2.3% + 0.8% |
| Allium tricoccum Aiton | Ramp (wild leeks) | 5 | 2.4% + 0.6% |
| Anemone quinquefolia L. | Wood anemone | 15 | 4.3% + 0.9% |
| Chimaphila maculata (L.) Pursh | Spotted wintergreen | 1 | t |
| Hepatica nobilis Schreb. | Round-lobed hepatica | 9 | t |
| Maianthemum canadense Desf. | Canada mayflower | 663 | 7.6% + 0.4% |
| Monotropa uniflora L. | Indian pipe | 5 | t |
| Oxalis stricta L. | Wood sorrel | 176 | 2.2% + 0.2% |
| Pyrola americana Sweet | Round-leaved pyrola | 1 | 3.0% + % |
| Pyrola elliptica Nutt. | Shinleaf | 3 | 2.0%+ 0.8% |
| Sanguinaria canadensis L. | Bloodroot | 19 | 2.3% + 0.6% |
| Trillium erectum L. | Red trillium | 37 | 2.3% + 0.4% |
| Uvularia spp. L. | Bellwort | 1 | t |
| Uvularia perfoliata L. | Perfoliate bellwort | 14 | 2.3% + 0.8% |
| Uvularia sessilifolia L. | Sessileleaf bellwort | 163 | 1.9% + 0.2% |
| Viola spp. L. | Violet spp. | 114 | 1.8% + 0.2% |
| Viola triloba Schwein | 3 lobed violet | 2 | 1.5% + 1.0% |
| Unknown forb | Unknown forb | 14 | 1.1% + 0.3% |
| Annual/biennial | |||
| Bidens frondosa L. | Devil’s beggartick | 4 | t |
| Epifagus virginiana (L.) W.P.C. Barton | Beechdrops | 7 | 2.0% + 0.4% |
| Galium aparine L. | Cleavers bedstraw | 9 | 2.6% + 1.2% |
| Lobelia inflata L. | Indian tobacco | 12 | 1.3% + 0.4% |
| Prenanthes alba L. | White lettuce | 1 | t |
| Pseudognaphalium helleri (Britton) Anderb. | Heller’s cudweed | 2 | t |
| Sanicula spp. L. | Snakeroot | 6 | t |
| Fern | |||
| Athyrium filix-femina (L.) Roth | Lady fern | 7 | 2.0% + 0.8% |
| Dennstaedtia punctilobula (Michx.) T. Moore | Hayscented fern | 54 | 16.9% + 3.1% |
| Polystichum acrostichoides (Michx.) Schott | Christmas fern | 104 | 10.0% + 0.7% |
| Thelypteris noveboracensis (L.) Nieuwl. | New York fern | 10 | 26.9% + 8.3% |
| Pteridophyta | Unknown fern | 2 | t |
References
- Nowacki, G.J.; Abrams, M.D. The demise of fire and “mesophication” of forests in the eastern United States. BioScience 2008, 58, 123–138. [Google Scholar] [CrossRef]
- Schlarbaum, S.E.; Hebard, F.; Spaine, P.C.; Kamalay, J.C. Three American tragedies: Chestnut blight, butternut canker, and Dutch elm disease. In Proceedings of the Exotic Pests of Eastern Forests Conference, Nashville, TN, USA, 8–10 April 1997; Britton, K.O., Ed.; U.S. Forest Service and Tennessee Exotic Pest Plant Council: Nashville, TN, USA, 1998; pp. 45–54. [Google Scholar]
- Schulz, B.K.; Gray, A.N. The new flora of northeastern USA: Quantifying introduced plant species occupancy in forest ecosystems. Environ. Monit. Assess. 2013, 185, 2931–3957. [Google Scholar] [CrossRef] [PubMed]
- McShea, W.J.; Rappole, J.H. White-tailed deer as keystone species with the forest habitats of Virginia. Va. J. Sci. 1992, 43, 177–186. [Google Scholar]
- Nuttle, T.; Yerger, E.H.; Stoleson, S.H.; Ristau, T.E. Legacy of top-down herbivore pressure ricochets back up multiple trophic levels in forest canopies over 30 years. Ecosphere 2011, 2, 397–409. [Google Scholar] [CrossRef]
- DeCalesta, D.S.; Stout, S.L. Relative deer density and sustainability: A conceptual framework for integrating deer management with ecosystem management. Wildl. Soc. Bull. 1997, 25, 252–258. [Google Scholar]
- Waller, W.M.; Alverson, W.S. The white-tailed deer: A keystone herbivore. Wildl. Soc. Bull. 1997, 25, 217–226. [Google Scholar]
- Castleberry, S.B.; Ford, W.M.; Miller, K.V.; Smith, W.P. Influences of herbivory and canopy opening size on forest regeneration in a southern bottomland hardwood forest. For. Ecol. Manag. 2000, 131, 57–64. [Google Scholar] [CrossRef]
- Rutherford, A.C.; Schmitz, O.J. Regional-scale assessment of deer impacts on vegetation within western Connecticut, USA. J. Wildl. Manag. 2010, 74, 1257–1263. [Google Scholar] [CrossRef]
