Intra-Annual Variation of Stem Radius of Larix principis-rupprechtii and Its Response to Environmental Factors in Liupan Mountains of Northwest China
Abstract
:1. Introduction
2. Materials and Methods
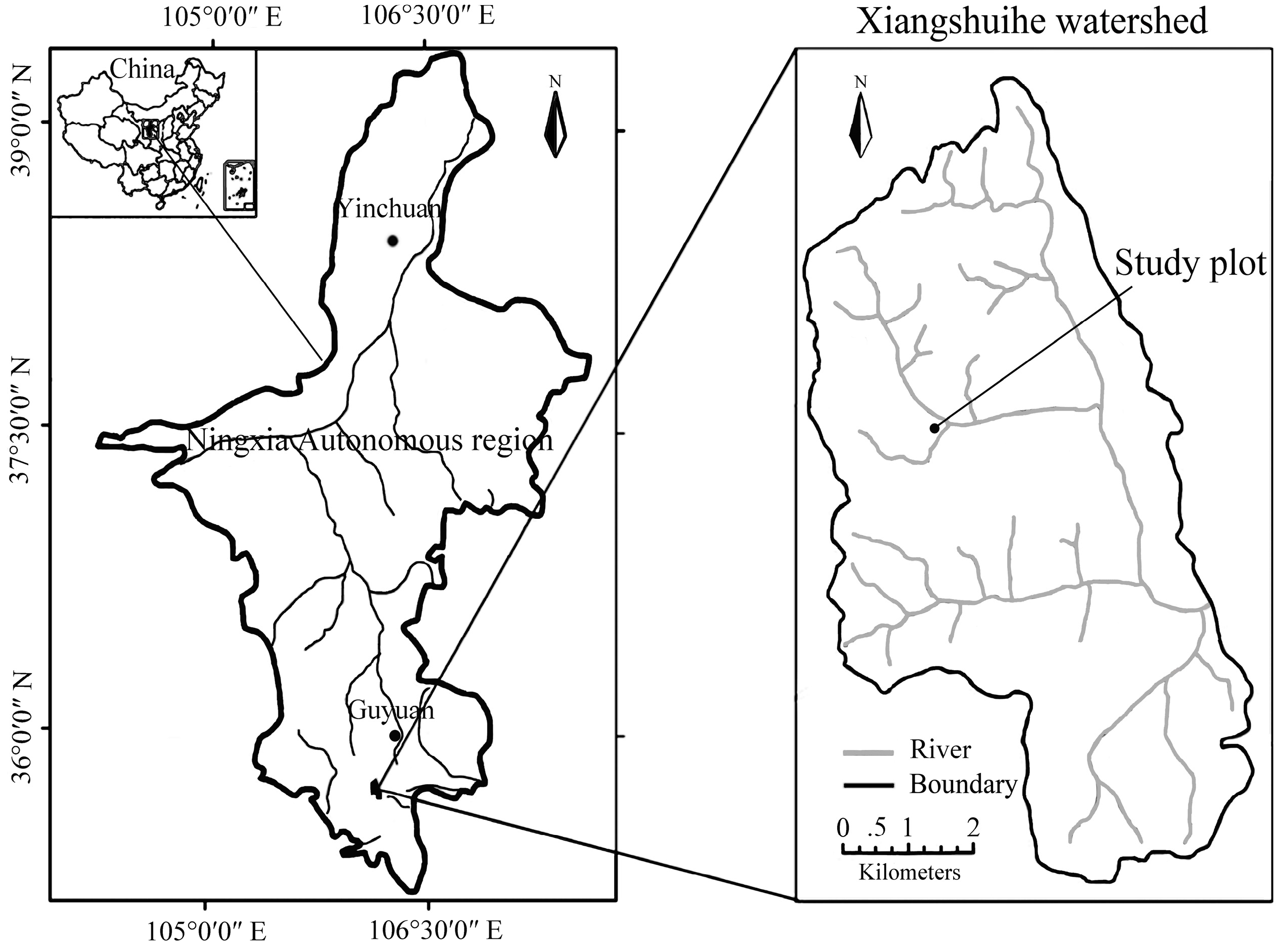
2.1. Study Site and Local Climate
2.2. Dendrometer Data Collection
2.3. Meteorological and Soil Moisture Measurements
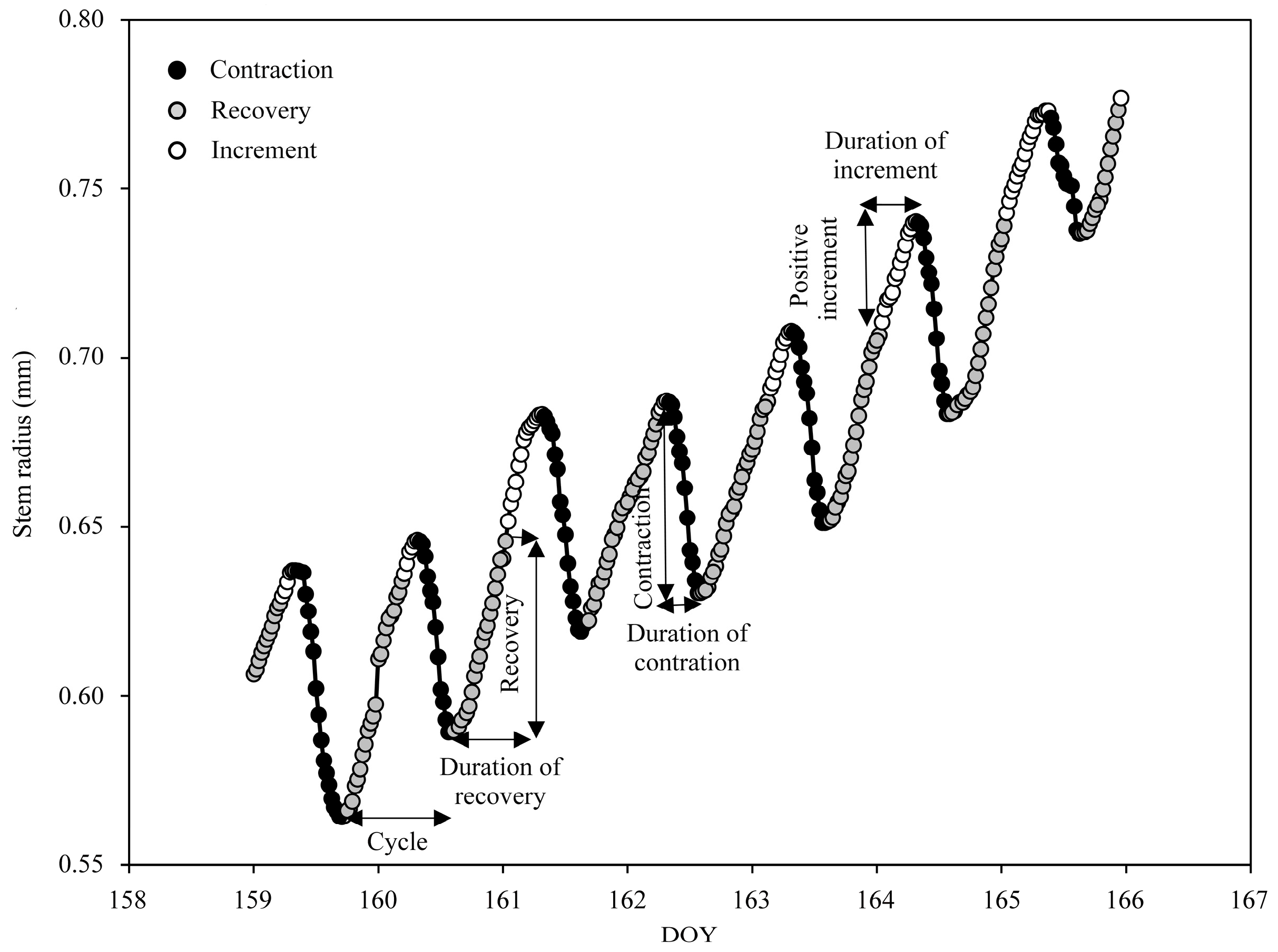
2.4. Data Analysis
3. Results
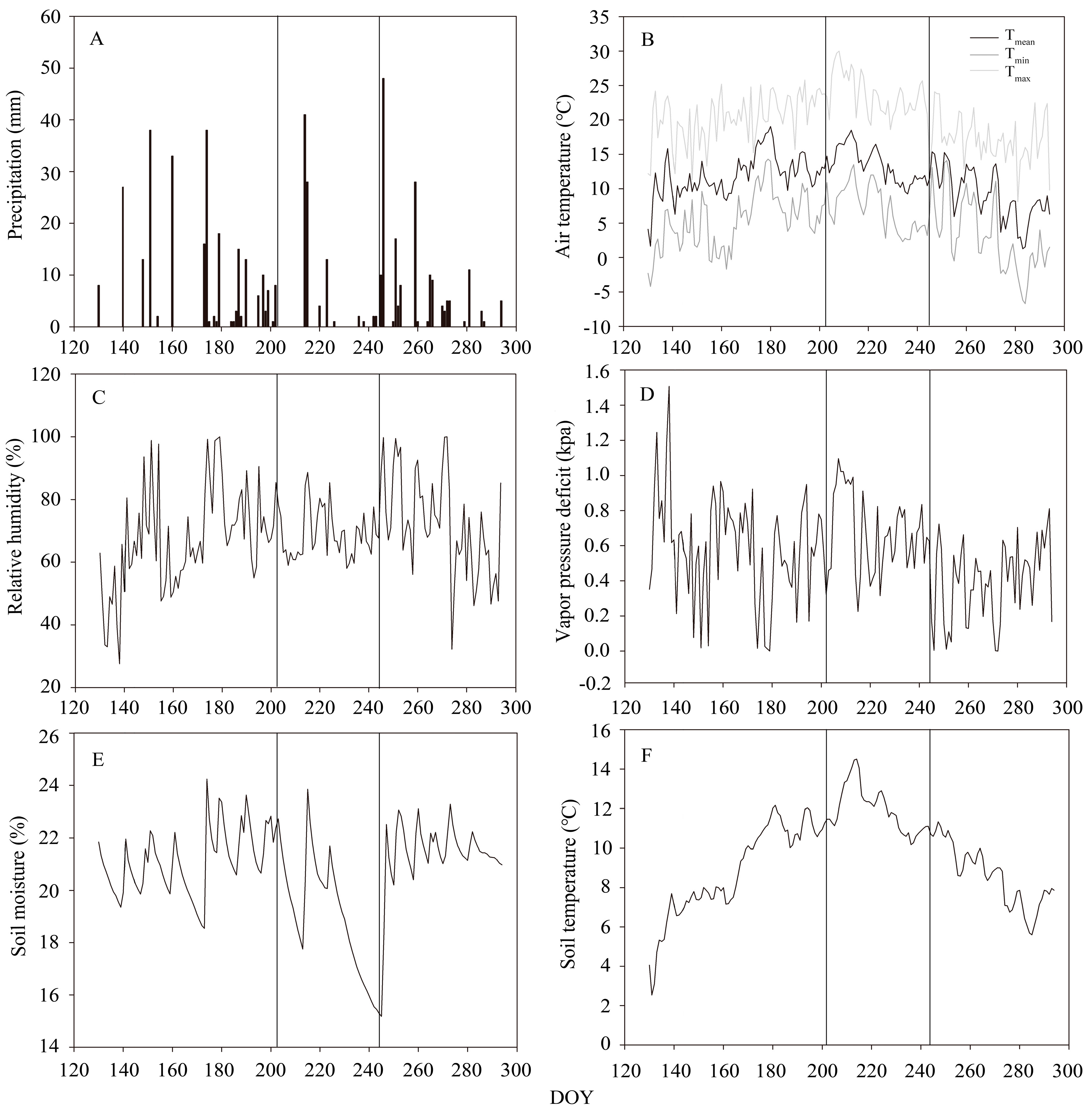
3.1. Meteorological Conditions
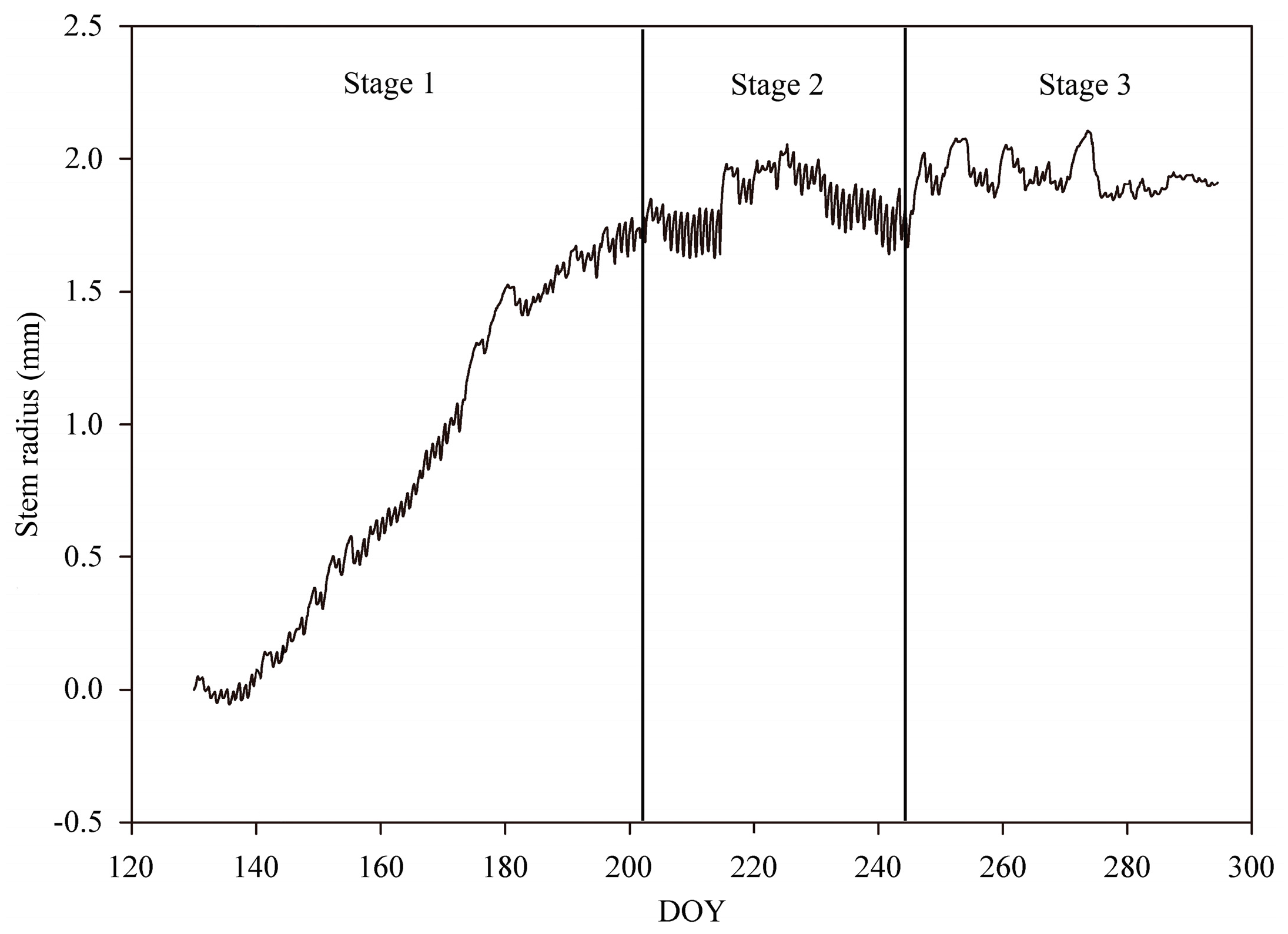
3.2. Stem Radius Variations
3.3. Seasonal Changes in the Amplitude and Duration of Radius Variation Phases
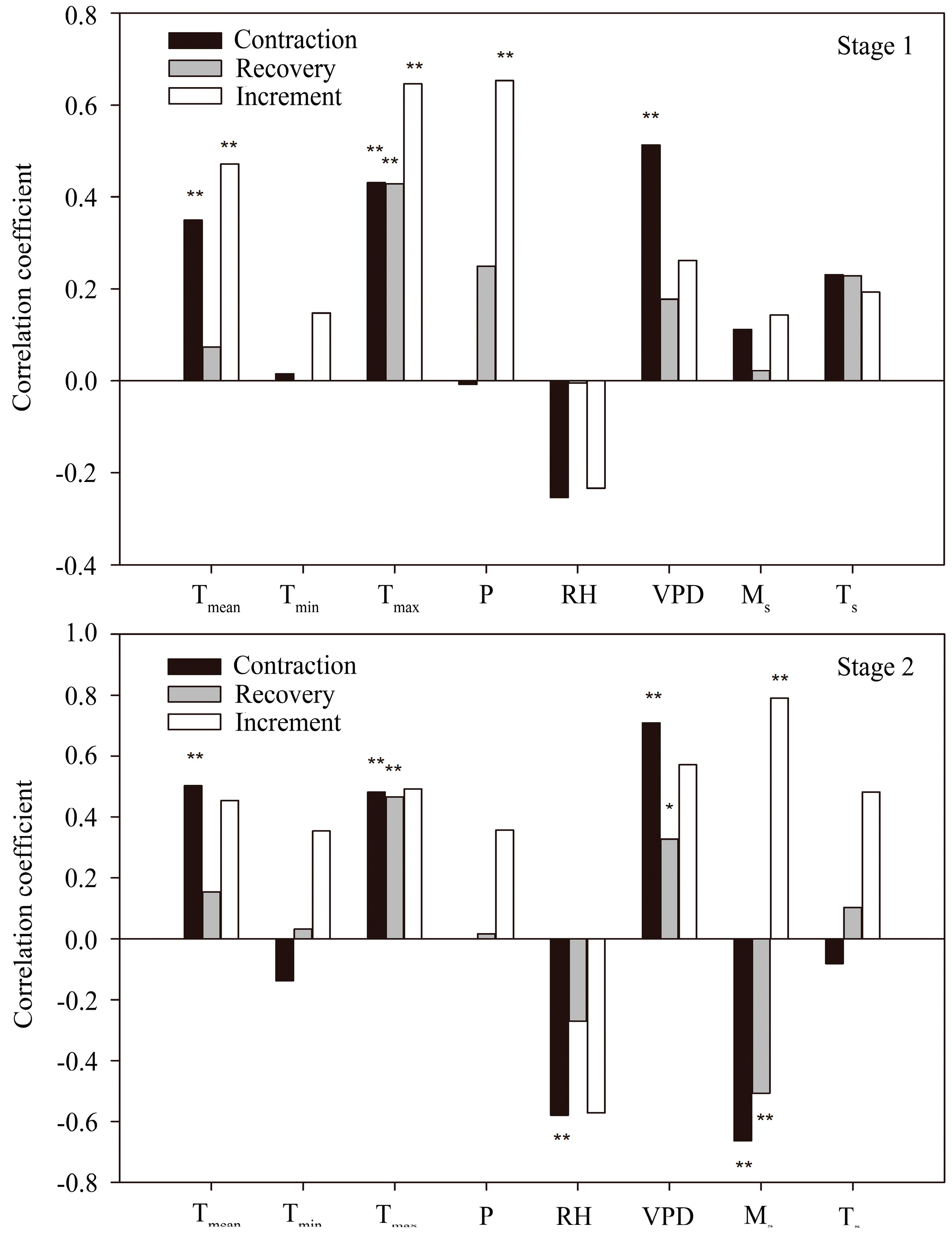
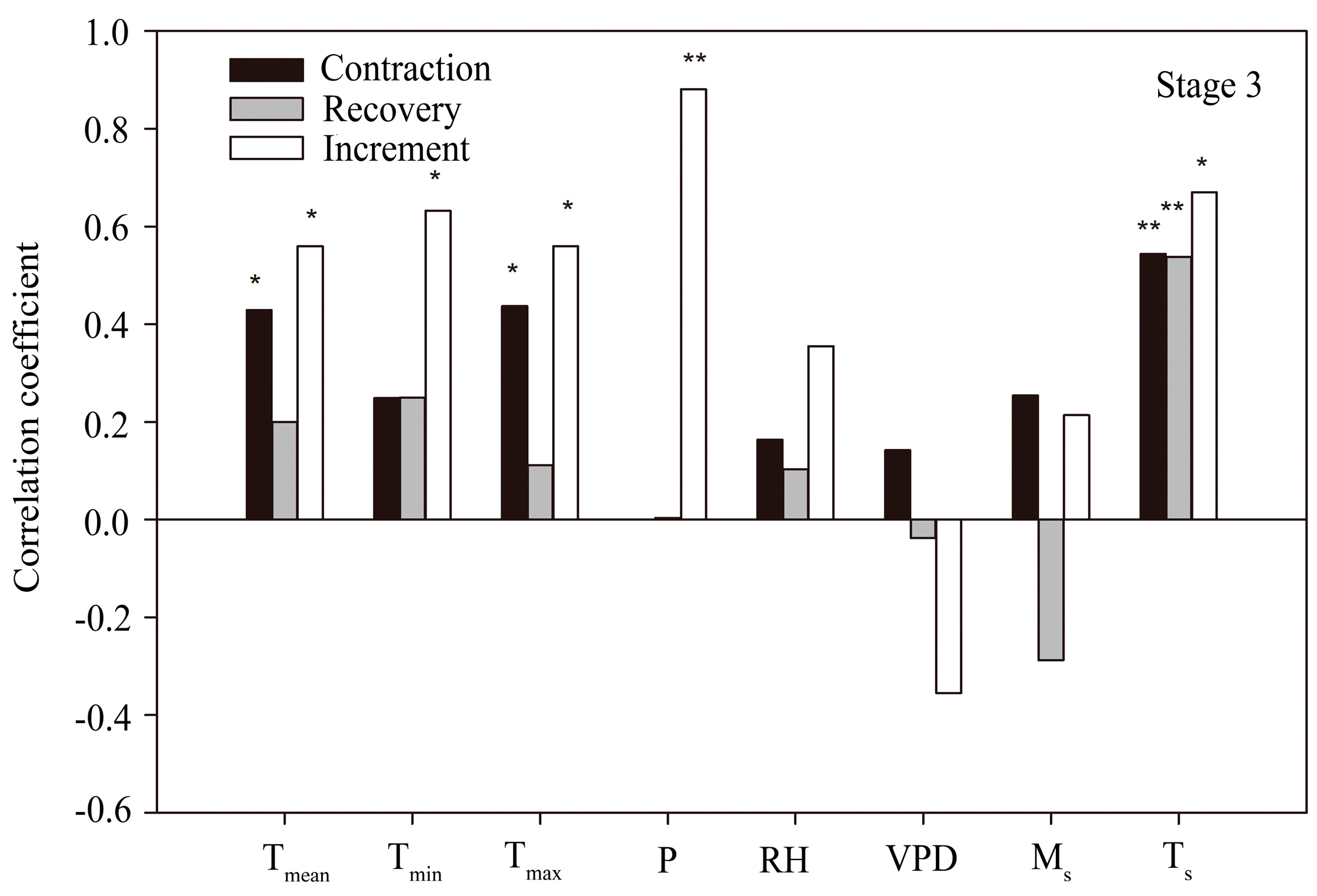
3.4. Correlation between Stem Radius and Environmental Factors
4. Discussion
4.1. Intra-Annual Variations of Stem Radius
4.2. Controlling Factors for Stem Radius Variation
4.2.1. Stem Radius Contraction
4.2.2. Stem Radius Recovery
4.2.3. Stem Radius Increment
4.3. Implications for Climate-Growth Relationship Studies
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guarín, A.; Taylor, A.H. Drought triggered tree mortality in mixed conifer forests in Yosemite National Park, California, USA. For. Ecol. Manag. 2005, 218, 229–244. [Google Scholar]
- Bigler, C.; Gavin, D.G.; Gunning, C.; Veblen, T.T. Drought induces lagged tree mortality in a subalpine forest in the Rocky Mountains. Oikos 2007, 116, 1983–1994. [Google Scholar]
- Peltola, H.; Kilpeläinen, A.; Kellomäki, S. Diameter growth of Scots pine (Pinus sylvestris) trees grown at elevated temperature and carbon dioxide concentration under boreal conditions. Tree Physiol. 2002, 22, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Kolb, T.E. Tree growth response to drought and temperature in a mountain landscape in northern Arizona, USA. J. Biogeogr. 2005, 32, 1629–1640. [Google Scholar] [CrossRef]
- Liu, H.; Williams, A.P.; Allen, C.D.; Guo, D.; Wu, X.; Anenkhonov, O.A.; Liang, E.; Sandanov, D.V.; Yin, Y.; Qi, Z.; et al. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob. Chang. Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Taschler, D.; Neuner, G. Summer frost resistance and freezing patterns measured in situ in leaves of major alpine plant growth forms in relation to their upper distribution boundary. Plant Cell Environ. 2004, 27, 737–746. [Google Scholar]
