Influence of Fuel Load Dynamics on Carbon Emission by Wildfires in the Clay Belt Boreal Landscape
Abstract
:1. Introduction
2. Materials and Methods
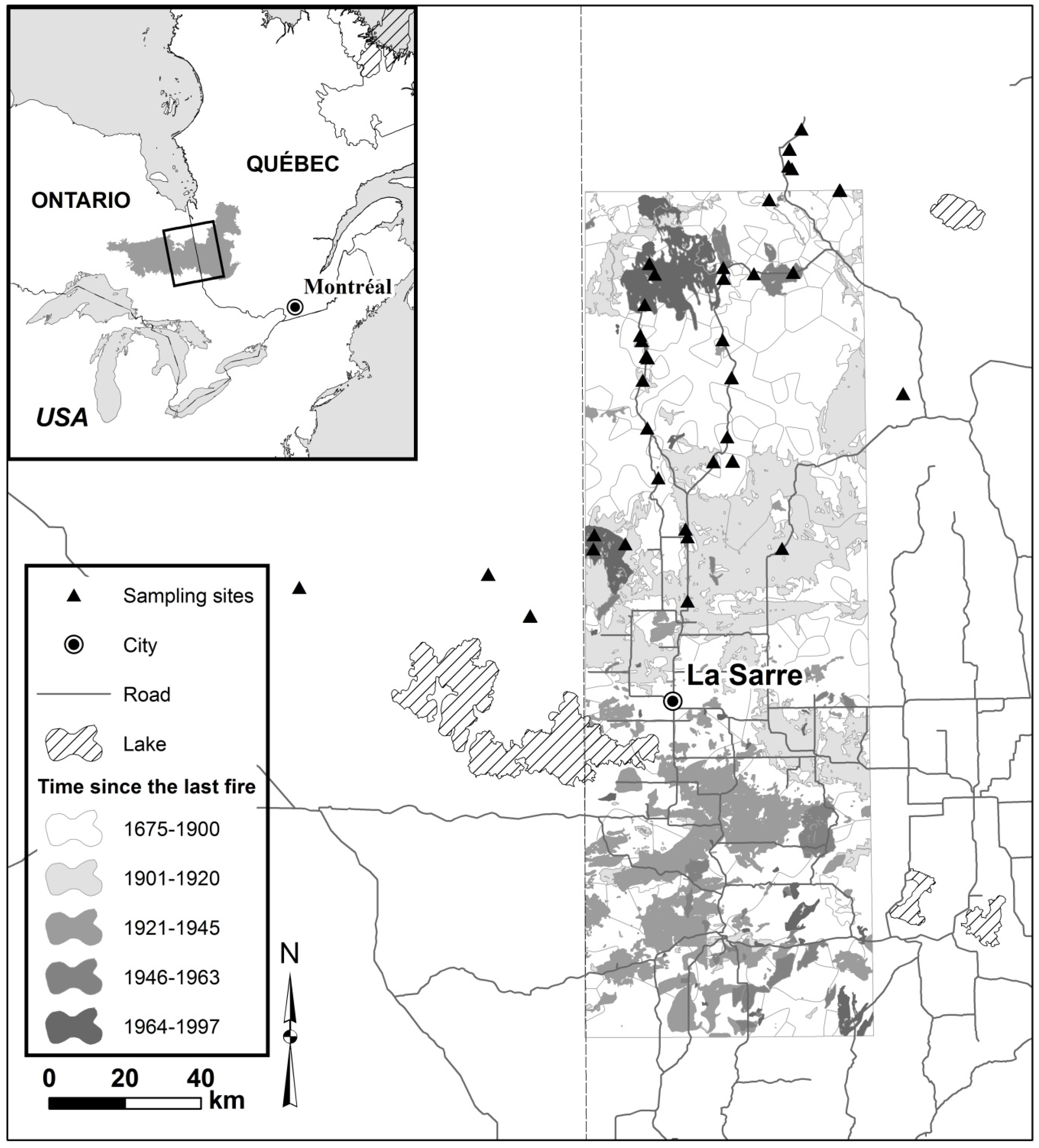
2.1. Study Area
2.2. Fuel Load Dynamics with TSF
2.3. Simulation of Carbon Emissions by Fire
3. Results
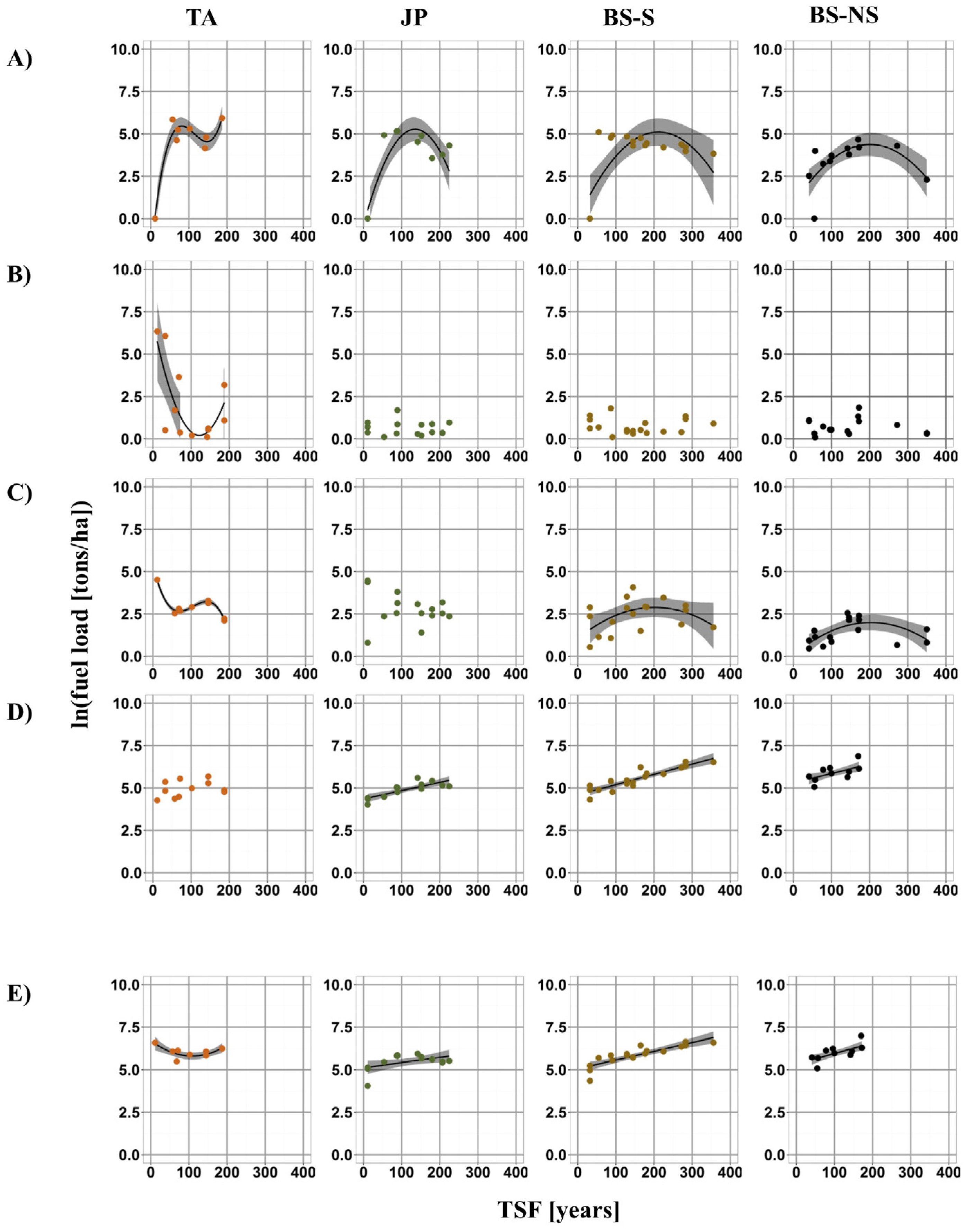
3.1. Fuel Load Dynamics with TSF
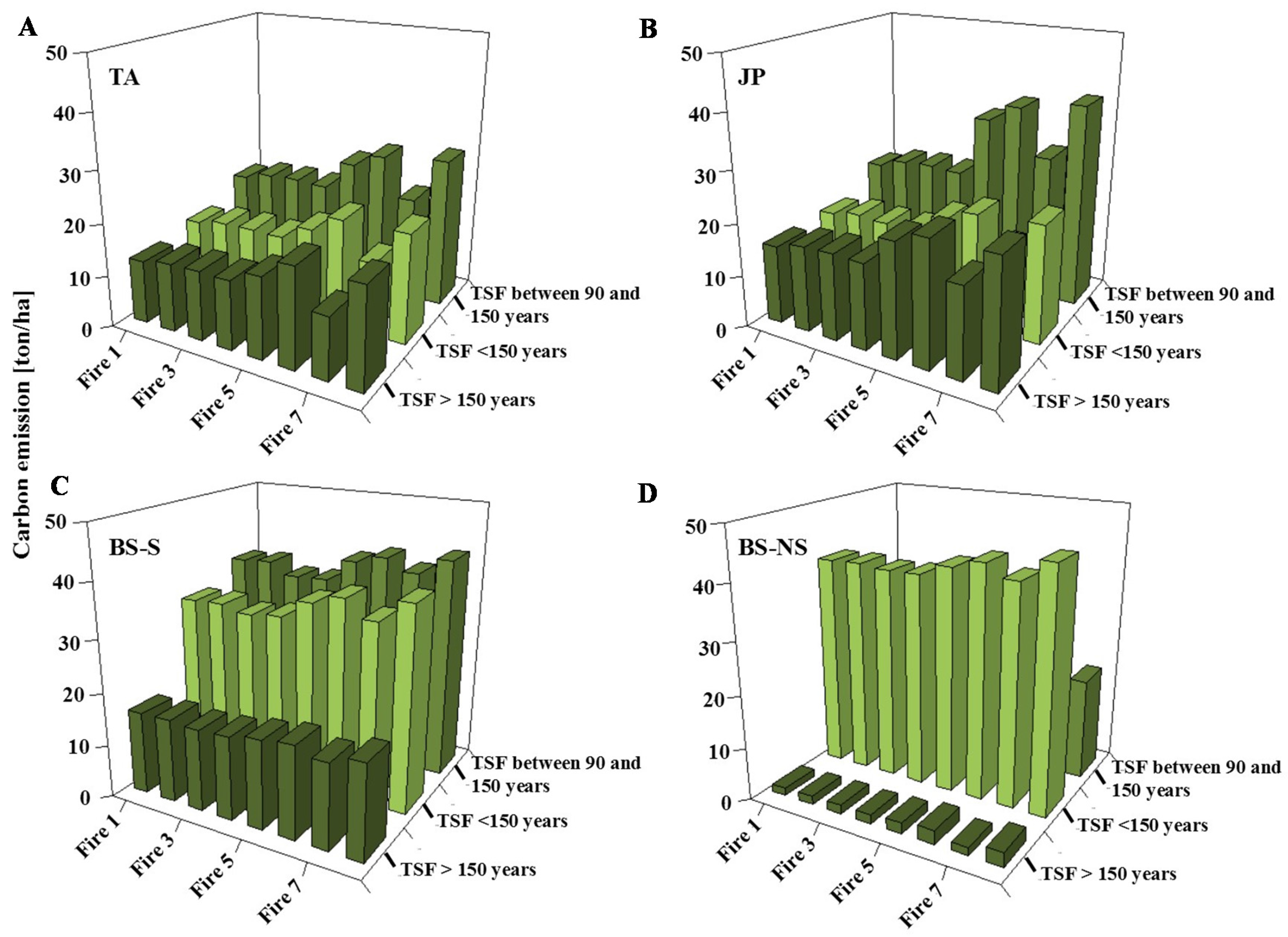
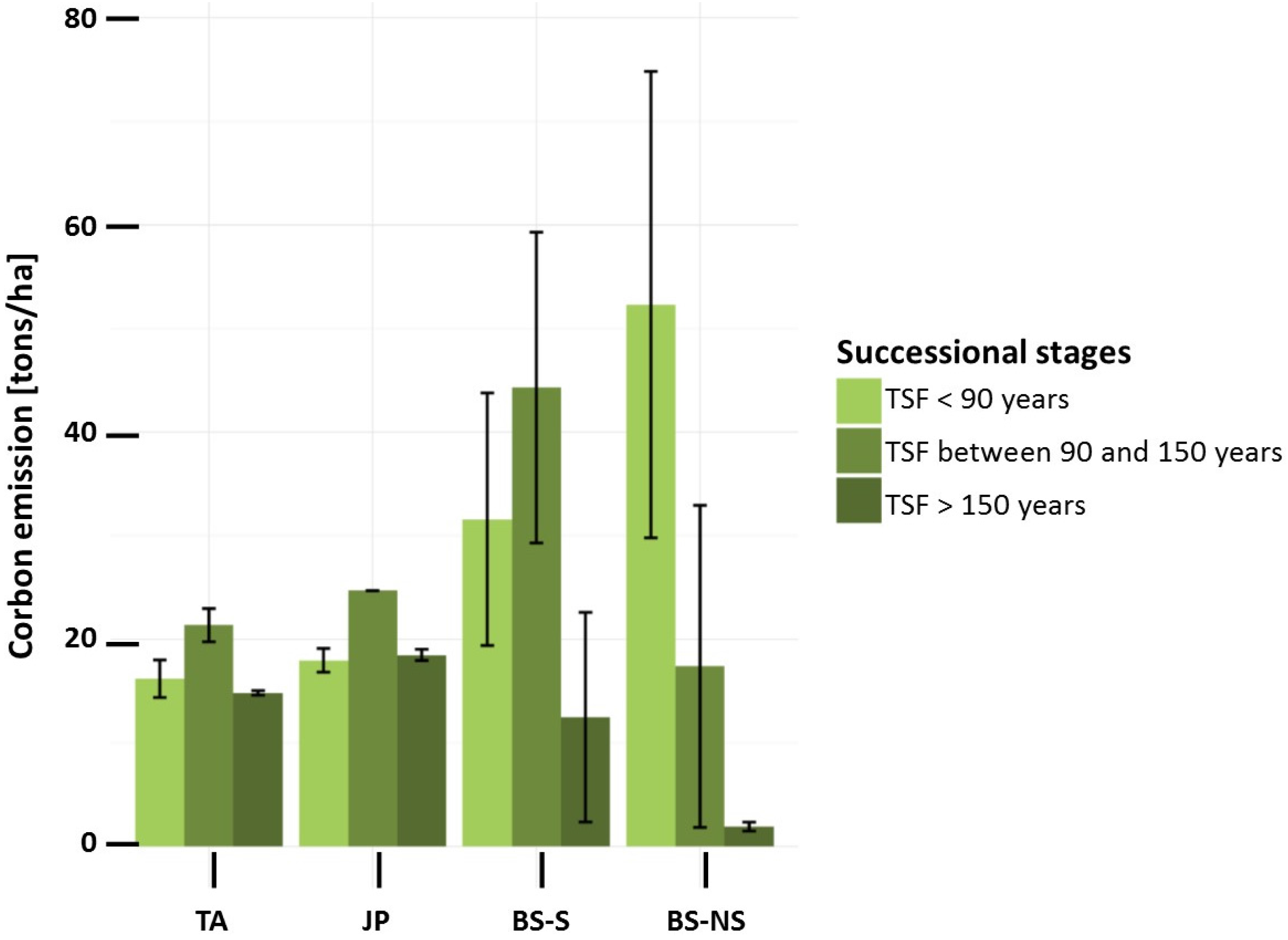
3.2. Simulation of Carbon Emission by Fire
4. Discussion
4.1. Fuel Load Dynamics with TSF
4.2. Simulation of Carbon Emission by Fire
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BS | Black spruce |
| BS-S | Black spruce forests originating from severe fire |
| BS-NS | Black spruce forests originating from non-severe fire |
| CWD | Coarse woody debris |
| DBH | Diameter at breast height |
| DWD | Dead woody debris |
| FFFC | Forest floor fuel consumption |
| FWI | Fire weather index |
| JP | Jack pine |
| TA | Trembling aspen |
| TSF | Time since the last fire |
Appendix A. Shrub Load Equations
| Species | Equations | Adjusted R2 |
|---|---|---|
| Kalmia angustifolia 2 | 0.91 | |
| Lonicera canadensis 1 | 0.82 | |
| Chamaedaphne calyculata 2 | 0.93 | |
| Rhododendron groenlandicum 2 | 0.94 | |
| Ribes sp. 1 | 0.79 | |
| Rosa acicularis 1 | 0.82 | |
| Vaccinium myrtilloides 1 | 0.65 | |
| Viburnum edule 2 | 0.84 |
Appendix B. Sensitivity Analysis of the Influence of Fire Weather Variations on Potential Carbon Emissions across Successional Stages
| Name | FFMC | DMC | DC | ISI | BUI | FWI |
|---|---|---|---|---|---|---|
| Fire 1 | 89 | 22 | 168 | 15 | 33 | 24 |
| Fire 2 | 93 | 42 | 85 | 17 | 41 | 30 |
| Fire 3 | 88 | 22 | 167 | 9 | 33 | 17 |
| Fire 4 | 90 | 50 | 125 | 15 | 49 | 29 |
| Fire 5 | 83 | 26 | 99 | 5 | 32 | 10 |
| Fire 6 | 82 | 24 | 101 | 4 | 30 | 8 |
| Fire 7 | 88 | 30 | 99 | 8 | 34 | 15 |
| Fire 8 | 88 | 31 | 88 | 6 | 33 | 13 |
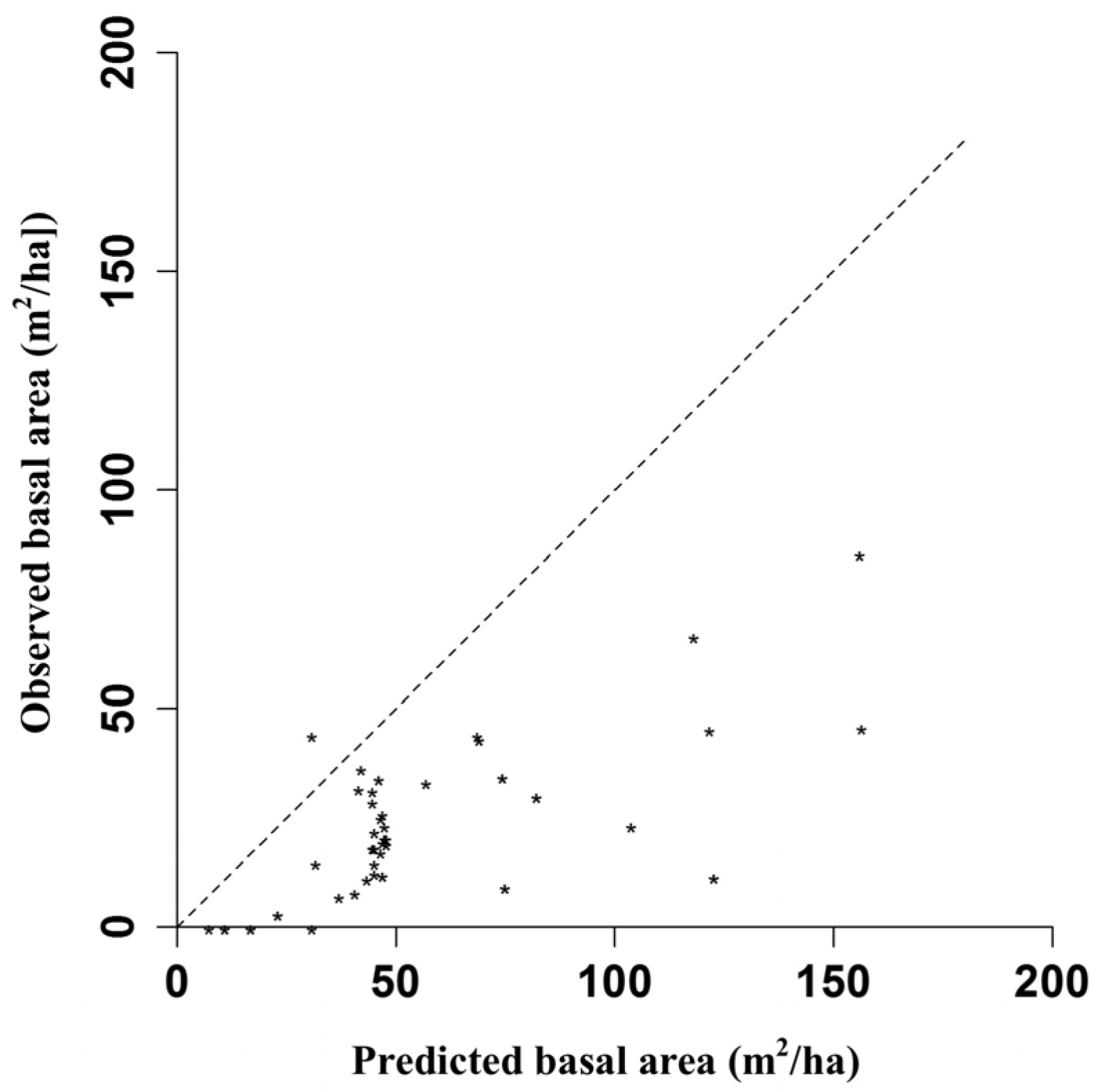
Appendix C. Comparison of Empirical Basal Area and Simulated Basal Area with Plonski’s Yield Table
Appendix D. Calibration of Bulk Density with Duff Fuel Loads
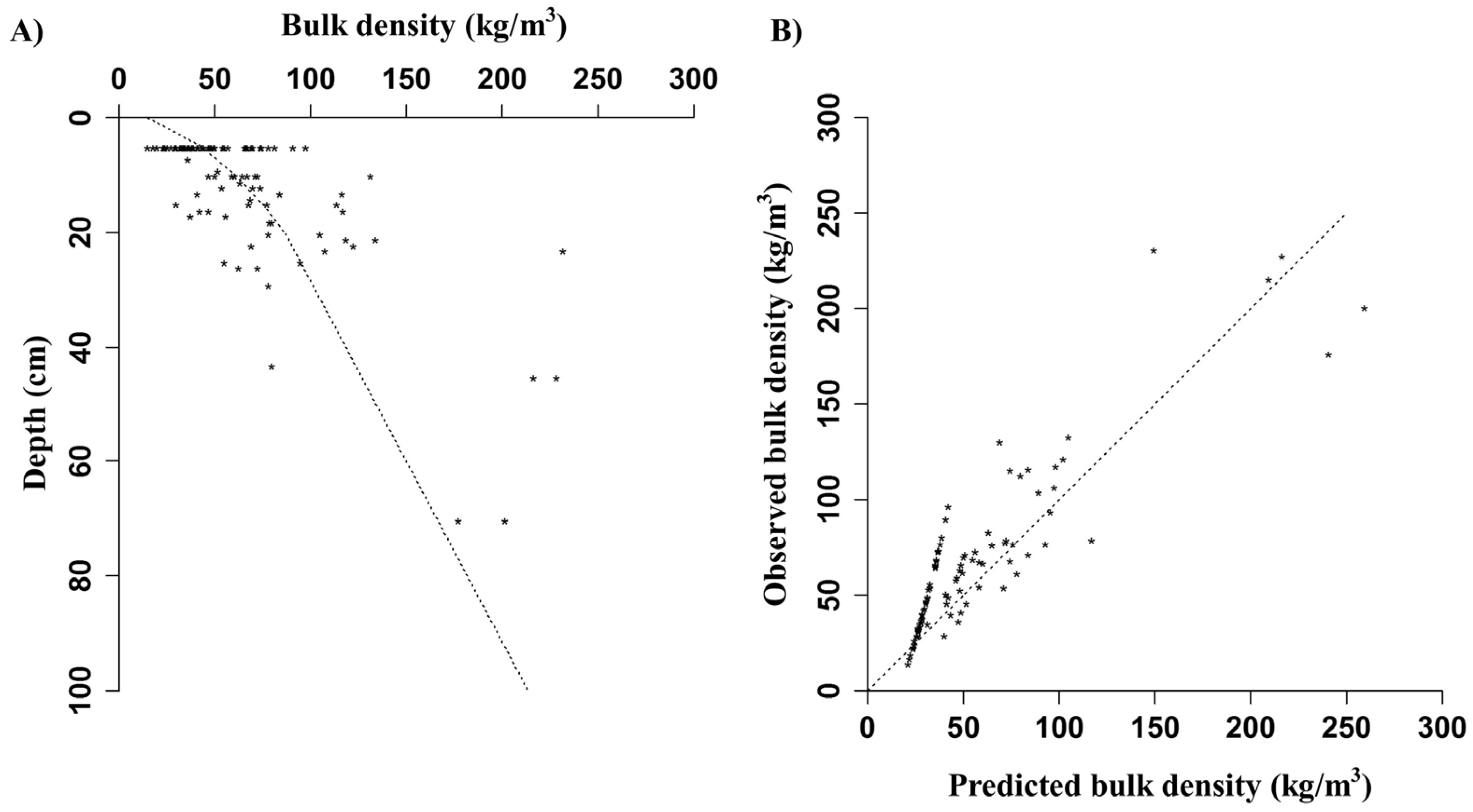
Appendix E. Calibration of Bulk Density with Duff Fuel Loads
| Fuel Material | ||||||
|---|---|---|---|---|---|---|