- Abrams, M.D.; Johnson, S.E. Long-term impacts of deer exclosures on mixed-oak forest composition at the Valley Forge National Historical Park, Pennsylvania, USA. J. Torrey. Bot. Soc. 2012, 139, 167–180. [Google Scholar] [CrossRef]
- Bressette, J.W.; Beck, J.; Beauchamp, V.B. Beyond the browse line: Complex cascade effects mediated by white-tailed deer. OIKOS 2012, 121, 1749–1760. [Google Scholar] [CrossRef]
- Horsley, S.B.; Stout, S.L.; DeCalesta, D.S. White-tailed deer impact on the vegetation dynamics of a northern hardwood forest. Ecol. Appl. 2003, 13, 98–118. [Google Scholar] [CrossRef]
- Rooney, T.P.; Waller, D.M. Direct and indirect effects of white-tailed deer in forest ecosystems. For. Ecol. Manag. 2003, 181, 165–176. [Google Scholar] [CrossRef]
- Duguay, J.P.; Farfaras, C. Overabundant suburban deer, invertebrates, the spread of an invasive exotic plant. Wildl. Soc. Bull. 2011, 35, 243–251. [Google Scholar] [CrossRef]
- Tremblay, J.-P.; Huot, J.; Potvin, F. Divergent nonlinear responses of the boreal forest field layer along an experimental gradient of deer densities. Oecologia 2006, 150, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Rooney, T.P. High white-tailed deer densities benefit graminoids and contribute to biotic homogenization of forest ground-layer vegetation. Plant Ecol. 2009, 202, 103–111. [Google Scholar] [CrossRef]
- Eschtruth, A.K.; Battles, J.J. Acceleration of exotic plant invasion in a forested ecosystem by a generalist herbivore. Conserv. Biol. 2009, 23, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.A.; Dunn, J.L.; Smith, L.A.; Davis, J.; Kalisz, S. Deer facilitate invasive plant success in a Pennsylvania forest understory. Nat. Areas J. 2009, 29, 110–116. [Google Scholar] [CrossRef]
- Williams, S.C.; Ward, J.S.; Ramakrishnan, U. Endozoochory by white-tailed deer (Odocoileus virginianus) across a suburban/woodland interface. For. Ecol. Manag. 2008, 255, 940–947. [Google Scholar] [CrossRef]
- Kalisz, S.; Spigler, R.B.; Horvitz, C.C. In a long-term experimental demography study, excluding ungulates reversed invader’s explosive population growth rate and restored natives. Proc. Nat. Acad. Sci. USA 2014, 111, 4501–4506. [Google Scholar] [CrossRef] [PubMed]
- Ashton, I.W.; Lerdau, M.T. Tolerance to herbivory, not resistance, may explain differential success of invasive, naturalized, native North American temperate vines. Divers. Distrib. 2008, 14, 169–178. [Google Scholar] [CrossRef]
- Shelton, A.L.; Henning, J.A.; Schultz, P.; Clay, K. Effects of abundant white-tailed deer on vegetation, animals, mycorrhizal fungi, soils. For. Ecol. Manag. 2014, 320, 39–49. [Google Scholar] [CrossRef]
- MacDougall, A.S.; Turkington, R. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 2005, 86, 42–55. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.J.; Mitchell, R.J.; Graham, S.A. Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology 2002, 83, 2328–2336. [Google Scholar] [CrossRef]
- Dorning, M.; Cipollini, D. Leaf and root extracts of the invasive shrub, Lonicera maackii, inhibit seed germination of three herbs with no autotoxic effects. Plant Ecol. 2006, 184, 287–296. [Google Scholar] [CrossRef]
- Orrock, J.L.; Witter, M.S.; Reichman, O.J. Apparent competition with an exotic plant reduces native plant establishment. Ecology 2008, 89, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.E. Effects of invasion by Lonicera tatarica L. on herbs and tree seedlings in four New England forests. Am. Midl. Nat. 1993, 130, 62–74. [Google Scholar] [CrossRef]