- Gruber, A.; Baumgartner, D.; Zimmermann, J.; Oberhuber, W. Temporal dynamic of wood formation in Pinus cembra along the alpine treeline ecotone and the effect of climate variables. Trees 2009, 23, 623–635. [Google Scholar]
- Gutiérrez, E.; Campelo, F.; Camarero, J.J.; Ribas, M.; Muntán, E.; Nabais, C.; Freitas, H. Climate controls act at different scales on the seasonal pattern of Quercus ilex L. stem radial increments in NE Spain. Trees 2011, 25, 637–646. [Google Scholar]
- Köcher, P.; Horna, V.; Leuschner, C. Environmental control of daily stem growth patterns in five temperate broad-leaved tree species. Tree Physiol. 2012, 32, 1021–1032. [Google Scholar]
- McMahon, S.M.; Parker, G.G. A general model of intra-annual tree growth using dendrometer bands. Ecol. Evol. 2015, 5, 243–254. [Google Scholar] [PubMed]
- Drew, D.M.; Downes, G.M. The use of precision dendrometers in research on daily stem size and wood property variation: A review. Dendrochronologia 2009, 27, 159–172. [Google Scholar] [CrossRef]
- Pérez, C.A.; Carmona, M.R.; Aravena, J.C.; Farina, J.M.; Armesto, J.J. Environmental controls and patterns of cumulative radial increment of evergreen tree species in montane, temperate rainforests of Chiloé Island, southern Chile. Austral Ecol. 2009, 34, 259–271. [Google Scholar] [CrossRef]
- Duchesne, L.; Houle, D. Modelling day-to-day stem diameter variation and annual growth of balsam fir (Abies balsamea (L.) Mill.) from daily climate. For. Ecol. Manag. 2011, 262, 863–872. [Google Scholar] [CrossRef]
- King, G.; Fonti, P.; Nievergelt, D.; Büntgen, U.; Frank, D. Climatic drivers of hourly to yearly tree radius variations along a 6 °C natural warming gradient. Agric. For. Meteorol. 2013, 168, 36–46. [Google Scholar] [CrossRef]
- Vieira, J.; Rossi, S.; Campelo, F.; Freitas, H.; Nabais, C. Seasonal and daily cycles of stem radial variation of Pinus pinaster in a drought-prone environment. Agric. For. Meteorol. 2013, 180, 173–181. [Google Scholar] [CrossRef]