| Forest Type | TSF (Years) | Tree (t/ha) | Fine Aerial (t/ha) | DWD (t/ha) | Duff (t/ha) | Total (t/ha) |
| TA | 11 | 0 | 569 | 90 | 70 | 730 |
| 57 | 346 | 4 | 12 | 78 | 440 | |
| 68 | 100 | 37 | 16 | 88 | 241 | |
| 71 | 190 | 0 | 14 | 257 | 460 | |
| 102 | 200 | 0 | 17 | 145 | 362 | |
| 145 | 120 | 1 | 22 | 292 | 435 | |
| 145 | 120 | 1 | 26 | 197 | 343 | |
| 187 | 375 | 23 | 8 | 117 | 524 | |
| 187 | 375 | 2 | 7 | 129 | 514 | |
| JP | 11 | 0 | 1 | 1 | 54 | 57 |
| 11 | 0 | 0 | 86 | 81 | 168 | |
| 11 | 0 | 2 | 79 | 77 | 158 | |
| 54 | 137 | 0 | 10 | 87 | 234 | |
| 87 | 173 | 0 | 12 | 154 | 339 | |
| 89 | 175 | 4 | 22 | 152 | 353 | |
| 89 | 175 | 1 | 44 | 115 | 335 | |
| 142 | 91 | 0 | 21 | 270 | 382 | |
| 152 | 132 | 0 | 3 | 182 | 317 | |
| 152 | 132 | 1 | 12 | 140 | 285 | |
| 180 | 34 | 1 | 15 | 225 | 276 | |
| 180 | 34 | 0 | 10 | 225 | 270 | |
| 207 | 42 | 0 | 12 | 171 | 225 | |
| 207 | 42 | 0 | 23 | 173 | 238 | |
| 225 | 74 | 2 | 10 | 163 | 248 | |
| BS-S | 32 | 0 | 1 | 17 | 170 | 188 |
| 32 | 0 | 3 | 10 | 131 | 144 | |
| 32 | 0 | 2 | 1 | 74 | 77 | |
| 55 | 164 | 1 | 2 | 131 | 298 | |
| 87 | 118 | 5 | 2 | 223 | 348 | |
| 91 | 134 | 0 | 7 | 117 | 258 | |
| 129 | 127 | 1 | 33 | 190 | 350 | |
| 129 | 127 | 1 | 16 | 232 | 376 | |
| 145 | 72 | 1 | 58 | 169 | 299 | |
| 145 | 94 | 0 | 11 | 204 | 310 | |
| 165 | 115 | 1 | 3 | 506 | 625 | |
| 177 | 74 | 2 | 18 | 289 | 383 | |
| 181 | 85 | 0 | 17 | 350 | 453 | |
| 225 | 66 | 1 | 31 | 338 | 436 | |
| 272 | 79 | 0 | 6 | 496 | 581 | |
| 283 | 52 | 2 | 14 | 698 | 767 | |
| 283 | 66 | 3 | 19 | 541 | 629 | |
| 356 | 45 | 1 | 4 | 682 | 733 | |
| BS-NS | 41 | 11 | 2 | 1 | 292 | 306 |
| 41 | 11 | 2 | 2 | 292 | 307 | |
| 41 | 11 | 2 | 1 | 292 | 306 | |
| 55 | 0 | 0 | 4 | 157 | 160 | |
| 57 | 53 | 0 | 2 | 239 | 295 | |
| 78 | 24 | 1 | 1 | 435 | 461 | |
| 96 | 29 | 1 | 2 | 483 | 515 | |
| 100 | 40 | 1 | 1 | 351 | 393 | |
| 142 | 63 | 1 | 12 | 281 | 356 | |
| 146 | 43 | 0 | 7 | 389 | 440 | |
| 146 | 43 | 0 | 9 | 389 | 441 | |
| 170 | 106 | 3 | 4 | 972 | 1084 | |
| 172 | 66 | 5 | 8 | 464 | 543 | |
| 172 | 66 | 2 | 10 | 464 | 542 | |
References