- Kourtev, P.S.; Ehrenfeld, J.G.; Huang, W.Z. Effects of exotic plant species on soil properties in hardwood forests of New Jersey. Water Air Soil Pollut. 1998, 105, 493–501. [Google Scholar] [CrossRef]
- Collier, M.H.; Vankat, J.L.; Hughes, M.R. Diminished plant richness and abundance below Lonicera maackii, an invasive shrub. Am. Midl. Nat. 2002, 147, 60–71. [Google Scholar] [CrossRef]
- Knight, K.S.; Kurylo, J.S.; Endress, A.G.; Stewart, J.R.; Reich, P.B. Ecology and ecosystem impacts of common buckthorn (Rhamnus cathartica): A review. Biol. Invasions 2007, 9, 925–937. [Google Scholar] [CrossRef]
- Runkle, J.R.; DiSalvo, A.; Graham-Gibson, Y.; Dorning, M. Vegetation release eight years after removal of Lonicera maackii in west-central Ohio. Ohio J. Sci. 2007, 107, 125–129. [Google Scholar]
- Hanula, J.L.; Horn, S.; Taylor, J.W. Chinese privet (Ligustrum sinense) removal and its effect on native plant communities of riparian forests. Invasive Plant Sci. Manag. 2009, 2, 292–300. [Google Scholar] [CrossRef]
- Luken, J.O.; Kuddes, L.M.; Tholemeier, T.C. Response of understory species to gap formation and soil disturbance in Lonicera maackii thickets. Restor. Ecol. 1997, 5, 229–235. [Google Scholar] [CrossRef]
- Love, J.P.; Anderson, J.T. Seasonal effects of four control methods on the invasive morrow’s honeysuckle (Lonicera morrowii) and initial responses of understory plants in a southwestern Pennsylvania old field. Restor. Ecol. 2009, 17, 549–559. [Google Scholar] [CrossRef]
- Gould, A.M.A.; Gorchov, D.L. Effects of the exotic invasive shrub Lonicera maackii on the survival and fecundity of three species of native annuals. Am. Midl. Nat. 2000, 144, 36–50. [Google Scholar] [CrossRef]
- Cipollini, K.; Ames, E.; Cipollini, D. Amur honeysuckle (Lonicera maackii) management method impacts restoration of understory plants in the presence of white-tailed deer (Odocoileus virginiana). Invasive Plant Sci. Manag. 2009, 2, 45–54. [Google Scholar] [CrossRef]
- Miller, K.E.; Gorchov, D.L. The invasive shrub, Lonicera maackii, reduces growth and fecundity of perennial forest herbs. Oecologia 2004, 139, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Nuzzo, V.; Blossey, B. Demographic responses of rare forest plants to multiple stressors: The role of deer, invasive species and nutrients. J. Ecol. 2014, 102, 1222–1233. [Google Scholar] [CrossRef]
- Leege, L.M.; Thompson, J.S.; Parris, D.J. The response of rare and common trilliums (Trillium reliquum, T. cuneatum, T. maculatum) to deer herbivory and invasive honeysuckle removal. Castanea 2010, 75, 433–443. [Google Scholar] [CrossRef]
- Christopher, C.D.; Matter, S.F.; Cameron, G.N. Individual and interactive effects of Amur honeysuckle (Lonicera maackii) and white-tailed deer (Odocoileus virginianus) on herbs in a deciduous forest in the eastern United States. Biol. Invasions 2014, 16, 2247–2261. [Google Scholar] [CrossRef]
- Ward, J.S.; Williams, S.C.; Worthley, T.E. Comparing effectiveness and impacts of Japanese barberry (Berberis thunbergii DC) control treatments and herbivory on plant communities. Invasive Plant Sci. Manag. 2013, 6, 459–469. [Google Scholar] [CrossRef]
- Beringer, J.; Hansen, L.P.; Sexton, O. Detection rates of white-tailed deer with a helicopter over snow. Wildl. Soc. Bull. 1998, 26, 24–28. [Google Scholar]
- Potvin, F.; Breton, L. Testing 2 aerial survey techniques on deer in fenced enclosures—Visual double-counts and thermal infrared sensing. Wildl. Soc. Bull. 2005, 33, 317–325. [Google Scholar] [CrossRef]
- Simberloff, D.; Dayan, T. The guild concept and the structure of ecological communities. Annu. Rev. Ecol. Syst. 1991, 22, 115–143. [Google Scholar] [CrossRef]