- Dong, M.; Jiang, Y.; Zhang, W.; Yang, Y.; Yang, H. Effect of alpine treeline conditions on the response of the stem radial variation of Picea meyeri Rebd. et Wils to environmental factors. Pol. J. Ecol. 2011, 59, 729–739. [Google Scholar]
- Bräuning, A.; Volland-Voigt, F.; Burchardt, I.; Ganzhi, O.; Nauss, T.; Peters, T. Climatic control of radial growth of Cedrela montana in a humid mountain rainforest in southern Ecuador. Erdkunde 2009, 63, 337–345. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, D. Point pattern analysis of different age-classes of Larix principis-rupprechtii in Luya Mountain Reserve, Shanxi Province, China. Front. Biol. China 2007, 2, 69–74. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, F.; Wang, W.; Wang, W.; Chen, Q.; Zhao, Y.; Ma, Y. Soil nutrients and enzyme activities in Larix principis-rupprechtii plantations in the Qinling Mountains, China. Acta Ecol. Sin. 2015, 35, 1086–1094, (In Chinese with English abstract). [Google Scholar]
- Lu, X.; He, K.; Wang, H.; Chen, C.; Wang, S.; Wang, W.; Chang, X.; Zhao, L.; Wang, X.; An, G. Simulation of canopy rainfall interception of the Larix principis-rupprechtii artificial forest with the Gash model in high-cold areas of Qinhai province. J. Soil Water Conserv. 2014, 28, 44–48, (In Chinese with English abstract). [Google Scholar]
- Liu, C.; Gu, J.; Li, J.; Chen, P.; Lu, G.; Tian, G. Correlated analysis between the growth of Larix principis-rupprechtii and climatic factors in Saihanba nature reserve, northern Heibei Province. J. Beijing. Fore. Univ. 2009, 31, 102–105, (In Chinese with English abstract). [Google Scholar]
- Liu, Z.; Wang, Y.; Xu, L.; Liu, Y.; Deng, X.; Wang, Y.; Zuo, H. Temporal stability of soil moisture on a slope covered by Larix principis-rupprechtii plantation in Liupan Mountains. J. Soil Water Conserv. 2017, 31, 153–159, (In Chinese with English abstract). [Google Scholar]
- Xiong, W.; Wang, Y.; Yu, P.; Liu, H.; Shi, Z.; Guan, W. Growth in stem diameter of Larix principis-rupprechtii and its response to meteorological factors in the south of Liupan Mountain, China. Acta Ecol. Sin. 2007, 27, 432–440. [Google Scholar] [CrossRef]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics, 2nd ed.; Springer: New York, NY, USA, 1998. [Google Scholar]