- Lemprière, T.C.; Kurz, W.A.; Hogg, E.H.; Schmoll, C.; Rampley, G.J.; Yemshanov, D.; McKenney, R.; Gilsenan, A.; Beatch, D.; Blain, J.S.; et al. Canadian boreal forests and climate change mitigation 1. Environ. Rev. 2013, 21, 293–321. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Carbon Sequestration in Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2010; pp. 5–11. [Google Scholar]
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexler, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Luyssaert, S.; Inglima, I.; Jung, M.; Richardson, A.D.; Reichstein, M.; Papale, D.; Piao, S.L.; Schulze, E.-D.; Wingate, L.; Matteucci, G.; et al. CO2 balance of boreal, temperate, and tropical forests derived from a global database. Glob. Chang. Biol. 2007, 13, 2509–2537. [Google Scholar] [CrossRef]
- Terrier, A.; Girardin, M.; Bergeron, Y. Les réservoirs de carbone en forêt boréale à l’est du Canada: Acquis et incertitudes dans la modélisation face aux changements climatiques. VertigO-la Revue Électronique Sci. L'environnement 2012, 11, 3. [Google Scholar] [CrossRef]
- Burton, P.J.; Messier, C.; Weetman, G.F.; Prepas, E.E.; Adamowicz, W.L.; Tittler, R. The current state of boreal forestry and the drive for change. In Towards Sustainable Management of the Boreal Forest; Burton, P.J., Messier, C., Smith, D.W., Adamowicz, W.L., Eds.; NRC Research Press: Ottawa, ON, Canada, 2003; pp. 1–40. [Google Scholar]
- Attiwill, P.M. The disturbance of forest ecosystems: The ecological basis for conservative management. For. Ecol. Manag. 1994, 63, 247–300. [Google Scholar] [CrossRef]
- Bergeron, Y.; Harvey, B. Basing sylviculture on natural ecosystems dynamics: An approach applied to the southern boreal mixedwood forest of Quebec. For. Ecol. Manag. 1997, 92, 235–242. [Google Scholar] [CrossRef]
- Angelstam, P.K. Maintaining and restoring biodiversity in European boreal forests by developing natural disturbance regimes. J. Veg. Sci. 1998, 9, 593–602. [Google Scholar] [CrossRef]
- Gauthier, S.; Vaillancourt, M.-A.; Leduc, A.; de GrandPré, L.; Kneeshaw, D.; Morin, H.; Drapeau, P.; Bergeron, Y. Ecosystem Management in the Boreal Forest; Presses de l’Uniersité du Québec: Québec, QC, Canada, 2009. [Google Scholar]