- Griggs, J.A.; Rock, J.H.; Webster, C.R.; Jenkins, M.A. Vegetative legacy of protected deer herd in Cades Cove, Great Smoky Mountains National Park. Nat. Areas J. 2006, 26, 126–136. [Google Scholar] [CrossRef]
- Hand, D.; Crowder, M. Practical Longitudinal Data Analysis; Chapman and Hall/CRC: New York, NY, USA, 1996; p. 232. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall, Inc.: Upper Saddle River, NJ, USA, 2010; p. 944. [Google Scholar]
- Gregonis, M. 2006/2007 aerial deer survey indicates stable population. Conn. Wildl. 2007, 27, 3. [Google Scholar]
- Milton, S.J. “Emerging ecosystems”: A washing-stone for ecologists, economists and sociologists? S. Afr. J. Sci. 2003, 99, 404–406. [Google Scholar]
- Hobbs, R.J.; Arico, S.; Aronson, J.; Baron, J.S.; Bridgewater, P.; Cramer, V.A.; Epstein, P.R.; Ewel, J.J.; Klink, C.A.; Lugo, A.E.; et al. Novel ecosystems: Theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 2006, 15, 1–7. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Fischer, J.; Felton, A.; Crane, M.; Michael, D.; Macgregor, C.; Montague-Drake, R.; Manning, A.; Hobbs, R.J. Novel ecosystems resulting from landscape transformation create dilemmas for modern conservation practice. Conserv. Lett. 2008, 1, 129–135. [Google Scholar] [CrossRef]
- Stromayer, K.A.K.; Warren, R.J. Are overabundant deer herds in the eastern United States creating alternate stable states in forest plant communities? Wildl. Soc. Bull. 1997, 25, 227–234. [Google Scholar]
- Niering, W.A.; Goodwin, R.H. Creation of relatively stable shrublands with herbicides: Arresting “succession” on rights-of-way and pastureland. Ecology 1974, 55, 784–795. [Google Scholar] [CrossRef]
- Ward, J.S.; Worthley, T.E.; Williams, S.C. Controlling Japanese barberry (Berberis thunbergii DC) in southern New England, USA. For. Ecol. Manag. 2009, 257, 561–566. [Google Scholar] [CrossRef]
- Richburg, J.A. Timing Treatments to the Phenology of Root Carbohydrate Reserves to Control Woody Invasive Plants. Ph.D. Dissertation, University of Massachusetts, Amherst, MA, USA, May 2005. [Google Scholar]
- Silander, J.A., Jr.; Klepeis, D.M. The invasion ecology of Japanese barberry (Berberis thunbergii) in the New England landscape. Biol. Invasions 1999, 1, 189–201. [Google Scholar] [CrossRef]
- Harrington, R.A.; Fownes, J.H.; Cassidy, T.M. Japanese barberry (Berberis thunbergii) in forest understory: Leaf and whole plant responses to nitrogen availability. Am. Midl. Nat. 2004, 151, 206–216. [Google Scholar] [CrossRef]
- Xu, C.-Y.; Griffin, K.L.; Schuster, W.S.F. Leaf phenology and seasonal variation of photosynthesis of invasive Berberis thunbergii (Japanese barberry) and two co-occurring native understory shrubs in a northeastern United States deciduous forest. Oecologia 2007, 154, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trend Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Eschtruth, A.K.; Battles, J.J. Assessing the relative importance of disturbance, herbivory, diversity, propagule pressure in exotic plant invasion. Ecol. Monogr. 2009, 79, 265–280. [Google Scholar] [CrossRef]
- Rooney, T.P.; Wiegmann, S.H.; Rogers, D.A.; Waller, D.M. Biotic impoverishment and homogenization in unfragmented forest understory communities. Conserv. Biol. 2009, 18, 787–798. [Google Scholar] [CrossRef]
- Martin, T.G.; Arcese, P.; Scheerder, N. Browsing down our natural heritage: Deer impacts on vegetation structure and songbird populations across an island archipelago. Biol. Conserv. 2011, 144, 459–469. [Google Scholar] [CrossRef]