- Downes, G.; Beadle, C.; Worledge, D. Daily stem growth patterns in irrigated Eucalyptus globutus and E. nitens in relation to climate. Trees 1999, 14, 102–111. [Google Scholar]
- Deslauriers, A.; Morin, H.; Urbinati, C.; Carrer, M. Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Québec (Canada). Trees 2003, 17, 477–484. [Google Scholar] [CrossRef]
- Deslauriers, A.; Rossi, S.; Turcotte, A.; Morin, H.; Krause, C. A three-step procedure in SAS to analyze the time series from automatic dendrometers. Dendrochronologia 2011, 29, 151–161. [Google Scholar] [CrossRef]
- Van der Maaten, E.; van der Maaten-Theunissen, M.; Smiljanić, M.; Rossi, S.; Simard, S.; Wilmking, M.; Deslauriers, A.; Fonti, P.; von Arx, G.; Bouriaud, O. dendrometeR: Analyzing the pulse of trees in R. Dendrochronologia 2016, 40, 12–16. [Google Scholar] [CrossRef]
- Volland-Voigt, F.; Bräuning, A.; Ganzhi, O.; Peters, T.; Maza, H. Radial stem variations of Tabebuia chrysantha (Bignoniaceae) in different tropical forest ecosystems of southern Ecuador. Trees 2011, 25, 39–48. [Google Scholar] [CrossRef]
- Gruber, A.; Zimmermann, J.; Wieser, G.; Oberhuber, W. Effects of climate variables on intra-annual stem radial increment in Pinus cembra (L.) along the alpine treeline ecotone. Ann. For. Sci. 2009, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Xiao, H.; Peng, X.; Tian, Q. Daily and seasonal stem radial activity of Populus euphratica and its association with hydroclimatic factors in the lower reaches of China’s Heihe River basin. Environ. Earth Sci. 2014, 72, 609–621. [Google Scholar] [CrossRef]
- Zweifel, R.; Item, H.; Häsler, R. Stem radius changes and their relation to stored water in stems of young Norway spruce trees. Trees 2000, 15, 50–57. [Google Scholar] [CrossRef]
- Améglio, T.; Cochard, H.; Ewers, F.W. Stem diameter variations and cold hardiness in walnut trees. J. Exp. Bot. 2001, 52, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T.T.; Winget, C.H. Diurnal and seasonal variation in radii of tree stems. Ecology 1964, 45, 149–155. [Google Scholar] [CrossRef]