- Franklin, J.F. Preserving biodiversity: Species, ecosystems, or landscapes? Ecol. Appl. 1993, 3, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Leduc, A.; Bergeron, Y. Forest dynamics modelling under natural fire cycles: A tool to define natural mosaic diversity for forest management. Environ. Monit. Assess. 1996, 39, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Perera, A.H.; Buse, L.J. Emulating Natural Disturbance in Forest Management. In Emulating Natural Forest Landscape Disturbances; Perera, A.H., Buse, L.J., Weber, M.G., Eds.; Columbia University Press: New York, NY, USA, 2004; pp. 3–7. [Google Scholar]
- Niemela, È.J. Management in relation to disturbance in the boreal forest. For. Ecol. Manag. 1999, 115, 127–134. [Google Scholar] [CrossRef]
- Bergeron, Y.; Fenton, N.J. Boreal forests of eastern Canada revisited: Old growth, nonfire disturbances, forest succession, and biodiversity. Botany 2012, 90, 509–523. [Google Scholar] [CrossRef]
- Bergeron, Y.; Harvey, B.; Leduc, A.; Gauthier, S. Forest management guidelines based on natural disturbances dynamics: Stand- and forest-level considerations. For. Chron. 1999, 75, 49–54. [Google Scholar] [CrossRef]
- Cyr, D.; Gauthier, S.; Bergeron, Y.; Carcaillet, C. Forest management is driving the eastern North American boreal forest outside its natural range of variability. Front. Ecol. Environ. 2009, 7, 519–524. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Amiro, B.D.; Cantin, A.; Flannigan, M.D.; de Groot, W.J. Future emissions from Canadian boreal forest fires. Can. J. For. Res. 2009, 39, 383–395. [Google Scholar] [CrossRef]
- De Groot, W.J.; Cantin, A.S.; Flannigan, M.D.; Soja, A.J.; Gowman, L.M.; Newbery, A. A comparison of Canadian and Russian boreal forest fire regimes. For. Ecol. Manag. 2013, 294, 23–34. [Google Scholar] [CrossRef]
- Chapin, F.; Woodwell, G.; Randerson, J.; Rastetter, E.; Lovett, G.; Baldocchi, D.; Clark, D.; Harmon, M.; Schimel, D.; Valentini, R.; et al. Reconciling carbon-cycle concepts, terminology, and methods. Ecosystems 2006, 9, 1041–1050. [Google Scholar] [CrossRef]
- Yang, G.; Di, X.-Y.; Guo, Q.-X.; Shu, Z.; Zeng, T.; Yu, H.-Z.; Wang, C. The impact of climate change on forest fire danger rating in China’s boreal forest. J. For. Res. 2011, 22, 249–257. [Google Scholar] [CrossRef]
- Flannigan, M.D.; Logan, K.A.; Amiro, B.D.; Skinner, W.R.; Stocks, B.D. Future area burned in Canada. Clim. Chang. 2005, 72, 1–16. [Google Scholar] [CrossRef]