- Côté, S.D.; Beguin, J.; de Bellefeuille, S.; Champagne, E.; Thiffault, N.; Tremblay, J.-P. Structuring effects of deer in boreal forest ecosystems. Adv. Ecol. 2014, 2014, 917834. [Google Scholar] [CrossRef]
- Gubanyi, J.A.; Savidge, J.A.; Hygnstrom, S.E.; VerCauteren, K.C.; Korte, S.P. Deer impact on vegetation in natural areas in southeastern Nebraska. Nat. Areas J. 2008, 28, 121–129. [Google Scholar] [CrossRef]
- Weber, J.F.; Fournet, A. Alkaloidal content of four Berberis species: Structure of berberilaurine, a new bisbenzyltetrahydroisoquinoline. J. Nat. Prod. 1989, 52, 81–84. [Google Scholar] [CrossRef]
- Ward, J.S.; Stephens, G.R. Protection of tree seedlings from deer browsing. In Proceedings of the 10th Central Hardwood Forestry Conference, Morgantown, WV, USA, 5–8 March 1995; Gottschalk, K.W., Fosbroke, S.L.C., Eds.; USDA Forest Service General Technical Report NE-197. USDA Forest Service: Radnor, PA, USA, 1995; pp. 507–514. [Google Scholar]
- Behrend, D.F.; Mattfeld, G.F.; Tierson, W.C.; Wiley, J.E., III. Deer density control for comprehensive forest management. J. For. 1970, 68, 695–700. [Google Scholar]
- Nixon, C.M.; McClain, M.W.; Russell, K.R. Deer food habits and range characteristics in Ohio. J. Wildl. Manag. 1970, 34, 870–886. [Google Scholar] [CrossRef]
- Rossell, C.R.; Patch, S.; Salmons, S. Effects of deer browsing on native and non-native vegetation in a mixed oak-beech forest on the Atlantic coastal plain. Northeast. Nat. 2007, 14, 61–72. [Google Scholar] [CrossRef]
- Webster, C.R.; Jenkins, M.A.; Rock, J.H. Long-term response of spring flora to chronic herbivory and deer exclusion in Great Smoky Mountains National Park, USA. Biol. Conserv. 2005, 125, 297–307. [Google Scholar] [CrossRef]
| Guild | Between Subjects | Within Subjects (Factor-by-Year) | ||
|---|---|---|---|---|
| Invasive | Exclosure | Invasive | Exclosure | |
| Invasive shrub | <0.001 *** | 0.247 | 0.007 ** | 0.305 |
| Native shrub | 0.004 ** | <0.001 *** | 0.009 ** | 0.018 * |
| Invasive vine | 0.672 | 0.011 * | 0.046 * | 0.026 * |
| Native vine | 0.098 | 0.009 ** | 0.001 *** | 0.255 |
| Graminoid | <0.001 *** | 0.012 * | 0.257 | <0.001 *** |
| Tall perennial forb | 0.017 * | 0.150 | 0.069 | 0.621 |
| Short perennial forb | <0.001 *** | 0.216 | 0.038 * | 0.111 |
| Annual/biennial | 0.010 ** | 0.443 | 0.205 | <0.001 *** |
| Invasive Control Treatments | ||||||
|---|---|---|---|---|---|---|
| I0-Dense | I1-Partial | I3-Controlled | ||||
| Invasive shrub | 75.4 (9.6) | a * | 35.1 (6.4) | b | 10.7 (7.5) | c |
| Native shrub | 0.1 (0.4) | b | 4.1 (4.6) | a | 7.3 (4.4) | a |
| Invasive vine | 10.3 (4.4) | a | 9.5 (4.6) | a | 12.3 (8.5) | a |
| Native vine | 3.8 (2.5) | b | 5.3 (2.4) | b | 12.5 (2.1) | a |
| Graminoid | 0.5 (0.3) | b | 6.9 (4.4) | a | 7.7 (3.6) | a |
| Tall perennial forb | 1.1 (1.0) | b | 4.6 (1.5) | a | 5.7 (1.4) | a |
| Short perennial forb | 0.8 (0.4) | b | 4.8 (1.3) | a | 4.6 (1.8) | a |
| Annual/biennial | 0.2 (0.2) | a | 0.8 (0.4) | a | 0.8 (0.6) | a |
| Fern | 1.9 (1.4) | a | 0.5 (0.9) | a | 1.6 (3.2) | a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ward, J.S.; Williams, S.C.; Linske, M.A. Independent Effects of Invasive Shrubs and Deer Herbivory on Plant Community Dynamics. Forests 2017, 8, 2. https://doi.org/10.3390/f8010002
Ward JS, Williams SC, Linske MA. Independent Effects of Invasive Shrubs and Deer Herbivory on Plant Community Dynamics. Forests. 2017; 8(1):2. https://doi.org/10.3390/f8010002
Chicago/Turabian StyleWard, Jeffrey S., Scott C. Williams, and Megan A. Linske. 2017. "Independent Effects of Invasive Shrubs and Deer Herbivory on Plant Community Dynamics" Forests 8, no. 1: 2. https://doi.org/10.3390/f8010002