- Li, Y.; Shi, H.; Zhang, A.; Tan, H. Daily changes in radial growth of several tree stems and their response to environmental factors in Loess Hilly Region. J. Soil Water Conserv. 2007, 21, 170–173, (In Chinese with English abstract). [Google Scholar]
- Liu, Z.; Wang, Y.; Liu, Y.; Tian, A.; Wang, Y.; Zuo, H. Spatiotemporal variation and scale effect of canopy leaf area index of larch plantation on a slope of the semi-humid Liupan Mountains, Ningxia, China. Chin. J. Plant Ecol. 2017, 41, 749–760, (In Chinese with English abstract). [Google Scholar]
- Jiang, Y.; Zhang, Y.; Guo, Y.; Kang, M.; Wang, M.; Wang, B. Intra-annual xylem growth of Larix principis-rupprechtii at its upper and lower distribution limits on the Luyashan Mountain in North-Central China. Forests 2015, 6, 3809–3827. [Google Scholar] [CrossRef]
- Oberhuber, W.; Gruber, A.; Kofler, W.; Swidrak, I. Radial stem growth in response to microclimate and soil moisture in a drought-prone mixed coniferous forest at an inner Alpine site. Eur. J. For. Res. 2014, 133, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, W.; Gruber, A. Climatic influences on intra-annual stem radial increment of Pinus sylvestris (L.) exposed to drought. Trees 2010, 24, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, R.; Häsler, R. Dynamics of water storage in mature subalpine Picea abies: Temporal and spatial patterns of change in stem radius. Tree Physiol. 2001, 21, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.M.; Downes, G.M.; Grzeskowiak, V.; Naidoo, T. Differences in daily stem size variation and growth in two hybrid eucalypt clones. Trees 2009, 23, 585–595. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yuan, Y.; Gou, X.; Zhang, T.; Zou, C.; Ji, C.; Fan, Z.; Qin, L.; Shang, H.; Li, X. Intra-annual radial growth of Schrenk spruce (Picea schrenkiana Fisch. et Mey) and its response to climate on the northern slopes of the Tianshan Mountains. Dendrochronologia 2016, 40, 36–42. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Yuan, L.; Wang, Q.; Zheng, K.; Zhao, C. Environmental factors effect on stem radial variations of Picea crassifolia in Qilian Mountains, Northwestern China. Forests 2016, 7, 210. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, B.; Dong, M.; Huang, Y.; Wang, M.; Wang, B. Response of daily stem radial growth of Platycladus orientalis to environmental factors in a semi-arid area of North China. Trees 2015, 29, 87–96. [Google Scholar] [CrossRef]
- Devine, W.D.; Harrington, C.A. Factors affecting diurnal stem contraction in young Douglas-fir. Agric. For. Meteorol. 2011, 151, 414–419. [Google Scholar] [CrossRef]
- Li, X.; Liu, R.; Mao, Z.; Song, Y.; Liu, L.; Sun, T. Daily stem radial variation of Pinus koraiensis and its response to meteorological parameters in Xiaoxinganling Mountains. Acta Ecol. Sin. 2014, 34, 1635–1644, (In Chinese with English abstract). [Google Scholar]
- Čermák, J.; Kučera, J.; Bauerle, W.L.; Phillips, N.; Hinckley, T.M. Tree water storage and its diurnal dynamics related to sap flow and changes in stem volume in old-growth Douglas-fir trees. Tree Physiol. 2007, 27, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Ripullone, F.; Guerrieri, M.R.; Nole’, A.; Magnani, F.; Borghetti, M. Stomatal conductance and leaf water potential responses to hydraulic conductance variation in Pinus pinaster seedlings. Trees 2007, 21, 371–378. [Google Scholar] [CrossRef]
- Jarvis, P.G.; McNaughton, K.G. Stomatal control of transpiration: Scaling up from leaf to region. Adv. Ecol. Res. 1986, 15, 1–49. [Google Scholar]
- Sun, L.; Guan, W.; Wang, Y.; Xu, L.; Xiong, W. Simulations of Larix principis-rupprechtii stand mean canopy stomatal conductance and its responses to environmental factors. Chin. J. Ecol. 2011, 30, 2122–2128, (In Chinese with English abstract). [Google Scholar]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lenz, A.; Hoch, G.; Körner, C. Early season temperature controls cambial activity and total tree ring width at the alpine treeline. Plant Ecol. Divers. 2013, 6, 365–375. [Google Scholar] [CrossRef]