- Bergeron, Y.; Cyr, D.; Girardin, M.P.; Carcaillet, C. Will climate change drive 21st century burn rates in Canadian boreal forest outside of its natural variability: Collating global climate model experiments with sedimentary charcoal data. Int. J. Wildl. Fire 2010, 19, 1127–1139. [Google Scholar] [CrossRef]
- Boulanger, Y.; Gauthier, S.; Burton, P.J.; Vaillancourt, M.A. An alternative fire regime zonation for Canada. Int. J. Wildl. Fire 2012, 21, 1052–1064. [Google Scholar] [CrossRef]
- Girardin, M.P.; Hogg, E.H.; Bernier, P.Y.; Kurz, W.A.; Guo, X.J.; Cyr, G. Negative impacts of high temperatures on growth of black spruce forests intensify with the anticipated climate warming. Glob. Chang. Boil. 2016, 22, 627–643. [Google Scholar] [CrossRef] [PubMed]
- De Groot, W.J. Modeling Canadian wildland fire carbon emissions with the Boreal Fire Effects (BORFIRE) model. For. Ecol. Manag. 2006, 234, S224. [Google Scholar] [CrossRef]
- Amiro, B.D.; Todd, J.B.; Wotton, B.M.; Logan, K.A.; Flannigan, M.D.; Stocks, B.J.; Mason, J.A.; Martell, K.G.; Hirsch, K.G. Direct carbon emissions from Canadian forest fires, 1959–1999. Can. J. For. Res. 2001, 31, 512–525. [Google Scholar] [CrossRef]
- Johnston, D.C.; Turetsky, M.R.; Benscoter, B.W.; Wotton, B.M. Fuel load, structure, and potential fire behaviour in black spruce bogs. Can. J. For. Res. 2015, 45, 888–899. [Google Scholar] [CrossRef]
- Scharlemann, J.P.; Hiederer, R.; Kapos, V.; Ravilious, C. Updated Global Carbon Map; UNEP-WCRC & EU-JRC: Cambridge, UK, 2009. [Google Scholar]
- Brown, J.K. Unatural Fuel Buildup Issue. In Proceedings of the Symposium and Workshop on Wilderness Fire, Missoula, MT, USA, 15–18 November 1983.
- Ryan, K.C.; Knapp, E.E.; Varner, J.M. Prescribed fire in North American forests and woodlands: History, current practice, and challenges. Front. Ecol. Environ. 2013, 11, e15–e24. [Google Scholar] [CrossRef]
- Johnson, E.A.; Miyanishi, K.; Bridge, S.R.J. Wildfire regime in the boreal forest and the idea of suppression and fuel buildup. Conserv. Biol. 2001, 15, 1554–1557. [Google Scholar] [CrossRef]
- Simard, M.; Lecomte, N.; Bergeron, Y.; Bernier, P.Y.; Paré, D. Forest productivity decline caused by successional paludification of boreal soils. Ecol. Appl. 2007, 17, 1619–1637. [Google Scholar] [CrossRef] [PubMed]
- Fenton, N.J.; Bergeron, Y. Facilitative succession in a boreal bryophyte community driven by changes in available moisture and light. J. Veg. Sci. 2006, 17, 65–76. [Google Scholar] [CrossRef]
- Terrier, A.; de Groot, W.J.; Girardin, M.P.; Bergeron, Y. Dynamics of moisture content in spruce-feather moss and spruce–Sphagnum organic layers during an extreme fire season and implications for future depths of burn in Clay Belt black spruce forests. Int. J. Wildl. Fire 2014, 23, 490–502. [Google Scholar] [CrossRef]
- Benscoter, B.W.; Thompson, D.K.; Waddington, J.M.; Flannigan, M.D.; Wotton, B.M.; de Groot, W.J.; Turetsky, M.R. Interactive effects of vegetation, soil moisture and bulk density on depth of burning of thick organic soils. Int. J. Wildl. Fire 2011, 20, 418–429. [Google Scholar] [CrossRef]
- Benscoter, B.W.; Wieder, R.K. Variability in organic matter lost by combustion in a boreal bog during the 2001 Chisholm fire. Can. J. For. Res. 2003, 33, 2509–2513. [Google Scholar] [CrossRef]
- Shetler, G.; Turetsky, M.R.; Kane, E.; Kasischke, E. Sphagnum mosses limit total carbon consumption during fire in Alaskan black spruce forests. Can. J. For. Res. 2008, 38, 2328–2336. [Google Scholar] [CrossRef]
- De Groot, W.J.; Bothwell, P.M.; Carlsson, D.H.; Logan, K.A. Simulating the effects of future fire regimes on western Canadian boreal forests. J. Veg. Sci. 2003, 14, 355–364. [Google Scholar] [CrossRef]
- De Groot, W.J.; Landry, R.; Kurz, W.A.; Anderson, K.R.; Englefield, P.; Fraser, R.H.; Hall, R.J.; Banfield, E.; Raymond, D.A.; Decker, V.; et al. Estimating direct carbon emissions from Canadian wildland fires. Int. J. Wildl. Fire 2007, 16, 593–606. [Google Scholar] [CrossRef]
- Vincent, J.S.; Hardy, L. L’évolution et l’extension des lacs glaciaires Barlow et Ojibway en territoire québécois. Géogr. Phys. Quat. 1977, 31, 357–372. [Google Scholar] [CrossRef]
- Bergeron, Y.; Gauthier, S.; Flannigan, M.D.; Kafka, V. Fire regimes at the transition between mixedwoods and coniferous boreal forest in northwestern Quebec. Ecology 2004, 85, 1916–1932. [Google Scholar] [CrossRef]
- Environment Canada. National Climate Data and Information Archive. 2016. Available online: http://climate.weatheroffice.gc.ca/ (accessed on 7 June 2016).