- Deslauriers, A.; Rossi, S.; Anfodillo, T.; Saracino, A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008, 28, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Gričar, J.; Zupančič, M.; Čufar, K.; Koch, G.; Schmitt, U.W.E.; Oven, P. Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies). Ann. Bot. 2006, 97, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; He, Z.; Xiao, S.; Peng, X.; Ding, A.; Lin, P. Response of stem radial growth of Qinghai spruce (Picea crassifolia) to environmental factors in the Qilian Mountains of China. Dendrochronologia 2017, 44, 76–83. [Google Scholar] [CrossRef]
- Gemmer, M.; Becker, S.; Jiang, T. Observed monthly precipitation trends in China 1951–2002. Theor. Appl. Climatol. 2004, 77, 39–45. [Google Scholar] [CrossRef]
- Kirdyanov, A.; Hughes, M.; Vaganov, E.; Schweingruber, F.; Silkin, P. The importance of early summer temperature and date of snow melt for tree growth in the Siberian Subarctic. Trees 2003, 17, 61–69. [Google Scholar] [CrossRef]
- Sun, C.; Liu, Y. Climate response of tree radial growth at different timescales in the Qinling Mountains. PLoS ONE 2016, 11, e0160938. [Google Scholar] [CrossRef]
- Steppe, K.; Lemeur, R. Effects of ring-porous and diffuse-porous stem wood anatomy on the hydraulic parameters used in a water flow and storage model. Tree Physiol. 2007, 27, 43–52. [Google Scholar] [CrossRef]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Pannatier, E.G.; Rigling, A. Drought alters timing, quantity, and quality of wood formation in Scots pine. J. Exp. Bot. 2011, 62, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Biondi, F.; Hartsough, P. Using automated point dendrometers to analyze tropical treeline stem growth at Nevado de Colima, Mexico. Sensors 2010, 10, 5827–5844. [Google Scholar] [CrossRef] [PubMed]
- Bouriaud, O.; Bréda, N.; Le Moguedec, G.; Nepveu, G. Modelling variability of wood density in beech as affected by ring age, radial growth and climate. Trees 2004, 18, 264–276. [Google Scholar] [CrossRef]
- Thabeet, A.; Vennetier, M.; Gadbin-Henry, C.; Denelle, N.; Roux, M.; Caraglio, Y.; Vila, B. Response of Pinus sylvestris L. to recent climatic events in the French Mediterranean region. Trees 2009, 23, 843–853. [Google Scholar] [CrossRef]
- Swinton, S.M. Drought survival tactics of subsistence farmers in Niger. Hum. Ecol. 1988, 16, 123–144. [Google Scholar] [CrossRef]