- Fenton, N.; Lecomte, N.; Légaré, S.; Bergeron, Y. Paludification in black spruce (Picea mariana) forests of eastern Canada: Potential factors and management implications. For. Ecol. Manag. 2005, 213, 151–159. [Google Scholar] [CrossRef]
- Lavoie, M.; Paré, D.; Fenton, N.; Groot, A.; Taylor, K. Paludification and management of forested peatlands in Canada: A literature review. Environ. Rev. 2005, 13, 21–50. [Google Scholar] [CrossRef]
- Harper, K.; Boudreault, C.; de Grandpré, L.; Drapeau, P.; Gauthier, S.; Bergeron, Y. Structure, composition and diversity of old-growth black spruce boreal forest of the Clay Belt region in Québec and Ontario. Environ. Rev. 2003, 11, S79–S98. [Google Scholar] [CrossRef]
- Lecomte, N.; Bergeron, Y. Successional pathways on different surficial deposits in the coniferous boreal forest of the Quebec Clay Belt. Can. J. For. Res. 2005, 35, 1984–1995. [Google Scholar] [CrossRef]
- Fenton, N.J.; Béland, C.; de Blois, S.; Bergeron, Y. Sphagnum establishment and expansion in black spruce (Picea mariana) boreal forests. Can. J. Bot. 2007, 85, 43–50. [Google Scholar] [CrossRef]
- Belleau, A.; Leduc, A.; Lecomte, N.; Bergeron, Y. Forest succession rate and pathways on different surface deposit types in the boreal forest of northwestern Quebec. Ecoscience 2011, 18, 329–340. [Google Scholar] [CrossRef]
- Lecomte, N.; Simard, M.; Bergeron, Y. Effects of fire severity and initial tree composition on stand structural development in the coniferous boreal forest of northwestern Québec, Canada. Ecoscience 2006, 13, 152–163. [Google Scholar] [CrossRef]
- Greene, D.F.; Macdonald, S.E.; Haeussler, S.; Domenicano, S.; Noël, J.; Jayen, K.; Charron, I.; Gauthier, S.; Hunt, S.; Gielau, E.T.; et al. The reduction of organic-layer depth by wildfire in the North American boreal forest and its effect on tree recruitment by seed. Can. J. For. Res. 2007, 37, 1012–1023. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Chapin, F.S., III; Hollingsworth, T.N.; Mack, M.C.; Romanovsky, V.; Turetsky, M. Fire, climate change, and forest resilience in interior Alaska. Can. J. For. Res. 2010, 40, 1302–1312. [Google Scholar] [CrossRef]
- Van Cleve, K.; Oliver, L.; Schlentner, R.; Viereck, L.A.; Dyrness, C. Productivity and nutrient cycling in taiga forest ecosystems. Can. J. For. Res. 1983, 13, 747–766. [Google Scholar] [CrossRef]
- Paré, D.; Bernier, P.; Lafleur, B.; Titus, B.D.; Thiffault, E.; Maynard, D.G.; Guo, X. Estimating stand-scale biomass, nutrient contents, and associated uncertainties for tree species of Canadian forests. Can. J. For. Res. 2013, 43, 599–608. [Google Scholar] [CrossRef]
- Brown, J.K.; Oberheu, R.D.; Johnston, C.M. Handbook for Inventorying Surface Fuels and Biomass in the Interior West; General Technical Report INT-GTR-129; U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experimental Station: Ogden, UT, USA, 1982; p. 48.
- Hély, C.; Université de Montpellier 2, Montpellier, France. Personal communication, 2001.
- McRae, D.J.; Alexander, M.E.; Stocks, B.J. Measurement and Description of Fuels and Fire Behavior on Prescribed Burns: A Handbook; Great Lakes Forest Research Centre: Sault Ste Mary, ON, Canada, 1979. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing, R Development Core Team: Vienna, Austria, 2010.
- Terrier, A.; Girardin, M.P.; Cantin, A.; Groot, W.J.; Anyomi, K.A.; Gauthier, S.; Bergeron, Y. Disturbance legacies and paludification mediate the ecological impact of an intensifying wildfire regime in the Clay Belt boreal forest of eastern North America. J. Veg. Sci. 2015, 26, 588–602. [Google Scholar] [CrossRef]
- Van Wagner, C.E. Development and Structure of the Canadian Forest Fire Weather Index System; Technical Report No. 35; Canadian Forestry Service: Ottawa, ON, Canada, 1987.
- Lambert, M.C.; Ung, C.H.; Raulier, F. Canadian national tree aboveground biomass equations. Can. J. For. Res. 2005, 35, 1996–2018. [Google Scholar] [CrossRef]
- Forestry Canada Fire Danger Group. Development and Structure of the Canadian Forest Fire Behavior Prediction System; Information Report ST-X-3; Forestry Canada Fire Danger Group: Ottawa, ON, Canada, 1992. [Google Scholar]
- De Groot, W.J.; Pritchard, J.M.; Lynham, T.J. Forest floor fuel consumption and carbon emissions in Canadian boreal forest fires. Can. J. For. Res. 2009, 39, 367–382. [Google Scholar] [CrossRef]
- Plonski, W.L. Normal Yield Tables (Metric) for Major Forest Species of Ontario; Ontario Ministry of Natural Resources: Toronto, ON, Canada, 1974; p. 40.
- Hély, C.; Bergeron, Y.; Flannigan, M.D. Coarse woody debris in the southeastern Canadian boreal forest: Composition and load variations in relation to stand replacement. Can. J. For. Res. 2000, 30, 674–687. [Google Scholar] [CrossRef]
- Légaré, S.; Bergeron, Y.; Paré, D. Influence of forest composition on understory cover in boreal mixedwood forests of western Quebec. Silva Fenn. 2002, 36, 353–366. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y. Stand structural dynamics of North American boreal forests. Crit. Rev. Plant Sci. 2006, 25, 115–137. [Google Scholar] [CrossRef]
- Hart, S.A.; Chen, H.Y. Understory vegetation dynamics of North American boreal forests. Crit. Rev. Plant Sci. 2006, 25, 381–397. [Google Scholar] [CrossRef]
- Greene, D.F.; Zasada, J.C.; Sirois, L.; Kneeshaw, D.; Morin, H.; Charron, I.; Simard, M.-J. A review of the regeneration dynamics of North. American boreal forest tree species. Can. J. For. Res. 1999, 29, 824–839. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Chapin, F.S., III. Effects of soil burn severity on post-fire tree recruitment in boreal forest. Ecosystems 2006, 9, 14–31. [Google Scholar] [CrossRef]
- Pothier, D.; Savard, F. Actualisation des Tables de Production Pour les Principales Espèces du Québec; RN98-3054; Gouvernement du Québec, Ministère des Ressources Naturelles, Bibliothèque Nationale du Québec: Québec, QC, Canada, 1998.
- Wardle, D.A.; Walker, L.R.; Bardgett, R.D. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 2004, 305, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Kneeshaw, D.D.; Bergeron, Y. Canopy gap characteristics and tree replacement in the southeastern boreal forest. Ecology 1998, 79, 783–794. [Google Scholar] [CrossRef]
- Chen, H.Y.; Popadiouk, R.V. Dynamics of North American boreal mixedwoods. Environ. Rev. 2002, 10, 137–166. [Google Scholar] [CrossRef]
- Pothier, D.; Raulier, F.; Riopel, M. Ageing and decline of trembling aspen stands in Quebec. Can. J. For. Res. 2004, 34, 1251–1258. [Google Scholar] [CrossRef]
- Messier, C.; Parent, S.; Bergeron, Y. Effects of overstory and understory vegetation on the understory light environment in mixed boreal forests. J. Veg. Sci. 1998, 9, 511–520. [Google Scholar] [CrossRef]
- Grandpré, L.; Gagnon, D.; Bergeron, Y. Changes in the understory of Canadian southern boreal forest after fire. J. Veg. Sci. 1993, 4, 803–810. [Google Scholar] [CrossRef]
- Pham, A.T.; Grandpré, L.D.; Gauthier, S.; Bergeron, Y. Gap dynamics and replacement patterns in gaps of the northeastern boreal forest of Quebec. Can. J. For. Res. 2004, 34, 353–364. [Google Scholar] [CrossRef]
- Pedlar, J.H.; Pearce, J.L.; Venier, L.A.; McKenney, D.W. Coarse woody debris in relation to disturbance and forest type in boreal Canada. For. Ecol. Manag. 2002, 158, 189–194. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Gower, S.T. Decomposition and fragmentation of coarse woody debris: Re-visiting a boreal black spruce chronosequence. Ecosystems 2008, 11, 831–840. [Google Scholar] [CrossRef]
- Brais, S.; Paré, D.; Lierman, C. Tree bole mineralization rates of four species of the Canadian eastern boreal forest: Implications for nutrient dynamics following stand-replacing disturbances. Can. J. For. Res. 2006, 36, 2331–2340. [Google Scholar] [CrossRef]
- Päätalo, M.-L. Factors influencing occurrence and impacts of fires in northern European forests. Silva Fenn. 1998, 32, 185–202. [Google Scholar] [CrossRef]
- Hély, C.; Flannigan, M.; Bergeron, Y.; McRae, D. Role of vegetation and weather on fire behavior in the Canadian mixedwood boreal forest using two fire behavior prediction systems. Can. J. For. Res. 2001, 31, 430–441. [Google Scholar] [CrossRef]
- Arienti, M.C.; Cumming, S.G.; Boutin, S. Empirical models of forest fire initial attack success probabilities: The effects of fuels, anthropogenic linear features, fire weather, and management. Can. J. For. Res. 2006, 36, 3155–3166. [Google Scholar] [CrossRef]
- Krawchuk, M.A.; Cumming, S.G.; Flannigan, M.D.; Wein, R.W. Biotic and abiotic regulation of lightning fire initiation in the mixedwood boreal forest. Ecology 2006, 87, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Harden, J.W.; Trumbore, S.E.; Stocks, B.J.; Hirsch, A.; Gower, S.T.; O’neill, K.P.; Kasischke, E.S. The role of fire in the boreal carbon budget. Glob. Chang. Biol. 2000, 6, 174–184. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Kane, E.S.; Harden, J.W.; Ottmar, R.D.; Manies, K.L.; Hoy, E.; Kasischke, E.S. Recent acceleration of biomass burning and carbon losses in Alaskan forests and peatlands. Nat. Geosci. 2011, 4, 27–31. [Google Scholar] [CrossRef]
- Whelan, R.J. The Ecology of Fire; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Fleming, R.A.; Candau, J.-N.; McAlpine, R.S. Landscape-Scale Analysis of Interactions between Insect Defoliation and Forest Fire in Central Canada. Clim. Chang. 2002, 55, 251–272. [Google Scholar] [CrossRef]
- Gignac, L.D.; Vitt, D.H. Responses of northern peatlands to climate change: Effects on bryophytes. Hattori Shokubutsu Kenkyujo Hokoku 1994, 75, 119–132. [Google Scholar]
- Breeuwer, A.; Robroek, B.J.; Limpens, J.; Heijmans, M.M.; Schouten, M.G.; Berendse, F. Decreased summer water table depth affects peatland vegetation. Basic Appl. Ecol. 2009, 10, 330–339. [Google Scholar] [CrossRef]
- Wickland, K.P.; Neff, J.C. Decomposition of soil organic matter from boreal black spruce forest: Environmental and chemical controls. Biogeochemistry 2008, 87, 29–47. [Google Scholar] [CrossRef]
| TSF (Years) | Forest Type | |||
|---|---|---|---|---|
| TA | JP | BS-S | BS-NS | |
| <90 | 6 | 7 | 5 | 5 |
| 90–150 | 4 | 1 | 5 | 5 |
| >150 | 2 | 7 | 8 | 6 |
| Total | 12 | 15 | 18 | 16 |
| Fuel | p-Value | R2 | Model | |
|---|---|---|---|---|
| TA | Trees | ≤0.005 | 0.92 | |
| Fine aerial | ≤0.05 | 0.47 | ||
| DWD | ≤0.001 | 0.84 | ||
| Duff | NS | NS | NS | |
| Total | ≤0.05 | 0.49 | ||
| JP | Trees | ≤0.005 1 | 0.78 | |
| Fine aerial | NS | NS | NS | |
| DWD | NS | NS | NS | |
| Duff | ≤0.001 | 0.65 | ||
| Total | ≤0.05 | 0.19 | ||
| BS-S | Trees | ≤0.001 1 | 0.52 | |
| Fine aerial | NS | NS | NS | |
| DWD | ≤0.05 1 | 0.16 | ||
| Duff | ≤0.001 | 0.81 | ||
| Total | ≤0.001 | 0.71 | ||
| BS-NS | Trees | ≤0.001 1 | 0.48 | |
| Fine aerial | NS | NS | NS | |
| DWD | ≤0.05 1 | 0.34 | ||
| Duff | ≤0.05 | 0.38 | ||
| Total | ≤0.005 | 0.48 |
| Forest Type | Successional Stage | Total (t/ha) | Contribution of Fuel Category | ||
|---|---|---|---|---|---|
| Tree (%) | DWD (%) | Duff (%) | |||
| TA | TSF < 90 years | 16.2 | 0 | 20 | 80 |
| TSF between 90 and 150 years | 21.4 | 0 | 11 | 89 | |
| TSF > 150 years | 14.8 | 0 | 10 | 90 | |
| JP | TSF < 90 years | 17.9 | 14 | 21 | 65 |
| TSF between 90 and 150 years | 24.7 | 0 | 9 | 91 | |
| TSF > 150 years | 18.5 | 0 | 6 | 94 | |
| BS-S | TSF < 90 years | 31.6 | 6 | 3 | 91 |
| TSF between 90 and 150 years | 44.3 | 6 | 5 | 89 | |
| TSF > 150 years | 12.5 | 2 | 12 | 86 | |
| BS-NS | TSF < 90 years | 52.3 | 3 | 1 | 96 |
| TSF between 90 and 150 years | 17.4 | 3 | 6 | 91 | |
| TSF > 150 years | 1.9 | 0 | 53 | 47 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrier, A.; Paquette, M.; Gauthier, S.; Girardin, M.P.; Pelletier-Bergeron, S.; Bergeron, Y. Influence of Fuel Load Dynamics on Carbon Emission by Wildfires in the Clay Belt Boreal Landscape. Forests 2017, 8, 9. https://doi.org/10.3390/f8010009
Terrier A, Paquette M, Gauthier S, Girardin MP, Pelletier-Bergeron S, Bergeron Y. Influence of Fuel Load Dynamics on Carbon Emission by Wildfires in the Clay Belt Boreal Landscape. Forests. 2017; 8(1):9. https://doi.org/10.3390/f8010009
Chicago/Turabian StyleTerrier, Aurélie, Mathieu Paquette, Sylvie Gauthier, Martin P. Girardin, Sylvain Pelletier-Bergeron, and Yves Bergeron. 2017. "Influence of Fuel Load Dynamics on Carbon Emission by Wildfires in the Clay Belt Boreal Landscape" Forests 8, no. 1: 9. https://doi.org/10.3390/f8010009