- Li, Y.; Ye, W.; Wang, M.; Yan, X. Climate change and drought: A risk assessment of crop-yield impacts. Clim. Res. 2009, 39, 31–46. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; Van der Schrier, G.; Scheffield, J.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees 2005, 19, 402–408. [Google Scholar] [CrossRef]
- Zhu, H.; Shao, X.; Yin, Z.; Xu, P.; Xu, Y.; Tian, H. August temperature variability in the southeastern Tibetan Plateau since AD 1385 inferred from tree rings. Palaeogeogr. Palaeocl. 2011, 305, 84–92. [Google Scholar] [CrossRef]
- Li, X.; Liang, E.; Gričar, J.; Prislan, P.; Rossi, S.; Čufar, K. Age dependence of xylogenesis and its climatic sensitivity in Smith fir on the south-eastern Tibetan Plateau. Tree Physiol. 2012, 33, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Deslauriers, A.; Griçar, J.; Seo, J.W.; Rathgeber, C.B.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]

| Mean DBH (cm) | Mean Tree Height (m) | Stand Density (Tree·hm−2) | Crown Density (%) | Mean Crown Diameter (m) | Soil Bulk Density of 0–60 cm Layer (g·cm−3) | Field Capacity of 0–60 cm Soil Layer (%) | Total Porosity of 0–60 cm Soil Layer (%) |
|---|---|---|---|---|---|---|---|
| 18.7 | 16.2 | 907 | 65 | 3.3 | 0.94 | 33.1 | 56.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, Y.; Tian, A.; Yu, P.; Xiong, W.; Xu, L.; Wang, Y. Intra-Annual Variation of Stem Radius of Larix principis-rupprechtii and Its Response to Environmental Factors in Liupan Mountains of Northwest China. Forests 2017, 8, 382. https://doi.org/10.3390/f8100382
Liu Z, Wang Y, Tian A, Yu P, Xiong W, Xu L, Wang Y. Intra-Annual Variation of Stem Radius of Larix principis-rupprechtii and Its Response to Environmental Factors in Liupan Mountains of Northwest China. Forests. 2017; 8(10):382. https://doi.org/10.3390/f8100382
Chicago/Turabian StyleLiu, Zebin, Yanhui Wang, Ao Tian, Pengtao Yu, Wei Xiong, Lihong Xu, and Yarui Wang. 2017. "Intra-Annual Variation of Stem Radius of Larix principis-rupprechtii and Its Response to Environmental Factors in Liupan Mountains of Northwest China" Forests 8, no. 10: 382. https://doi.org/10.3390/f8100382






